Abstract
Aims
To determine the effects of sex and age on the pharmacokinetics of alosetron.
Methods
Single oral and intravenous 2 mg doses of alosetron were administered on separate occasions to 48 healthy, young and elderly, males and females. Serum was sampled for 12 h post-dose to measure alosetron concentrations.
Results
Serum concentrations of alosetron were higher in females than in males, resulting from a sex difference in clearance by metabolism. Mean clearance values were 504 vs 677 ml min−1 in young females vs males (mean ratio 0.75), and 461 vs 670 ml min−1 in elderly females vs males (mean ratio 0.69). The sex difference in alosetron pharmacokinetics achieved statistical significance in the elderly, but not in the young. Irrespective of sex, alosetron clearance was increased by smoking. Serum concentrations tended to be higher in the elderly, although the effect of age was generally not significant. Volume of distribution was smaller in females (approximately 63 l) compared with males (approximately 84 l), regardless of age or the sex difference in body weight.
Conclusions
A significant difference in clearance by metabolism of alosetron between the sexes, and possibly between the young and elderly was observed.
Keywords: age, alosetron, pharmacokinetics, sex
Introduction
Alosetron is a potent and selective 5-HT3-receptor antagonist developed to treat irritable bowel syndrome (IBS) pain and discomfort in females whose predominant bowel habit is diarrhoea [1]. Although IBS occurs in both sexes, it is twice as prevalent in females [2]. As with many drugs during early clinical development, the pharmacokinetics of alosetron were initially examined in only healthy young males. Alosetron undergoes rapid oral absorption (tmax at 1 h) with a bioavailability of 50–60%. It is rapidly eliminated with a 1.5 h half-life and a clearance of 600 ml min−1, predominantly (94%) by hepatic cytochrome P450-mediated metabolism to a variety of products. Only a small portion of the dose (6%) is excreted unchanged renally.
Initial studies indicated that alosetron exhibited preferential efficacy in women [3]. Thus, it was important to characterize the disposition of alosetron in females in attempting to explain the sex difference in efficacy. Therefore, this study was performed to characterize the effects of sex as well as age on the pharmacokinetics of alosetron.
Methods
Subjects and procedures
This open-label, single dose, parallel group, randomized two-period crossover study (Glaxo Wellcome protocol C92-058) was carried out at LAB GmbH & Co., Neu Ulm, Germany and the protocol was approved by the Freiburg Ethics Committee. All subjects provided written informed consent prior to participation.
Forty-eight subjects completed the study. Twelve subjects enrolled into each of four groups, composed of young (aged 18–40 years) males, young females, elderly (aged over 65 years) males, and elderly females. All subjects were healthy, had normal vital signs, ECG, and clinical laboratory tests, and had taken no regular medication within 2 weeks or any medication within 2 days of the first dose. Females of childbearing potential were requested to use adequate means of contraception and were tested for pregnancy at screening and before each dose. Subjects who smoked more than 15 cigarettes per day were not enrolled in the study, and smoking was not permitted while on the unit from the evening before to 12 h after dosing.
On two occasions separated by at least 7 days, each subject received a 2 mg dose of alosetron, consisting of either a single oral tablet or an intravenous injection in 15 ml saline given over 15 min, administered in random order. Subjects entered the unit the evening prior to each dosing and remained until the last blood sample was taken. Alcohol and caffeine were prohibited from 24 h prior to dosing and throughout the time spent in the unit. Subjects fasted overnight and for 4 h after each dose, and remained recumbent for 2 h after dosing.
Sample collection and bioanalysis
Blood samples (10 ml) were taken prior to dosing and at 15, 30, 45 min, and 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h after initiating dosing, and additionally at 20 and 25 min after initiating intravenous dosing. Blood was allowed to coagulate and serum was separated for bioanalysis.
Serum alosetron concentration was determined by reverse-phase h.p.l.c. with fluorescence detection using a previously described method [4]. The lower limit of quantification was 0.1 ng ml−1. Concentrations were linear (r≥0.996) over the 0.1–20 ng ml−1 standard range. Analytical precision was within 9%, and accuracy ranged from 95% to 103% of nominal.
