Abstract
Aims
To assess if the inhibitory potency of nonsteroidal anti-inflammatory drugs (NSAIDs) on cyclooxygenase (COX) isoenzymes, when given therapeutically in humans, can be predicted from their in vitro concentration-response curves using the whole blood assay.
Methods
Twenty-four healthy male volunteers aged 20–27 years were recruited. Inhibition of blood COX isoenzymes was determined in vitro before any drug intake and ex vivo after single and repeated intake of either 7.5 mg meloxicam once, 400 mg ibuprofen three times daily or 75 mg diclofenac SR once, taken in a randomized cross-over design. Production of thromboxane B2 (TXB2) during clotting and of prostaglandin E2 (PGE2) during endotoxin exposure served as indicators of platelet COX-1 and monocyte COX-2 activity, respectively. Drugs were determined in plasma by h.p.l.c., with a chiral separation of ibuprofen and free fractions after equilibrium dialysis.
Results
Intra-subject variation for COX-1 and COX-2 at baseline was at 26±18% and 18±13% respectively, and intersubject variation at 39% and 36%, respectively. The ratios of IC50s and, at best, of IC80s revealed diclofenac and meloxicam as selective COX-2 inhibitors and ibuprofen as a preferential COX-1 inhibitor in vitro. However, after oral intake, ibuprofen inhibited ex vivo COX-2 by 80% whereas diclofenac inhibited COX-1 by 70%. Meloxicam inhibited COX-1 from 30 to 55% depending on the repetition of the dose and increase in plasma concentrations. Using in vitro dose–response curves, the in vivo inhibitory potency of diclofenac was estimated adequately from its circulating concentration ([−0.18, 0.21] for COX-1 and [−0.13, −0.03] for COX-2) but this was not the case for ibuprofen on COX-2 ([−0.14, 0.27]) and meloxicam on COX-1 ([0.31, 1.05]). The limited predictability of the system was not improved through considering the unbound fraction of the drugs or the variable chiral inversion of ibuprofen.
Conclusions
Assessment of COX-2 selectivity based on in vitro studies and pharmacological modelling has a limited clinical relevance. There is a need to investigate COX selectivity at therapeutic plasma concentrations of NSAIDs using the ex vivo whole blood assay.
Keywords: COX isoenzymes, enantioselectivity, NSAIDs, protein binding, whole blood assay
Introduction
The widely accepted mechanism of action of nonsteroidal anti-inflammatory drugs (NSAIDs) is to inhibit the conversion of arachidonic acid (AA) into cyclic endoperoxides by the enzyme cyclooxygenase (COX, prostaglandin endoperoxide synthase, PGHS) [1]. Since this last decade, we know that COX exists in at least two isoforms, COX-1 and COX-2, encoded by separate genes and thought to participate differently in physiological situations and disease processes [2, 3]. This new dichotomy, between the ‘mostly physiological’ COX-1 and the ‘mostly pathological’ COX-2, has generated a lot of studies comparing NSAIDs for their ability to inhibit both COX enzymes [4, 5]. A variety of in vitro enzyme and cell-based assay models have been developed for that purpose and to guide the discovery of new molecules [4, 5], based on the assumption that a marked inhibition of COX-2 without any significant inhibition of COX-1 may characterize NSAIDs with an improved side-effect profile [6]. In these systems, the selectivity of NSAIDs on COX activity is determined generally by the ratio of their IC50 (concentration of the drug inhibiting enzyme activity by 50%) values for COX-1 and COX-2 [4, 5, 7]. As a consequence, NSAIDs were classified by their relative potency on cyclooxygenase isoenzymes rather than by their chemical structure [8], although this new classification was mainly based on in vitro data collected from miscellaneous biological systems [8]. Unfortunately, each in vitro assay system has clear advantages but also certain drawbacks, inherent to the system itself, i.e. its clinical relevance [9], or related to the way the system has been manipulated, i.e. the experimental conditions used (reviewed in [7, 10]).
In an effort to be closer to the therapeutic use of NSAIDs, an extended classification of cyclooxygenase inhibitors has been proposed, considering the level of COX inhibition at pharmacologically relevant doses in animal models and humans, in addition to enzymatic or biochemical assays [11]. From that point of view, the whole blood assay using thromboxane B2 (TXB2) production during clotting, as an index of platelet COX-1 activity to endogenously formed thrombin, and prostaglandin E2 (PGE2) production during LPS stimulation, as an index of leucocytes COX-2 expression to bacterial endotoxins [12], has become a widely accepted system [13]. For in vitro pharmacological studies, this assay takes advantage of using whole cells that are pathophysiological targets for NSAIDs, of considering the intracellular transport of drugs, of providing a physiological plasma protein level and of checking for inhibition of both COX enzymes on a single sample [10]. Of primary importance for estimating the selectivity of NSAIDs towards COX enzymes, the whole blood assay can also be used to determine the degree of COX inhibition in vivo after an oral intake of therapeutic doses of the drug [12, 14]. As a close correlation has been reported between the inhibitory potency of NSAIDs on thromboxane synthesis by platelets and COX-1 activity in gastric mucosa [15], such ex vivo assay has a clear clinical relevance as long as the tissue concentrations of the drug are considered.
As an in vitro assay is easy to perform and spares time in comparison with clinical studies, authors have proposed to use the in vitro whole blood data for estimating the expected levels of COX inhibition from the circulating levels of NSAIDs [10, 12]. Such a predictive approach is based on: (i) the use of COX inhibition curves obtained by the addition of a range concentration of NSAIDs to donated blood from few healthy subjects; (ii) the extrapolation of plasma concentrations into whole blood concentrations of drugs, assuming that NSAIDs do not enter red cells and that the haematocrit is 45% [10, 12]. However, it has been underlined recently that the ex vivo whole blood assay is somewhat variable and that the level of COX inhibition depends clearly on the pharmacokinetics of NSAIDs [11]. As a consequence, an appropriate number of subjects might be required for a meaningful determination of NSAIDs selectivity [11] and COX inhibition should be reported at pharmacologically relevant times [14, 16] instead of an arbitrary time following drug administration [17].
