Abstract
Aims
To investigate the safety, tolerability and pharmacokinetics of the novel NMDA antagonist CNS 5161 in humans. Excessive activation of glutamate receptors, especially of the N-methyl-d-aspartate (NMDA) subtype has been associated with neuropathic pain, and brain damage caused by focal ischaemia in mature brain or hypoxia-ischaemia (HI) in neonates. CNS 5161 is a novel NMDA ion-channel antagonist that interacts with the NMDA receptor/ion channel site to produce a noncompetitive blockade of the actions of glutamate. Pre-clinical studies have demonstrated neuroprotective effects of CNS 5161 in the adult rat model of focal cerebral ischaemia, as well as anticonvulsant and analgesic effects. This study reports the first administration of CNS 5161 to man. Its objectives were to investigate the haemodynamic effects of the compound, to assess its safety and tolerability in healthy male volunteers, and to provide some preliminary human pharmacokinetic data.
Methods
We performed a randomized, double-blind placebo controlled phase 1 dose escalation study of CNS 5161. Volunteers were randomized to receive CNS 5161 or placebo in a ratio of 3 : 1. Twenty-four of 32 healthy volunteers received intravenous infusion of CNS 5161 over 15 min, followed by serial measurements of plasma drug concentration and haemodynamic observations over 24 h. A dose escalation design was adopted and the volunteers were stratified into eight dosage groups, ranging from 30 µg to 2000 µg.
Results
The drug was well tolerated by recipients. Side-effects were dose-related, self limiting and comprised minor subjective sensory symptoms. A dose dependent rise in systolic, mean arterial and diastolic blood pressure was seen in subsequent dosage groups, reaching 23/19 mmHg. Maximal effects were seen between 60 and 120 min after commencement of infusion. All subjects returned to baseline haemodynamic values within 24 h. Putative neuroprotective concentrations of CNS 5161 were achieved transiently, although these levels were not sustained. The pharmacokinetic data were best described by a two compartment model. The mean half-life was 2.95 h (s.d. 0.75). Mean clearance was 106 l h−1 (s.d. 17.8) mean volume of distribution was 296 l (s.d. 69). These parameters were not significantly affected by body weight.
Conclusions
This study suggests that CNS 5161 is well tolerated in healthy volunteers within the dose range studied. In addition, information concerning the pharmacokinetics of the compound has been acquired. Studies to investigate the efficacy of the compound in man may now be justified.
Keywords: glutamate receptor, neuroprotection, stroke
Introduction
Cerebral infarction accounts for 80% of all strokes and is the major cause of long-term disability. Whilst cerebral ischaemia per se is responsible for neuronal death, it is believed that excessive release of the neurotransmitter glutamate during ischaemia causes further neurological damage. The excessive release of glutamate causes overstimulation of the N-methyl-d-aspartate (NMDA) subclass of glutamate receptors. When activated these receptors contribute to intracellular calcium overload [1, 2], a process implicated in ischaemic cell death [3]. Similar mechanisms are suspected to contribute to neurological dysfunction following certain cardiovascular surgical procedures, and to underlie the development or exacerbation of some chronic pain syndromes. Inhibitors of this receptor channel complex may therefore have potential therapeutic use in reducing neuronal injury associated with stroke, trauma, or cardiovascular surgery, and in preventing or ameliorating certain forms of intractable pain.