Data analyses
Serum concentration data were analysed to determine peak concentration (Cmax) and the time at which it occurred (tmax). Area under the concentration vs time curve (AUC) was determined using linear (ascending) and logarithmic (descending) trapezoidal interpolation and extrapolation to infinity. Elimination half-life (t1/2) was calculated by log-linear least-squares regression of the terminal phase. Data from the intravenous dose (D) were used to calculate clearance (CL) as D÷AUC, and steady-state volume of distribution (Vss) as [D×AUMC÷AUC2]−[D÷(2×AUC)], where AUMC is the area under the first moment of the concentration-time curve. Absolute bioavailability (F) was calculated as the ratio of oral AUC to intravenous AUC.
Pharmacokinetic parameters (except tmax) were log-transformed and assessed using an analysis of variance (aov) model accounting for the effects of group, sequence, body weight, and smoking status (expressed as cigarettes smoked per day). Additionally, the effects of period, treatment, and subject within group and sequence were accounted for in the aov for F using log-transformed values of oral and intravenous AUC. Parameters (except tmax) were compared between groups, based on the aov of log-transformed data, by calculating the estimated difference using contrasts, and exponentiating to obtain the least-squares mean ratio and associated 90% confidence interval (CI). Values of tmax were compared between groups using the Wilcoxon Rank Sum test, and each median difference and associated 90% CI was estimated using the method of Hodges-Lehmann.
Results
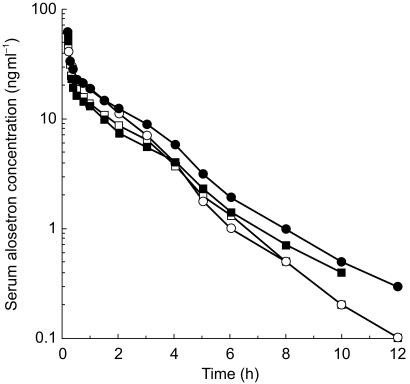
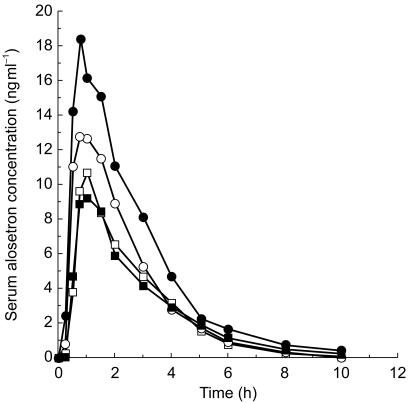
Subject demographic characteristics are summarized in Table 1. Median alosetron serum concentration vs time plots for all groups are displayed in Figure 1 (intravenous dose) and Figure 2 (oral dose). Pharmacokinetic parameters are summarized for all groups and both doses in Table 2. Statistical comparisons of these parameters between groups are summarized in Table 3.
Table 1.
Summary of subject demographic characteristics.
| Young males | Young females | Elderly males | Elderly females | |
|---|---|---|---|---|
| Age (years)* | 28 (19–39) | 30 (19–39) | 69 (66–78) | 70 (66–75) |
| Weight (kg)* | 74 (61–87) | 61 (53–70) | 77 (61–91) | 65 (58–77) |
| Smoking status (cigarettes/day) | 0 (n = 8) | 0 (n = 6) | 0 (n = 9) | 0 (n = 10) |
| 4 (n = 1) | 4 (n = 1) | 4 (n = 1) | 7 (n = 1) | |
| 5 (n = 1) | 10 (n = 3) | 5 (n = 1) | 10 (n = 1) | |
| 15 (n = 2) | 15 (n = 2) | 10 (n = 1) | ||
| Hormone use | – | No (n = 12) | – | No (n = 10) |
| Yes (n = 2) |
median (range); all groups n = 12.
Figure 1.
Median (n = 12) alosetron serum concentration vs time following a 2 mg intravenous dose in young males (□), young females (^), elderly males (▪), and elderly females (•).
Figure 2.
Median (n = 12) alosetron serum concentration vs time following a 2 mg oral dose in young males (□), young females (^), elderly males (▪), and elderly females (•).
Table 2.