In the present study, we investigated whether the data obtained with the in vitro whole blood assay provided an accurate estimation of the COX selectivity of NSAIDs in clinical use. For that purpose, we enrolled a sufficient number of healthy volunteers to establish the in vitro COX response curves of ibuprofen, diclofenac or meloxicam, chosen as non selective to COX-2 selective inhibitors, respectively [13]. We compared, subsequently, the levels of COX inhibition anticipated from these in vitro assays with the levels measured ex vivo in the same subjects after a single or a repeated oral intake of the drugs. Finally, as protein binding and drug metabolism may vary in pathological situations such as inflammation, we investigated the predicting value of the free concentration of NSAIDs and, in the case of ibuprofen, the possible contribution of chiral inversion.
Methods
Subjects
Twenty-four male volunteers (age, 23±2 years; height, 1.80±0.08 m; mean±s.d.) were enrolled in this study. Subjects were within 20% of ideal (Metropolitan Life Insurance tables) body-weight (mean weight, 68.8 kg; weight range, 52.0–96.0 kg) and generally healthy, based on physical examination and routine laboratory screening. Subjects with acute or chronic diseases, medical history of gastrointestinal ulcers or allergy to NSAIDs were not included. Subjects were not permitted to receive any other medication (including aspirin or NSAIDs) for 2 weeks before and throughout the duration of the study. The study was approved by the Ethics Committee of Nancy (Nancy, France). Written informed consent was obtained from all study participants.
In vitro study
The effects of meloxicam (Bœhringer Ingelheim, Biberach, Germany), ibuprofen (Sigma, Saint-Quentin Fallavier, France) and diclofenac (Sigma, Saint-Quentin Fallavier, France) on platelet COX-1 and monocyte COX-2 activities were assessed after incubating increasing concentrations of each NSAID with peripheral whole blood samples drawn from all subjects. Drugs were dissolved in dimethylsulfoxide (DMSO; Sigma, Saint-Quentin Fallavier, France) and transferred into test tubes as 5 µl aliquots to provide final concentrations of NSAID ranging from 10−4 m to 10−9 m after addition of whole blood.
COX-1 activity was determined as previously described [12]. Briefly, fresh blood was collected from all volunteers, before any drug intake, into vacutainers containing no anticoagulants. Whole blood aliquots of 1 ml were immediately transferred into nonsiliconized glass tubes preloaded with DMSO alone or NSAID and allowed to clot for 1 h at 37°C. Serum was separated by centrifugation at 2000 rev min−1 for 10 min and stored at −80°C until assay. Serum TXB2 levels were determined with an immunoassay kit according to the manufacturer's procedure (Cascade Biochem Limited, Bershire, UK).
COX-2 activity was determined as previously described [12]. Briefly, whole blood drawn from the same donors was immediately divided into 1 ml aliquots in test tubes containing 10 IU of sodium heparin (Choay, France) and 10 µg of aspirin (Sigma, Saint-Quentin Fallavier, France). After a 15 min incubation at 37°C to allow aspirin to inactivate irreversibly platelet COX-1, blood aliquots were further incubated for 24 h at 37°C in the presence or absence of 10 µg ml−1 LPS, derived from Escherichia coli O55 : B5 (Sigma, Saint-Quentin Fallavier, France), and NSAID or vehicle alone (DMSO). Plasma was separated by centrifugation at 2000 rev min−1 for 10 min and stored at −80°C until assay. Plasma PGE2 levels were determined with an immunoassay kit according to the manufacturer's procedure (Cascade Biochem Limited, Bershire, UK).
Ex vivo study
All volunteers received randomly in a cross-over design 7.5 mg meloxicam (Mobic®, Bœhringer Ingelheim, Paris, France) once for 5 days, 400 mg ibuprofen (Brufen®, Knoll, Levallois-Perret, France) three times daily for 3 days or 75 mg of diclofenac SR (Voltarène®, Novartis, Rueil-Malmaison, France) once for 3 days. Drug regimens, corresponding to half of the maximal recommended dosages [18–20], were chosen to mimic the clinical practice even if lower doses of NSAIDs would probably have helped to define more precisely their in vivo selectivity. Study duration was about 24 weeks and treatments were separated by adapted wash-out periods of minus 7 days after meloxicam and 2 days after ibuprofen or diclofenac.
Intake of meloxicam or diclofenac was at 08.30 h and intake of ibuprofen was at 09.00 h, noon and 20.00 h with a light meal. Drug intake was controlled by telephone and absence of occult intake of NSAID was confirmed by chromatographic analysis as described below. Peripheral venous blood samples were drawn before any drug administration and after a single or a repeated intake of NSAID. Sampling times allowed each NSAID to enter the circulation importantly [18–20]: 5.5 h after meloxicam, 2.5 h after ibuprofen and 3.5 h after diclofenac. Samples were assessed for TXB2 and PGE2 production in whole blood, as described above, and relative changes after drug intake were expressed as percentage of predose levels. Blood samples were also used for the determination of NSAIDs concentration in plasma.
C-reactive protein and creatinine serum levels, liver function tests, platelets and blood cells counts were controlled throughout the study.
Plasma drug concentrations
Total plasma concentrations of meloxicam, diclofenac and racemic ibuprofen were determined by high-performance liquid chromatography (h.p.l.c.) as described elsewhere with minor modifications [21]. The h.p.l.c. system consisted of a Model 510 pump, a Model 486 variable wavelength u.v. detector and a computer loaded with the Millenium software, all from Waters Associates (St Quentin en Yvelines, France). Briefly, plasma or serum samples (200 µl) were added with 1 µg of (benzoyl-4-phenyl)-2-butyric acid (Specia, Vitry, France) as internal standard, then extracted with diethyl ether (3 ml) after acidification with 1 m hydrochloric acid (0.1 ml). After evaporation under a nitrogen stream, the dry residues were dissolved in 100 µl of the mobile phase (acetonitrile-0.3% acetic acid [50 : 50, v/v]) and chromatographed isocratically in the following conditions: 100×8 mm internal diameter reversed-phase Radial Pak C18 end-capped column enclosed in a RCM-10 compression module (Waters), 1 ml min−1 of flow rate, peak detection at 230 nm for diclofenac, 280 nm for ibuprofen and 360 nm for meloxicam.