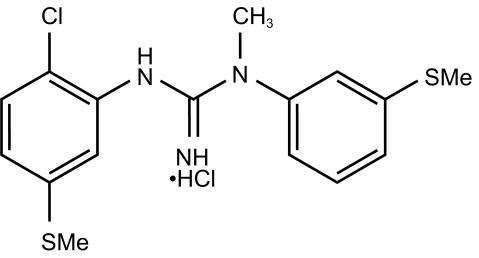
CNS 5161 [N-(2-chloro-5-(methylmercapto) phenyl)-N′-(3-(methylmercapto) phenyl)-N′-methylguanidine monohydrochloride] is a novel and selective noncompetitive antagonist of the NMDA subset of glutamate receptors in the mammalian brain (Figure 1). It has potent inhibitory activity in vitro at the NMDA ion channel and is able to displace [3H] MK-801 binding with a Ki of 1.8 nm in synaptosomal membrane preparations from rat brain [4]. In the neonatal rat NMDA excitotoxicity model in vivo, CNS 5161 protects against the necrotic effects of exogenous N-methyl-d-aspartate with an ED80 of 4 mg kg−1 by the intraperitoneal (i.p.) route [4]. CNS 5161 also shows a 91% inhibition of audiogenic seizures in DBA/2 mice at 4 mg kg−1 i.p. [5]. and has a neuroprotective effect following hypoxix/ischaemic brain injury in neonatal rats [6]. The effects of CNS 5161 in a focal model of rat cerebral ischaemia, have also been studied [7]. A total of 64 adult male Sprague-Dawley rats underwent intraluminal suture middle cerebral artery occlusion (MCAO) followed by i.v. administration of vehicle (0.3 m mannitol) or CNS 5161 at three different boluses: 0.275, 0.55 or 1.1 mg kg−1 followed by a 3 h i.v. infusion of 0.2, 0.4, or 0.8 mg kg−1 h−1 (total doses of 0.88 (n = 11), 1.75 (n = 15) or 3.5 (n = 12) mg kg−1), respectively, in a blinded fashion. The vehicle or CNS dosing began at 10 min after MCAO. The total infarct volume in the control group with permanent intraluminal suture MCAO was 296±17 mm [3] (n = 26). CNS significantly reduced the total brain infarct volume by 35%, 42% and 46%, and the cortical infarct volume by 43%, 50% and 52%, respectively, in a dose-dependent manner, compared with infarct volumes obtained from vehicle-treated rats. There were no significant changes in mean arterial blood pressure and heart rate up to 3 h before and after the MCA occlusion in these anaesthetized animals treated either with vehicle or CNS 5161. On recovery from anaesthesia, the animals treated with CNS 5161 displayed notable dose-dependent behavioural changes such as excitation and impairment of locomotor co-ordination.
Figure 1.
Chemical structure of CNS 5161 (N-(2-chloro-5-(methylmercapto) phenyl)-N′-(3-(methylmercapto) phenyl)-N′-methylguanidine monohydrochloride).
Some dose-dependent mortality was seen in the animal groups treated with CNS 5161, up to 25% in the highest dose group. These deaths appeared to be related to sudden respiratory failure after continuing i.v. drug infusion under anaesthesia without the assistance of mechanical ventilation. After recovery from anaesthesia no animal died.
The plasma concentration of CNS 5161 was determined in samples from vehicle, 0.88 and 1.75 mg kg−1 (total dose) treated animals using a standard h.p.l.c. assay using a C8 column, an isocratic elution with a mobile phase of acetonitrile: methanol: buffer and an electrochemical detector. The assay was validated to a limit of detection of 0.25 ng ml−1. All analyses were performed blinded to treatment allocation. At the end of the infusion, the mean plasma concentration for the 0.88 mg kg−1 dose group was 9.7±0.9 ng ml−1 (n = 11), while the mean plasma concentration for the 1.75 mg kg−1 dose group was 19.6±1.2 ng ml−1 (n = 14). The CNS 5161 concentration in the samples taken from the vehicle-treated rats did not exceed the limit of quantification of the assay (n = 12).
On the basis of its activity in these different animal models of NMDA excitotoxicity, seizure and neuropathic pain, CNS 5161 appears to have good CNS penetration and efficacy.
The main potential clinical applications of the compound are neuroprotection and analgesia. This report documents a double-blind, dose escalating safety and tolerability study of CNS 5161 in normal healthy male volunteers. It was designed to provide initial information on the pharmacokinetics, pharmacodynamics, safety and tolerability of CNS 5161 in man.