Summary of serum alosetron pharmacokinetic parametersa following a 2 mg dose.
| Young males | Young females | Elderly males | Elderly females | |
|---|---|---|---|---|
| Intravenous | ||||
| Cmax (ng ml−1) | 40.2 (34.2, 47.4) | 43.1 (35.5, 52.3) | 51.0 (42.5, 61.1) | 63.7 (54.3, 74.8) |
| AUC(0, ∞) (ng ml−1 h) | 49.3 (40.5, 59.9) | 66.1 (52.4, 83.4) | 49.7 (40.0, 61.8) | 72.2 (59.6, 87.6) |
| t1/2 (h) | 1.52 (1.36, 1.69) | 1.65 (1.45, 1.88) | 1.69 (1.49, 1.90) | 1.79 (1.61, 1.99) |
| CL (ml min−1) | 677 (556, 823) | 504 (400, 636) | 670 (539, 833) | 461 (381, 559) |
| Vss (l) | 82.6 (72.3, 94.2) | 63.1 (53.9, 73.8) | 84.5 (73.0, 97.8) | 62.4 (54.8, 71.1) |
| Oral | ||||
| Cmax (ng ml−1) | 9.35 (6.85, 12.8) | 14.6 (10.1, 21.1) | 8.67 (6.13, 12.2) | 16.1 (11.9, 21.9) |
| tmax (h) | 1.00 (0.75, 2.00) | 1.00 (0.50, 2.00) | 0.75 (0.50, 2.00) | 0.75 (0.50, 1.50) |
| AUC(0, ∞) (ng ml−1 h) | 24.6 (17.5, 34.7) | 36.6 (24.4, 55.0) | 23.6 (16.1, 34.5) | 44.2 (31.6, 61.9) |
| t1/2 (h) | 1.45 (1.28, 1.64) | 1.44 (1.24, 1.68) | 1.58 (1.37, 1.82) | 1.67 (1.48, 1.89) |
| F | 0.50 (0.41, 0.62) | 0.49 (0.40, 0.61) | 0.51 (0.41, 0.63) | 0.63 (0.51, 0.79) |
Geometric least-square mean (95% confidence interval), except tmax: median (range); all groups n = 12.
Table 3.
Statistical comparison of serum alosetron pharmacokinetic parameters between groups.
| Sex comparsion(female/male)a | Age comparsion(Elderly/Young)a | |||
|---|---|---|---|---|
| Young subjects | Elderly subjects | Male subjects | Female subjects | |
| Intravenous | ||||
| Cmax (ng ml−1) | 1.07 (0.85, 1.35) | 1.25 (1.01, 1.54) | 1.27 (1.05, 1.53)* | 1.48 (1.21, 1.81)* |
| AUC(0, ∞) (ng ml−1 h) | 1.34 (1.02, 1.77) | 1.45 (1.13, 1.87)* | 1.01 (0.81, 1.26) | 1.09 (0.86, 1.39) |
| t1/2 (h) | 1.09 (0.93, 1.26) | 1.06 (0.92, 1.22) | 1.11 (0.98, 1.26) | 1.08 (0.95, 1.24) |
| CL (ml min−1) | 0.75 (0.57, 0.98) | 0.69 (0.53, 0.89)* | 0.99 (0.79, 1.24) | 0.92 (0.72, 1.16) |
| Vss (l) | 0.76 (0.63, 0.92)* | 0.74 (0.62, 0.88)* | 1.02 (0.88, 1.19) | 0.99 (0.84, 1.16) |
| Oral | ||||
| Cmax (ng ml−1) | 1.56 (1.01, 2.41) | 1.86 (1.24, 2.78)* | 0.93 (0.65, 1.32) | 1.11 (0.76, 1.62) |
| tmax (h) | 0.00 (−0.25, 0.25) | 0.00 (−0.25, 0.00) | −0.13 (−0.25, 0.00) | −0.25 (−0.25, 0.00) |
| AUC(0, ∞) (ng ml−1 h) | 1.49 (0.92, 2.41) | 1.87 (1.20, 2.92)* | 0.96 (0.65, 1.42) | 1.21 (0.79, 1.83) |
| t1/2 (h) | 1.00 (0.83, 1.19) | 1.06 (0.90, 1.24) | 1.09 (0.95, 1.26) | 1.16 (0.99, 1.35) |
| F | 0.98 (0.76, 1.27) | 1.24 (0.96, 1.60) | 1.01 (0.79, 1.31) | 1.28 (0.99, 1.65) |
Geometric least-square mean ratio (90% confidence interval), except tmax: median difference (90% confidence interval).