In the case of ibuprofen, R- and S-enantiomers were evaluated using the same methodology with the following modifications. The dry residues were derivatized, as described previously for ketoprofen [22], by adding 100 µl of 50 mm triethylamine in acetonitrile, then 50 µl of 60 mm ethyl chloroformate (Merck) in acetonitrile for 30 s followed by 50 µl of 1 m l-leucinamide in 1 m triethylamine in methanol for 2 min at room temperature. The reaction was stopped by addition of 50 µl distilled water. Derivatized samples were then eluted isocratically using the following mobile phase (Na2HPO4 50 mm-acetonitrile-triethylamine [58 : 42 : 0.02, v/v], where pH was adjusted at 7.0 with orthophosphoric acid) and enantiomers were detected at 223 nm.
The total plasma concentration of NSAIDs was determined both in the ex vivo and the in vitro study. For the latter, the chromatographic measurement of drugs added exogenously served to assess the ability of NSAIDs to partition in blood cells. Due to the limits of detection of the chromatographic method, plasma NSAIDs concentrations were detected over 10−6m only and the mean correcting factor calculated on the three highest doses was subsequently applied to the entire concentration range. If NSAIDs did not enter into red cells, the following dividing factor [100/(100−haematocrit)] had to be used for transforming whole blood into plasma concentrations.
Protein binding
Unbound fractions of NSAIDs were determined by equilibrium dialysis with subsequent h.p.l.c. analysis. Equilibrium dialysis was performed on a Dianorm apparatus (Braun ScienceTec, Les Ulis, France), equipped with 1 ml cells and Spectrapor 2 membranes (cut-off 12–14 kDa), at 37°C in a thermostat-controlled bath. Samples (1 ml) were dialysed for 3 h against 1 ml of 67 mmol l−1 phosphate buffer pH 7, a time sufficient to reach equilibration for each NSAID. Samples collected ex vivo after ibuprofen intake and samples added in vitro with NSAIDs were analysed after a subsequent addition of 20 µg ml−1 of either diclofenac, meloxicam or racemic ibuprofen.
Statistical analysis
Data were expressed as mean±s.d. or s.e. mean when specified. Inhibition of whole blood TXB2 and PGE2 production by NSAIDs was calculated as the percentage of baseline levels in the same patient. The inhibition of COXs was modelled using the following equation mathematically analogous to the Hill function: Y = [a−d/(1+(X/c)b] + d, where X and Y represent the molar concentration of NSAID and the percentage of inhibition, respectively; a, b, c and d are the parameters of the equation [23]. Fitting of the experimental data to this model was carried out by minimization of the objective function, defined as the sum of square deviations between predicted and experimental response. The best fit was with a weight at 1/Y. This equation was used to fit the sigmoidal concentration-response curves and to estimate the IC50 and IC80 values from the values of the parameters. Fitting was made with all drugs, both for each patient and for mean curves, allowing to calculate individual and mean ICs for COX-1 and COX-2.
Having excluded a sequence or carry-over effects by a multivariate anova, the ex vivo whole blood data were combined regardless of the treatment sequence. For each NSAID, statistical comparisons (plasma drug concentration, COX inhibition) between single and repeated intake were done using the Student's paired t-test. The statistical level of significance was chosen at 5%.
Based on the individual fitting of COXs inhibition in whole blood and the determination of a plasma-to-whole blood correcting factor for each NSAID, in vitro sigmoidal concentration-response curves were reconstructed for plasma NSAIDs concentration. Using these plasma concentration-COXs inhibition curves and the circulating levels of NSAIDs measured after single and repeated intake, we determined the theoretical levels of COX-1 and COX-2 inhibition. These theoretical levels of COXs inhibition were calculated on individual and mean concentration-response curves. Their comparison with the level of COX inhibition really observed ex-vivo was performed using the 95% confidence interval of the centred relative difference [(theoretical−observed)/observed] to the interval [−0.25, +0.25] chosen for statistical significance.
Results
In vitro study
Plasma PGE2 and serum TXB2 averaged 77.2±28.4 ng ml−1 (mean±s.d., n = 24) and 391.2± 152.6 ng ml−1, respectively, at baseline. Intra-subject coefficients of variation, determined on three different measurements of basal COX activities (before each treatment phase of the clinical study), averaged 18±13% for PGE2 and 26±18% for TXB2. The mean IC50 values for COX-1 and COX-2 calculated from the individual dose–response curves (individual IC50) showed that ibuprofen inhibited COX-1 more efficiently than COX-2 (IC50COX-2/COX-1 = 3.3) whereas diclofenac and meloxicam inhibited COX-2 with a higher potency than COX-1 (IC50COX-2/COX-1 = 0.16 and 0.23, respectively) (Table 1). Similar data were obtained from the mean dose–response curves showing IC50COX-2/COX-1 ratios of 2.3 for ibuprofen, 0.16 for diclofenac and 0.19 for meloxicam. In all case, diclofenac was approximately one order of magnitude more potent than meloxicam for inhibiting COX activities (6–7-fold on COX-1 and 8–10-fold on COX-2). The overall COX selectivity of NSAIDs was unchanged when considering the IC80 ratios, although diclofenac appeared slightly more selective for COX-2 than meloxicam in this case (Table 1).
Table 1.
In vitro potencies of diclofenac, ibuprofen and meloxicam to inhibit the synthesis of prostanoid by human whole blood COX isoenzymes. Data are expressed as concentration inhibiting COX activity by 50% [IC50] or 80% [IC80].
| COX-1 | COX-2 | Ratios | COX-1 | COX-2 | Ratios | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50a | IC80a | IC50a | IC80a | COX-2/COX-1a | IC50b | IC80b | IC50b | IC80b | COX-2/COX-1b | |||
| NSAID | (µm) | (µm) | (µm) | (µm) | IC50 | IC80 | (µm) | (µm) | (µm) | (µm) | IC50 | IC80 |
| Diclofenac | 0.35 ± 0.26 | 1.32 ± 0.10 | 0.06 ± 0.05 | 0.13 ± 0.13 | 0.17 | 0.10 | 0.25 | 1.17 | 0.04 | 0.16 | 0.16 | 0.13 |
| Ibuprofen | 6.05 ± 4.82 | 27.0 ± 15.90 | 19.90 ± 24.01 | 221.0 ± 710.0 | 3.29 | 8.19 | 4.30 | 40.80 | 10.00 | 74.90 | 2.33 | 1.84 |
| Meloxicam | 2.64 ± 2.49 | 8.92 ± 8.68 | 0.60 ± 0.82 | 2.51 ± 2.04 | 0.23 | 0.28 | 1.60 | 12.30 | 0.31 | 4.16 | 0.19 | 0.34 |
Data obtained from the arithmetic mean± s.d. of individual IC50 or IC80 values.