The primary endpoints of the study were the effects of CNS 5161 on heart rate and blood pressure and changes in baseline laboratory values attributable to treatment. This endpoint was chosen as previous studies of NMDA receptor antagonists have revealed significant haemodynamic effects in both healthy volunteers and patients. Secondary endpoints comprised drug tolerability (as assessed by recording the nature, onset time, severity and duration of each complaint and clinical sign during treatment and for 24 h thereafter) and pharmacokinetics, specifically clearance and plasma drug half-life.
Local ethics approval for the study was granted, and written, informed consent was obtained from all volunteers. A dose escalation design was chosen, starting with 5% of the expected pharmacologically active dose.
Methods
Study population
Thirty-two healthy male volunteers participated in groups of four (three drug and one placebo) receiving a single intravenous administration of test medication over 15 min The inclusion criteria comprised:
Males aged 18–35 years
Body mass index between 21 and 29
Healthy (by medical history and physical examination)
Supine blood pressure 100–145 mmHg systolic and 50–90 mmHg diastolic
Written informed consent
The exclusion criteria comprised:
Exposure to any unlicensed pharmacological substance within 6 months of the study
Blood donation or venesection exceeding 200 ml in the preceding 6 months
Previous history of drug or alcohol abuse
Smoking
History of psychiatric illness
History of serious allergy, asthma or drug intolerance
Taking any regular medication
Clinically significant abnormality of any screening investigations
Any additional factor which, in the opinion of the investigator, would compromise the conduct, safety or validity of the study
Drugs
The test drug was provided in sterile vials containing 1 mg ml−1 CNS 5161 in an acetate buffered solution. The required dose was prepared in a blinded fashion by a registered pharmacist in the local sterile production unit by further dilution in normal saline under sterile conditions. The final volume to be infused was 20 ml, although 30 ml of solution was provided to allow for priming of dead space within the infusion system. Randomization was also performed by the pharmacy department. The same volume of normal saline alone was provided to act as a placebo comparator.
The test solution was administered by slow intravenous injection over 15 min via an ante cubital vein at a rate of 80 ml h−1. Subjects were studied in groups of four with three subjects receiving a single active dose and one subject receiving saline placebo. All actively treated subjects within each group received the same dose. Escalations in dose as the study progressed were decided following analysis of the pharmacokinetic and tolerability data of the preceding group. Dose escalations are shown in Table 1.
Table 1.
Dose escalation.
| Group | Dose |
|---|---|
| Group 1 | 30 µg or placebo |
| Group 2 | 100 µg or placebo |
| Group 3 | 300 µg or placebo |
| Group 4 | 600 µg or placebo |
| Group 5 | 750 µg or placebo |
| Group 6 | 1000 µg or placebo |
| Group 7 | 1250 µg or placebo |
| Group 8 | 2000 µg or placebo |
No concomitant therapy was permitted during the study. Water was provided as required throughout the dosing day and food was permitted after the 4 h observational period that followed cessation of infusion. No medications were taken by the subjects during the 7 day follow up period.
Observations
Safety observations up to 24 h post treatment included vital signs, physical examinations and laboratory studies. Any emergent side-effects were closely monitored.
Plasma samples for pharmacokinetic analysis were collected at 5, 10, 20, 40 min, 1, 2, 3, 4, 6, 8, and 24 h after cessation of infusion.
Pharmacokinetic analysis
CNS 5161 was infused at constant rate for 15 min. Plasma samples for pharmacokinetic analysis were collected at defined intervals following cessation of the infusion, however, no samples were taken during the infusion.
Blood samples drawn from the contralateral arm to the infusion at defined intervals following cessation of infusion were centrifuged promptly and frozen until analysed.
Plasma samples were analysed by means of high performance liquid chromatography at the clinical pharmacology laboratory of Primedica, Worcester, MA, USA. Similar methodology to the preclinical h.p.l.c. assay described above was employed. Extraction recoveries of CNS 5161 using this method range from 85% to 100%. Intra-day coefficients of variation for quality control standards ranged from 0.6% to 6.5%. Inter-day coefficients of variation ranged from 2.6% to 7.5%. During validation of the method employed, interday accuracy ranged from +0.3% to +2.2%.