Significant difference between subject groups (P < 0.05).
Although the characteristic sex difference in body weight was apparent in both young and elderly subjects, body weight (or body mass index) was not a significant factor in the analysis of any pharmacokinetic parameter (P > 0.4). Smoking was accounted for, independent of other subject characteristics, as a significant factor (P < 0.03), increasing CL, and decreasing F, intravenous and oral AUC, and oral Cmax. Although none of the females used oral contraceptives during the study, two elderly females were receiving hormone replacement therapy. It was determined that including these two subjects made no important difference in the statistical analysis. Post-hoc analysis indicated that there was 80% power to detect the observed between-group differences in each pharmacokinetic parameter.
During conduct of the study, no serious adverse events were reported, and no clinically relevant changes in laboratory tests attributable to alosetron were observed.
Sex comparison
In females who were elderly, compared with males of similar age, statistically significantly higher values were observed for intravenous AUC (45%), and oral AUC (87%) and Cmax (86%). These higher serum concentrations reflected the statistically significantly lower clearance (31%) observed in elderly females. Consistent with these sex differences, absolute bioavailability tended to be higher in elderly females, although this did not achieve statistical significance. The approximately two-fold larger sex difference in serum concentrations after oral dosing compared with intravenous dosing reflected the first-pass component of metabolic clearance. In young females compared with young males, a similar pattern of contrasts was observed, although these did not achieve statistical significance.
Volume of distribution was statistically significantly lower (approximately 25%) in females, irrespective of age or body weight. As a consequence of parallel differences in volume of distribution and clearance, half-life did not significantly differ between the sexes.
Age comparison
In elderly subjects of either sex, compared with young subjects of the same sex, intravenous Cmax was statistically significantly higher (27% in males, 48% in females). No statistically significant age-related differences were observed in any other pharmacokinetic parameter, although there was a tendency toward higher bioavailability and oral AUC with increased age in females.
Discussion
The pharmacokinetics of alosetron are not greatly affected by age. Although bioavailability tended to be higher with age in females, no statistically significant effect of age was observed after oral dosing. The only observed statistically significant effect of age was higher peak concentrations after intravenous dosing, which bears no direct clinical relevance for this orally administered drug.
In contrast to age, sex was associated with significant differences in the pharmacokinetics of alosetron, independent of smoking habit and body weight. The clearance of alosetron by metabolism was lower in women than in men. However, this difference in pharmacokinetics does not explain the greater efficacy of alosetron in women. However, it may be inferred that the activity of at least one of the enzymes that metabolize alosetron differs between the sexes. Preliminary in vitro experiments indicate that alosetron is metabolized by several human hepatic cytochrome P450 enzymes including CYPs 2C9, 3A4, and 1A2 (unpublished data).
Although sex differences in human drug metabolism are not common, a number have been identified [5]. One particularly relevant example is the metabolism of ondansetron, which is chemically and pharmacologically related to alosetron. Ondansetron exhibits lower clearance in women, especially elderly women [6], and is also metabolized by CYP3A4 and 1A2. Sex differences in the activity of both these enzymes have been reported, with both higher [7–9] and lower [10, 11] metabolism of some CYP3A4 substrates, and lower metabolism of some CYP1A2 substrates [12–16] in women. Thus, either or both of these enzymes may be responsible for the sex difference in alosetron metabolism.
The sex difference in alosetron metabolism was statistically significant in the elderly but not in the young. A larger sex difference in metabolism in the elderly has been reported for some substrates of CYP3A4 [5, 9] and CYP2C9 [17]. Thus, the differential effect of age on the variation between men and women in alosetron metabolism may also be related to the activity of one or more enzymes.
The involvement of CYP1A2 in alosetron metabolism had been demonstrated in vitro, although the in vivo importance of this smoking-inducible [18] enzyme is not known. The effect of smoking on alosetron clearance, independent of those of sex or other subject characteristics, provides indirect evidence of the involvement of CYP1A2 in the in vivo metabolism of alosetron.