Data obtained from the mean concentration response curves.
Ex vivo study
Tolerability of the medications
The 24 volunteers completed the study in accordance with the study protocol. Neither serious nor minor adverse events were observed or reported. Liver function tests, serum creatinine and C-reactive protein levels (<5 mg l−1), haematocrit, platelet and blood cell counts did not vary significantly over study duration.
Plasma concentration of NSAIDs
Measurement of plasma drug concentrations after a single and a repeated intake are shown in Table 2. Drug monitoring confirmed the good compliance of the patients (only four abnormally low NSAIDs concentrations over 144 assays) and the lack of any occult intake of NSAID over study duration. Plasma drug concentrations were similar after a single and a repeated oral intake for both ibuprofen and diclofenac while a 1.8-fold increase in plasma concentration was noted for meloxicam at steady state (P < 0.05).
Table 2.
Monitoring of plasma NSAIDs concentrations after oral intake of 75 mg day−1 of diclofenac SR, 400 mg three times daily of ibuprofen and 7.5 mg day−1 of meloxicam. H.p.l.c. analysis was done as described in the method section. Concentration was determined 3.5 h after diclofenac, 2.5 h after ibuprofen and 5.5 h after meloxicam intake and steady-state was evaluated after 3 days of diclofenac, 2 days of ibuprofen and 5 days of meloxicam. Data are expressed as mean ± s.d. of 24 volunteers.
| Diclofenac (µg ml−1) ([µm]) | Ibuprofen (µg ml−1) ([µm]) | Meloxicam (µg ml−1) ([µm]) | |||
|---|---|---|---|---|---|
| Single | Repeated | Single | Repeated | Single | Repeated |
| 0.38 ± 0.35 | 0.27 ± 0.18 | 24.0 ± 8.0 | 14.8 ± 5.9 | 0.62 ± 0.13 | 1.09 ± 0.37* |
| [1.19 ± 1.10] | [0.85 ± 0.57] | [116.2 ± 38.7] | [70.4 ± 1.28] | [1.76 ± 0.37] | [3.11 ± 1.06]* |
P < 0.05 vs single intake.
Inhibition of COXs activity
The relative changes of prostanoid formation after NSAID intake are shown in Table 3. Ibuprofen inhibited almost completely COX-1 activity after single and repeated intake whereas COX-2 inhibition averaged 80% at corresponding times. Diclofenac reduced COX-1 activity by about 70% and COX-2 by about 96% whatever treatment duration. In contrast, a significant increase (P < 0.05) in COX inhibition was observed after repeated intake of meloxicam, which was more marked on COX-1 (1.8-fold change) than on COX-2 (1.3-fold change). It is worth noting that four subjects in the meloxicam phase of the study failed to show any detectable inhibition of prostanoid synthesis, despite drug plasma levels in the therapeutic range. This explains, as least in part, the higher scattering of values observed with meloxicam than with other NSAIDs, especially for COX-1 inhibition.
Table 3.
Ex vivo inhibition of prostanoid formation in whole blood after single and repeated oral intake of diclofenac, ibuprofen or meloxicam. Results are expressed as percentage inhibition of TXB2 production, as a reflection of platelet COX-1, and PGE2 production, as a reflection of monocyte COX-2, relative to the predose values.
| COX-1 inhibition (%) | COX-2 inhibition (%) | |||
|---|---|---|---|---|
| NSAID (dose) | Single | Repeated | Single | Repeated |
| Diclofenac (75 mg day−1) | 69±22 | 71±18 | 95±16 | 97±5 |
| Ibuprofen (400 mg three times daily) | 96±3 | 90±16 | 83±12 | 76±20 |
| Meloxicam (7.5 mg day−1) | 30±16 | 55±20* | 63±16 | 83±13* |
All values are mean±s.d.
P < 0.05 vs single.
In vivo relevance of in vitro data
Transformation of whole blood into plasma drug concentration
Haematocrit averaged 0.44±0.02 l l−1 throughout study duration and the corresponding dividing factor was 1.81±0.07 for the transformation of whole blood into plasma concentrations. The comparison between the expected whole blood concentration of NSAIDs and the concentration really measured by h.p.l.c. in plasma showed that the correcting factor averaged 1.65±0.24 for ibuprofen, 1.34±0.20 for diclofenac and 1.49±0.13 for meloxicam. Thus, the transformation of whole blood into plasma concentrations differed slightly between NSAIDs and was not fitted adequately when considering haematocrit only. These data suggested that NSAIDs entered, at least in part, into whole blood cells.
Overall predicting value of the in vitro assay
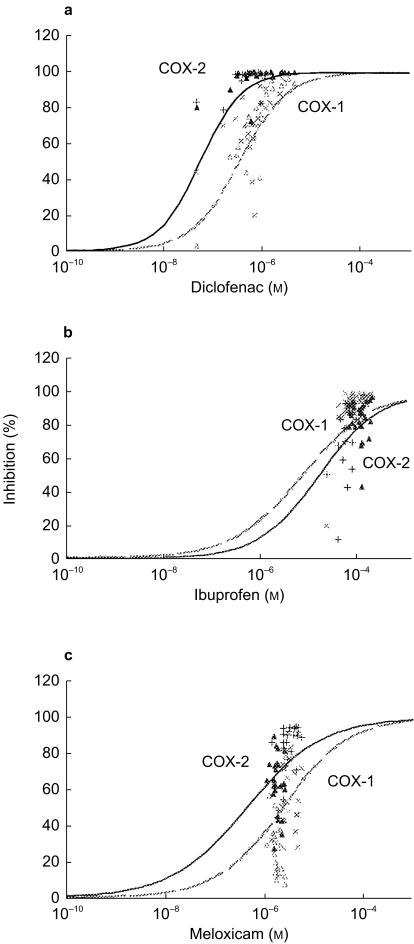
The mean dose–response curves reconstructed by considering the plasma concentration of NSAIDs measured in vitro are depicted in Figure 1(a,b,c). When considering the plasma concentrations measured after single and repeated drug intake, the expected (theoretical) levels of COX inhibition were quite similar if they were calculated from the individual or the mean concentration-response curves (data not shown). However, when these theoretical levels were matched to those measured after NSAID intake, a larger than expected scattering of experimental data was observed (Figure 1a,b,c). The in vivo inhibitory potency of diclofenac was predicted by the in vitro assay: [−0.18, 0.21] for COX-1 and [−0.13, −0.03] for COX-2. For ibuprofen, the in vitro assay was relevant for COX-1 [−0.18, 0.03] but not for COX-2 [−0.14, 0.27] whereas it was relevant for COX-2 ([−0.05, 0.15]) but not for COX-1 ([0.31, 1.05]) in the case of meloxicam. When existing, the predicting value of the in vitro assay was better for repeated intake than for single intake of diclofenac ([−0.10, −0.02]vs[−0.19, −0.01] for COX-2, respectively) or meloxicam ([−0.15, −0.01]vs[−0.01, 0.35] for COX-2, respectively), whereas the contrary was observed for ibuprofen ([−0.24, 0.18]vs[−0.13, −0.10] for COX-1, respectively) (Figure 1). These data were consistent both with the lowest scattering of plasma concentrations of diclofenac and ibuprofen at corresponding times and with the significant increase in circulating meloxicam between single and repeated intake (Table 2).