Analysis
A full biochemical profile was performed using a local routine biochemistry analyser. Haematology analyses were performed by the hospital automated counter. Dip-stick urine analyses for blood, protein, ketones and glucose were carried out. Standard urinary screen for drugs of abuse (amphetamines, benzodiazepines and opiates) was performed at the discretion of the investigators.
Study procedure
Subjects were screened by clinical history, physical examination and laboratory tests up to 10 days prior to treatment. At 09.00 h on the dosing day, following baseline measurement of vital signs, resting 12 lead ECG and initiation of continuous ECG monitoring, a constant infusion of CNS 5161 or placebo was initiated. The dose was delivered over 15 min and continuous ECG recording continued for 45 min after cessation of infusion. Blood samples were taken at the time points detailed above, with measurement of supine blood pressure in triplicate immediately before each blood sample. Erect blood pressure and heart rate were also recorded from the 1 h timepoint onwards. Repeat resting 12 lead ECG was performed at 4 and 24 h post infusion.
Volunteers were encouraged to report any spontaneous symptoms, and in addition they were asked ‘How are you feeling?’ at 0.5, 1, 2, 3, 4, 6, 8, and 24 h post dosing and the response recorded.
Subjects were observed overnight in the research unit and allowed home after the 24 h time point. Each subject's response to treatment was evaluated and recorded. At 7 days post treatment, the subjects returned for evaluation by physical examination, symptom review and repeat laboratory tests.
Results
Thirty-two subjects completed the study in accordance with the protocol. There were no withdrawals. The mean age of the volunteers was 26.7 years (s.d. 2.2 years), mean height was 181.1 cm (s.d. 3.2 cm) and mean weight 77.7 kg (s.d. 5 kg).
Blood pressure
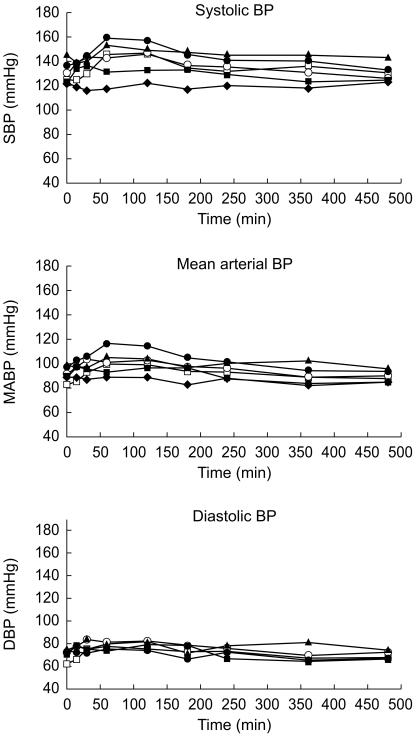
No consistent effect of CNS 5161 was seen in the lowest (30 µg) dose group. A dose dependent rise in systolic, mean arterial and diastolic blood pressure was seen in subsequent dosage groups, reaching 23/19 mmHg. Maximal effects were seen between 60 and 120 min after commencement of infusion. All subjects returned to baseline haemodynamic values within 24 h (Figure 2).
Figure 2.
Blood pressure changes following infusion of CNS 5161 (♦ placebo; ▪ 600 µg; ▴ 750 µg; ^ 1000 µg; □ 1250 µg; • 2000 µg).
Heart rate
The changes in heart rate were less closely related to drug dose than changes in blood pressure. As subjects were ambulant to a variable degree from 4 h after dosing, no inference concerning the effect of dosing on heart rate can be drawn.