Although volume of distribution has no clinical relevance for an orally administered drug, the observed sex difference in this parameter was unexpected. This difference was not related to body weight, but may be influenced by body composition (e.g. a difference in lean body mass), which will apply to both the young and elderly.
In conclusion, alosetron provides an example of a drug that exhibits a sex difference in both metabolism and distribution, independent of smoking habit. The contribution of various CYP enzymes to the metabolism of alosetron, and the role these may play in producing the sex difference in alosetron clearance will require further investigation.
References
- 1.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 2.Sandler RS. Epidemiology of Irritable Bowel Syndrome in the United States. Gastroenterology. 1990;99:409–415. doi: 10.1016/0016-5085(90)91023-y. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Alimentary Pharmacol Ther. 1999;13:1149–1159. doi: 10.1046/j.1365-2036.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd TL, Gupta SK, Gooding AE, Alianti JR. Determination of alosetron in human plasma or serum by high-performance liquid chromatography with robotic sample preparation. J Chromatogr B Biomed Appl. 1996;678:261–267. doi: 10.1016/0378-4347(95)00491-2. [DOI] [PubMed] [Google Scholar]
- 5.Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 6.Glaxo Wellcome. Physician's Desk Reference. 54. Medical Economics Company: Montvale (New Jersey, USA); 2000. Ondansetron (prescribing information/product label) [Google Scholar]
- 7.Hunt CM, Westerkam WR, Stave GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992;44:275–283. doi: 10.1016/0006-2952(92)90010-g. [DOI] [PubMed] [Google Scholar]
- 8.Watkins PB, Murray SA, Winkelman LG, Heuman DM, Wrighton SA, Guzelian PS. Erythromycin breath-test as an assay of glucocorticoid-inducible liver cytochromes P-450: Studies in rats and patients. J Clin Invest. 1989;83:688–697. doi: 10.1172/JCI113933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA, O'Mara EM, Hall SD. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 10.Barbhaiya RH, Buch AB, Greene DS. A study of the effect of age and gender on the pharmacokinetics of nefazodone after single and multiple doses. J Clin Psychopharmacol. 1996;16:19–25. doi: 10.1097/00004714-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Warrington SJ. Clinical implications of the pharmacology of sertraline. Int Clin Psychopharmacol. 1991;6(Suppl 2):11–21. doi: 10.1097/00004850-199112002-00004. [DOI] [PubMed] [Google Scholar]
- 12.Relling MV, Lin JS, Ayers GD, Evans WE. Racial and gender differences in N-acetyltransferase, xanthine oxidase, and CYP1A2 activities. Clin Pharmacol Ther. 1992;52:643–658. doi: 10.1038/clpt.1992.203. [DOI] [PubMed] [Google Scholar]
- 13.Ford JM, Truman CA, Wilcock GK, Roberts CJ. Serum concentrations of tacrine hydrochloride predict its adverse effects in Alzheimer's disease. Clin Pharmacol Ther. 1993;53:691–695. doi: 10.1038/clpt.1993.91. [DOI] [PubMed] [Google Scholar]
- 14.Haring C, Fleischhacker WW, Schett P, Humpel C, Barnas C, Saria A. Influence of patient-related variables on clozapine plasma levels. Am J Psychiatry. 1990;147:1471–1475. doi: 10.1176/ajp.147.11.1471. [DOI] [PubMed] [Google Scholar]
- 15.Ereshefsky L, Saklad SR, Watanabe MD, Davis CM, Jann MW. Thiothixene pharmacokinetic interactions. A study of hepatic enzyme inducers, clearance inhibitors, and demographic variables. J Clin Psychopharmacol. 1991;11:296–301. [PubMed] [Google Scholar]
- 16.Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine pharmacokinetic and pharmacodynamic profile. Clin Pharmacokin. 1999;37:177–193. doi: 10.2165/00003088-199937030-00001. [DOI] [PubMed] [Google Scholar]
- 17.Richardson CJ, Blocka KLN, Ross SG, Verbeeck RK. Effects of age and sex on piroxicam disposition. Clin Pharmacol Ther. 1985;37:13–18. doi: 10.1038/clpt.1985.4. [DOI] [PubMed] [Google Scholar]
- 18.Butler MA, Lang NP, Young JF, et al. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992;2:116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]