Figure 1.
Comparison of NSAID concentrations and inhibition of COX activities in human blood assessed in vitro and ex vivo. The in vitro dose–response curves of platelet COX-1 (grey hatched line) and monocyte COX-2 (black line) activities were done for diclofenac (a), ibuprofen (b) and meloxicam (c). Increasing concentrations of NSAIDs were incubated with 1 ml whole blood samples allowed to clot for 1 h, and serum TXB2 levels were measured as a reflection of thrombin-induced platelet thromboxane A2 production. NSAIDs were also incubated with 1 ml heparinized whole blood samples in the presence of aspirin (10 µg ml−1) and LPS (10 µg ml−1) for 24 h, and plasma PGE2 levels were assayed as a reflection of endotoxin-induced monocyte COX-2 activity. In vitro whole blood concentrations of NSAIDs were corrected into plasma concentrations using a dividing factor of 1.34 for diclofenac, 1.65 for ibuprofen and 1.49 for meloxicam. Results, depicted as percentage inhibition, were obtained from triplicates of the 24 volunteers and concentration-response curves were fitted according to the Hill function as described in the method section. In the ex vivo study, plasma NSAIDs concentrations measured after single and repeated intake of diclofenac 75 mg once, ibuprofen 400 mg three times daily or meloxicam 7.5 mg once were plotted on the same graph vs the percentage of inhibition of COX-1 (▵: single; ×: repeated) and COX-2 (▴: single; +: repeated) activities measured ex vivo at the same time.
Influence of protein binding on the predicting value of the in vitro assay
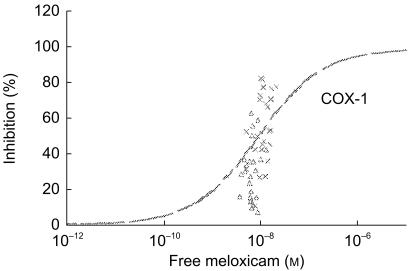
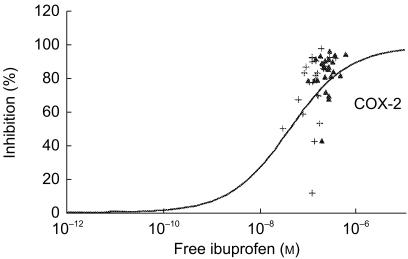
The unbound fraction mirrored the total concentration of NSAIDs, since the concentrations of free diclofenac or ibuprofen were similar after single and repeated intake whereas that of free meloxicam increased with the repetition of the dose (data not shown). In the case of ibuprofen, the unbound fraction was identical in vitro (0.27±0.07%) and in vivo (0.23±0.07% after single intake, 0.22±0.08% after repeated intake), highlighting that circulating metabolites did not contribute to protein binding. When the predicting value of the in vitro assay was tested using the free concentration of NSAIDs, the data were similar to those obtained with the total concentration of drugs: (i) predictability was achieved in the [−0.25, +0.25] interval except for meloxicam on COX-1 (Figure 2) and ibuprofen on COX-2 (Figure 3); (ii) predictability was at best after a repeated intake of diclofenac or meloxicam but a single intake of ibuprofen.
Figure 2.
Discrepancy between inhibition of platelet COX-1 activity by free meloxicam in vitro and percentage of COX-1 inhibition observed ex vivo after an oral intake of 7.5 mg day−1 (▵: single intake; ×: repeated intake).
Figure 3.
Discrepancy between inhibition of monocyte COX-2 activity by free ibuprofen racemate in vitro and percentage of COX-2 inhibition observed ex vivo after an oral intake of 400 mg three times daily (▴: single intake; +: repeated intake).
Influence of stereoselectivity on the predicting value of the in vitro assay
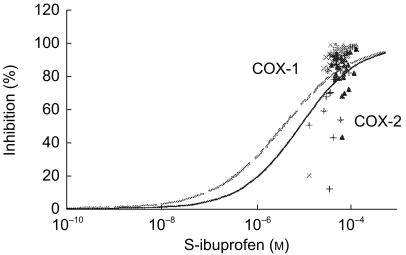
As the drug added in vitro to donated blood samples was ibuprofen racemate, the dose–response curves were easily refitted by considering S-(+)-enantiomer as half of the concentration applied (Figure 4). In vivo, the ratio of S-(+)/R-(−) ibuprofen enantiomers varied between individuals (range [0.87–3.89]) and increased from 1.45±0.41 (mean±s.d.) after single intake to 2.18±1.03 after repeated intake. When considering the plasma levels of the S-(+)-enantiomer only, the in vitro whole blood assay was predictive of the in vivo COX inhibition after single intake of ibuprofen ([−0.12, −0.10] for COX-1, [−0.12, 0.07] for COX-2). After repeated drug intake, the inhibitory potency was estimated adequately on COX-1 ([−0.22, 0.21]) but not on COX-2 ([−0.25, 0.68]) (Figure 4). Similar data were obtained when considering the unbound fraction of the S-(+)-enantiomer (data not shown), demonstrating that this limitation was not supported by a variable stereoselective protein binding.
Figure 4.
Influence of enantioselectivity on the inhibitory potency of ibuprofen towards COX activities in human blood assessed in vitro and ex vivo. The in vitro dose–response curves of platelet COX-1 (grey hatched line) and monocyte COX-2 (black line) activities were done considering that the S-(+)-enantiomer represented half of the racemate concentration. Results, depicted as percentage inhibition, were obtained from triplicates of the 24 volunteers and concentration-response curves were fitted according to the Hill function as described in the method section. In the ex vivo study, plasma concentrations of S-(+)-ibuprofen measured after single and repeated intake of 400 mg three times daily of ibuprofen racemate were plotted on the same graph vs the percentage of inhibition of COX-1 (▵: single; ×: repeated) and COX-2 (▴: single; +: repeated) activities measured ex vivo at the same time.
Discussion
The present work investigated if the COX inhibitory potency of NSAIDs determined with the in vitro whole blood assay was relevant for clinical practice. The rationale was that the whole blood system is unique for comparing in vitro and in vivo data in the same subject [10, 12, 14], and is often considered as a reference system for establishing the selectivity of NSAIDs towards COX isoenzymes [11, 13]. The clinical relevance of any in vitro assay is of primary importance since efforts were made to evidence a link between the COX selectivity ratio of NSAIDs and their ability to produce severe gastro-intestinal complications [13, 24], a meaning supported further by the low incidence of clinically important upper gastrointestinal events with ‘selective COX-2 inhibitors’[25, 26]. As all in vitro systems, the whole blood assay is limited by its relevance to the level of COX inhibition in target tissues, i.e. inflamed joints and gastric mucosa, but offers advantage of investigating biochemical selectivity ex vivo at therapeutic plasma levels of NSAIDs [14, 27].
In our experimental conditions, we pointed out that the level of COX-1 and COX-2 inhibition achieved in vivo cannot be predicted universally from corresponding in vitro dose–response curves. Indeed, the predictability of the whole blood assay was suitable for diclofenac, acceptable for ibuprofen, although with some limitations relative to COX-2 inhibition, but unadapted to meloxicam. As we did not study range doses of each NSAID, we cannot rule out that the predictability of the system would be influenced by drug dosage, since a variable selectivity profile could have appeared at lower doses of NSAIDs. Consistent with this are both the quite complete inhibition of COX-1 by ibuprofen and COX-2 by diclofenac from the first drug intake and the well known dose-dependent inhibition of COX isoenzymes by NSAIDs [16, 27]. This quite unexpected finding was favoured by our choice of sampling blood at a time consistent with a high plasma concentration of drugs, since COX inhibition follows the pharmacokinetics of NSAIDs [16, 28]. However, one must underline that clinical trials demonstrated an equivalent efficacy of daily doses of 7.5 mg of meloxicam and 100 mg of diclofenac in osteoarthritis [18], whereas the time for blood sampling was shown as critical for interpreting the inhibitory potency of meloxicam on platelet functions [16, 17, 27]. As the limitation of the in vitro whole blood assay was observed mainly with meloxicam, whose lower doses are ineffective [18], we think that our study protocol allowed a convenient though not exhaustive analysis of the clinical relevance of the system. Furthermore, the plasma drug concentration was 28–40-fold higher for diclofenac, 8–14-fold higher for meloxicam, and 12–19-fold higher for ibuprofen than their corresponding in vitro IC50 on COX-2, confirming that the doses could be assumed to have at least an equal anti-inflammatory efficacy.
In an effort to explain the variable predictability of the whole blood system, we searched for possible causes related to the biological system itself. As our in vitro IC50 COX-2/COX-1 ratios were close to those reported with the same methodology [12, 13, 27], such limitation is unlikely to result from our experimental conditions. However, the intrasubject variation reached 26±18% for COX-1 and 18±13% for COX-2, whereas the intersubject variation reached 39% for COX-1 and 36% for COX-2. These results are in good accordance with previous clinical studies [12, 16, 27, 28], but confirm that biological variability is a possible limitation of the whole blood assay [11] since scattering in baseline levels of prostanoids may exceed that of purified enzymes or cell-based assays [4, 5, 10]. Furthermore, this intersubject variation makes it hazardous to study the inhibitory potency of NSAIDs in a small number of patients and to extrapolate the individual level of COX inhibition from the dose–response curve of foreign patients. As the number of patients used for establishing the dose–response curves of NSAIDs varied from 5 [10] to 20 [29], the comparison of published selectivity ratios could be disturbed by the variability of the system, even if we failed to improve its clinical relevance when considering individual instead of mean dose–response curves. Since each of our 24 subjects balanced the mean dose–response with the same weight, this finding was not unexpected although it highlighted that the predicting value of the whole blood system remained limited even when the intersubject variation was taken into account.
Estimation of the in vivo extent of COX inhibition from the plasma concentration of NSAIDs is based generally on the prerequisite that drugs do not enter red cells and that haematocrit is 45% [10, 12]. Unfortunately, we demonstrated that the drugs partitioned differently into whole cells, although the difference remained moderate between molecules since they were acidic in nature. The factor accounting for the transformation of whole blood into plasma concentrations followed a different rank order from the drug pKa, suggesting that it was influenced by the lipophilicity of the molecules. Despite having a limited influence for interpreting data with classical NSAIDs, we suggest that it could be a major flaw to neglect the ability of non acidic molecules to enter red cells since, for example, celecoxib is thought to be evenly distributed between erythrocytes and plasma [30]. In addition, the use of a normalized haematocrit has no influence as long as the studies are made in healthy subjects of the same sex, but it adds further limitation to the predicting value of the whole blood system in patients with chronic inflammation.
From a pharmacokinetic point of view, inhibition of COX isoenzymes by NSAIDs may differ between in vitro and in vivo conditions because drugs can accumulate in the target cells, can be degraded variably between individuals or may act through metabolites [10, 12]. We demonstrated that diclofenac exhibited a higher COX-2 selectivity than meloxicam in vitro although it inhibited COX-1 more efficiently than meloxicam after single and repeated oral intake. As their metabolites are inactive [18, 20], such discrepancy cannot be explained by a slower metabolism of meloxicam than of diclofenac in liver. A more likely explanation is that, when drugs were administered at half the maximal recommended doses, the plasma concentration of diclofenac exceeded its inhibitory potency on COX isoenzymes more than that of meloxicam, as we discussed before. As meloxicam 7.5 mg day−1 was as effective as a higher dose of diclofenac in clinical studies [18], this finding challenges further the exclusive contribution of COX inhibition in the therapeutic effects of some NSAIDs. Besides, Panara and coworkers reported that the 35% inhibition of platelet COX-1 activity by 15 mg day−1 of meloxicam corresponded to a 70% inhibition of monocyte COX-2 activity at steady-state [27], which confirms that oral doses of meloxicam were not excessive in terms of COX inhibition. For ibuprofen, we investigated the contribution of the S-(+)-enantiomer because it is several orders of magnitude more potent than the R-(−)-enantiomer in inhibiting prostaglandin synthesis in vitro [31] and can be generated in vivo by enantiomeric inversion via the formation of coenzyme A thioesters [32]. We showed that the S-(+)/R-(−) ratio was highly variable at a given time and increased with repetition of the dose in the same subject. Although this ratio is not an ideal index of fractional inversion, as it depends also on protein binding, one must underline that the predicting value of the in vitro assay on COX-2 was not changed when considering the S-(+)-enantiomer only. As the weakest predictability was observed at steady-state, that is when the S-(+)/R-(−) ratio was the highest, it is tempting to speculate that the higher than expected inhibition of COX-2 could be supported by the additional contribution of the R-ibuprofenoyl-CoA thioester [33].
Protein binding is another key limiting factor of the pharmacokinetics of NSAIDs which is thought to be accurately reproduced by the in vitro whole blood assay [10, 12]. As NSAIDs are highly bound to albumin, we searched for intersubject variability in protein binding as a possible limitation of the system. We demonstrated that the inability of the whole blood assay to predict the inhibitory potency of meloxicam on COX-1 cannot be ascribed to an abnormal protein binding. This appears logical when considering that unbound fraction of NSAIDs is a faithful reflection of their total concentration in healthy volunteers, that protein binding of meloxicam is linear in its therapeutic range [18], and that any occult intake NSAID was excluded by a careful plasma monitoring. For ibuprofen, the measurement of free drug concentration was of particular interest since protein binding can vary from linear to non linear depending on the dose and includes a stereoselective competition for albumin sites [19]. The concentration of free S-(+)-ibuprofen, the less protein bound enantiomer [32], varied differently from that of unbound racemate, with a higher proportion of the S-enantiomer after a repeated (81%) than after a single intake (72%). This finding agreed well with the concomitant increase in the S-(+)/R-(−) ratio suggesting that it was reflecting the chiral inversion rather than the enantiomeric protein binding. However, despite this relative increase in the unbound active fraction, the in vitro system still underestimated the inhibitory potency of ibuprofen on COX-2 at steady state. Thus, our data are consistent with the possible contribution of additional metabolites to the in vivo COX-2 inhibitory potency of ibuprofen.
The inability of the whole blood assay to estimate the inhibitory potency of meloxicam on COX-1 needs further discussion. It is noticeable that inhibition of COX-1 by meloxicam showed the highest scattering of values, with coefficients of variation averaging 53% after single and 36% after repeated drug intake. This variability was unexpected since it contrasted with the homogeneity of ex vivo COX-2 inhibition and plasma concentration of meloxicam. Surprisingly, the increase in plasma concentration of meloxicam with repetition of the dose paralleled its inhibitory potency on COX-1 (1.8-fold increase) but not on COX-2 (1.3-fold increase). A similar dissociation between circulating drug levels and COX inhibition was reported after repeated intake of increasing doses of meloxicam, although both COX iso-enzymes were affected similarly [27]. Interestingly, a possible cumulative inhibition of COX-1 was suggested to explain the higher than expected inhibition of platelet activity [27]. In contrast, an adaptation of platelet COX-1 was proposed to explain the lower inhibition of COX-1 by meloxicam at steady-state than would be expected from its level of inhibition after a single dose [16]. Our results support none of these proposals since inhibition of COX-1 followed the plasma concentration of meloxicam between single and repeated intake. We suggest that the inability of the whole blood assay to estimate COX-1 inhibition by meloxicam was partly attributable to the lack of inhibition observed in some subjects despite drug plasma concentrations in the therapeutic range. Although it remains unexplained, this finding contributes most likely to the scattering of values in the most critical, i.e. the linear, part of the sigmoidal dose–response curve. The variable influence of plasma concentration of meloxicam on COX-1 and COX-2 activity may reflect a higher drug accumulation in monocytes than in platelets. Alternatively, a different mechanism of inhibition of COX iso-enzymes by meloxicam cannot be excluded [34], since we did not performed recovery experiments.
Conclusions
The present cross-over study revisited the clinical relevance of the whole blood assay through comparing the COX inhibitory potency of NSAIDs in vitro and in vivo in the same subjects. We confirmed that the biological variability of the system was high, although this does not exceed its advantages as long as a sufficient (high) number of subjects is considered. We showed further that the selectivity ratios obtained in vitro using IC50s gave a somewhat misleading view of the selectivity of NSAIDs in clinical use, with diclofenac and meloxicam being more COX-2 selective in vitro than in vivo. In addition, the in vitro whole blood assay estimated adequately the ex vivo inhibition of COXs iso-enzymes by diclofenac but was not adapted to this of meloxicam on COX-1 and ibuprofen on COX-2, even when considering variability due to protein binding or chiral inversion. These data extend previous work with the whole blood assay [14, 16, 27] to underline the limitations of assessing COX-2 selectivity based on in vitro studies and pharmacological modelling, even with a ‘more physiological’ system. We conclude that there is a need to investigate COX selectivity at therapeutic plasma concentrations of NSAIDs, as assessed ex vivo with the same assays.
Acknowledgments
The authors would like to thank Françoise Groussin for her excellent technical assistance and Pr Pierre Gillet for his helpful comments on the manuscript.
This work was supported by grant n°971016 from the ‘Agence Française de Sécurité Sanitaire des Produits de Santé’ of the French Minister for Health.
References
- 1.Vane JR. Inhibition of prostaglandins as a mechanism of action for aspirin-like drugs. Nature New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 2.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 3.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 4.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isoenzymes by aspirin and other non-steroidal anti inflammatory drugs. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 5.Battistini B, Botting R, Bakhle YS. Cox-1 and Cox-2: Towards the development of more selective NSAIDs. Drug News Perspectives. 1994;7:501–512. [Google Scholar]
- 6.Vane JR. Towards a better aspirin. Nature. 1994;367:215–216. doi: 10.1038/367215a0. [DOI] [PubMed] [Google Scholar]
- 7.Jouzeau J-Y, Terlain B, Abid A, Nédélec E, Netter P. Cyclooxygenase isoenzymes: how recent findings affect thinking about non steroidal anti-inflammatory drugs. Drugs. 1997;53:563–582. doi: 10.2165/00003495-199753040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Frölich JC. A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. Trends Pharmacol Sci. 1997;18:30–34. doi: 10.1016/s0165-6147(96)01017-6. [DOI] [PubMed] [Google Scholar]
- 9.Griswold DE, Adams JL. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): rationale for selective inhibition and progress to date. Med Res Rev. 1996;16:181–206. doi: 10.1002/(SICI)1098-1128(199603)16:2<181::AID-MED3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Pairet M. Inhibition of cyclooxygenase-1 and cyclooxygenase-2. Analysis of the in vitro test systems and their clinical relevance. J Clin Rheumatol. 1998;4:S17–S25. doi: 10.1097/00124743-199810001-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lipsky PE, Abramson SB, Crofford L, DuBois RN, Simon L, Van de Putte LBA. The classification of cyclooxygenase inhibitors. J Rheumatol. 1998;25:2298–2303. [PubMed] [Google Scholar]
- 12.Patrignani P, Panara MR, Sciulli MG, Santini G, Renda G, Patrono C. Differential inhibition of human prostaglandin endoperoxide synthase-1 and -2 by nonsteroidal anti-inflammatory drugs. J Physiol Pharmacol. 1997;48:623–631. [PubMed] [Google Scholar]
- 13.Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drugs selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panara MR, Padovano R, Sciulli MG, et al. Effects of nimesulide on constitutive and inducible prostanoid biosynthesis in man. Clin Pharmacol Ther. 1998;63:672–681. doi: 10.1016/S0009-9236(98)90091-1. [DOI] [PubMed] [Google Scholar]
- 15.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 16.Tegeder I, Lötsch J, Krebs S, Muth-Selbach U, Brune K, Geisslinger G. Comparison of inhibitory effects of meloxicam and diclofenac on human thromboxane biosynthesis after single doses and at steady state. Clin Pharmacol Ther. 1999;65:533–544. doi: 10.1016/S0009-9236(99)70073-1. [DOI] [PubMed] [Google Scholar]
- 17.Stichtenoth DO, Wagner B, Frölich JC. Effects of meloxicam and indomethacin on cyclooxygenase pathways in healthy volunteers. J Invest Med. 1997;45:44–49. [PubMed] [Google Scholar]
- 18.Degner F, Türck D, Pairet M. Meloxicam. Pharmacological, pharmacokinetic and clinical profile. Drugs of Today. 1998;34(ASupplement):1–22. [Google Scholar]
- 19.Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34:101–154. doi: 10.2165/00003088-199834020-00002. [DOI] [PubMed] [Google Scholar]
- 20.Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Clin Pharmacokinet. 1997;33:184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 21.Lapicque F, Netter P, Bannwarth B, et al. Identification and simultaneous determination of non-steroidal anti-inflammatory drugs using high performance liquid chromatography. J Chromatogr Biomed Applic. 1989;496:301–320. doi: 10.1016/s0378-4347(00)82579-7. [DOI] [PubMed] [Google Scholar]
- 22.Presle N, Lapicque F, Gillet P, Hermann MA, Bannwarth B, Netter P. Effect of dimethicone (polysilane gel) on stereoselective pharmacokinetics of ketoprofen. Eur J Clin Pharmacol. 1998;54:351–354. doi: 10.1007/s002280050473. [DOI] [PubMed] [Google Scholar]
- 23.De Lean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose–response curve. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- 24.Vane JR, Botting RM. Mechanism of action of aspirin-like drugs. Semin Arthritis Rheum. 1997;26:2–10. doi: 10.1016/s0049-0172(97)80046-7. [DOI] [PubMed] [Google Scholar]
- 25.Bombardier C, Laine L, Reicin D, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 26.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 27.Panara MR, Renda G, Sciulli MG, et al. Dose-dependent inhibition of platelet cyclooxygenase-1 and monocyte cyclooxygenase-2 by meloxicam in healthy subjects. J Pharmacol Exp Ther. 1999;290:276–280. [PubMed] [Google Scholar]
- 28.Patrono C, Ciabattoni G, Patrignani P, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation. 1985;72:1177–1184. doi: 10.1161/01.cir.72.6.1177. [DOI] [PubMed] [Google Scholar]
- 29.Cullen L, Kelly LO, Connor S, Fitzgerald DJ. Selective cyclooxygenase-2 inhibition by nimesulide in man. J Pharmacol Exp Ther. 1998;287:578–582. [PubMed] [Google Scholar]
- 30.Davies NM, McLachlan AJ, Day RO, Williams KM. Clinical pharmacokinetics and pharmacodynamics of celecoxib, a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38:225–242. doi: 10.2165/00003088-200038030-00003. [DOI] [PubMed] [Google Scholar]
- 31.Boneberg EM, Zou M-H, Ullrich V. Inhibition of cyclooxygenase-1 and -2 by R(−) and S(+)-ibuprofen. J Clin Pharmacol. 1996;36:16S–9S. [PubMed] [Google Scholar]
- 32.Evans AM. Pharmacodynamics and pharmacokinetics of the profens: enantioselectivity, clinical implications, and special reference to S(+)-ibuprofen. J Clin Pharmacol. 1996;36:7S–15S. [PubMed] [Google Scholar]
- 33.Neupert W, Brugger R, Euchenhofer C, Brune K, Geisslinger G. Effects of ibuprofen enantiomers and its coenzyme A thioesters on human prostaglandin endoperoxide synthases. Br J Pharmacol. 1997;122:487–492. doi: 10.1038/sj.bjp.0701415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem J. 1999;339:607–614. [PMC free article] [PubMed] [Google Scholar]