Electrocardiographic analysis
Analysis of PR, QRS and QTc intervals was performed using both two sample t-test and anova. These data were collected by manual examination of each ECG trace. Corrected QT interval was calculated using Bazett's method. No significant differences between parameters of control subjects and dosed subjects were observed. No differences between predose and postdose ECG parameters were noted, nor was there any dose-related effect of CNS 5161. No significant disturbances of ECG morphology or rhythm were observed during ECG monitoring in the peri-infusion period or on any subsequent 12-lead ECGs.
Laboratory determinations
All subjects completed assessment at 7 days following the study. No significant abnormalities in biochemistry, haematology or urinalysis were identified.
Biochemistry
Two subjects had mild, isolated elevation of serum bilirubin concentration, attributed to Gilbert's Syndrome in both cases. Serum bilirubin concentration was unchanged by the study drug.
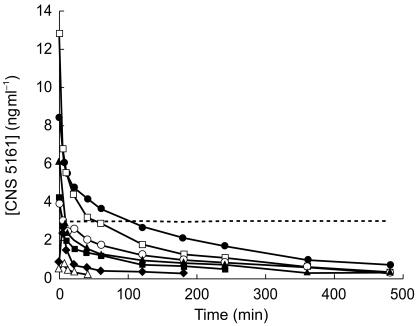
Pharmacokinetics
A graph representing the mean concentration-time profiles of each panel of subjects is presented (Figure 3). Computerized pharmacokinetic modelling was performed using least squares nonlinear regression analysis. The data were best described by a two-compartment pharmacokinetic model.
Figure 3.
Change in plasma concentration of CNS 5161 (▵ 100 µg; ♦ 300 µg; ▪ 600 µg; ▴ 750 µg; ^ 1000 µg; □ 1250 µg; • 2000 µg). ---- neuroprotective concentration.
Maximum concentration
A linear correlation between Cmax and dose was noted. The strength of the relationship between absolute dose and Cmax (correlation coefficient 0.626) did not differ substantially from the relationship between dose in µg kg−1 and Cmax (correlation coefficient 0.680).
AUC
Area under the concentration-time curve for the first 10 h after infusion was calculated using the trapezoidal rule and expressed in ng ml−1 h. It was found to rise with dose in a linear fashion (correlation coefficient 0.911). There was little difference between the correlation of absolute dose with AUC and the correlation of dose per kilogram body weight.
Half-life
The mean half-life was 2.95 h (s.d. 0.75).
Clearance
Mean clearance was 106 l h−1 (s.d. 17.8).
Volume of distribution
The mean volume of distribution was 296 l (s.d. 69).
Other pharmacokinetic parameters (stratified by dose group) are given in Table 2.
Table 2.
Pharmacokinetic parameters.
| Cmax (ng ml−1) | AUC (ng ml−1 h) | Clearance (ml min−1) | Vss (l) | t1/2 (min) | |
|---|---|---|---|---|---|
| 600 µg dose | |||||
| Mean | 4.31 | 268.8 | 2285.3 | 250.9 | 163.5 |
| s.e. mean | 2.5 | 27.6 | 259.8 | 50.7 | 39.2 |
| n | 3 | 3 | 3 | 3 | 3 |
| % CV | 1.0 | 0.17 | 0.19 | 0.35 | 0.41 |
| High | 9.31 | 303.9 | 2801.4 | 344.0 | 234.3 |
| Low | 1.21 | 214.1 | 1973.9 | 169.5 | 98.7 |
| 750 µg dose | |||||
| Mean | 6.09 | 485.1 | 1723.5 | 289.0 | 179.6 |
| s.e. mean | 1.6 | 98.5 | 437.7 | 30.6 | 20.7 |
| n | 3 | 3 | 3 | 3 | 3 |
| % CV | 0.46 | 0.35 | 0.43 | 0.18 | 0.20 |
| High | 7.7 | 595.9 | 2598.3 | 327.0 | 201.4 |
| Low | 2.81 | 288.6 | 1258.4 | 228.3 | 138.0 |
| 1000 µg dose | |||||
| Mean | 4.3 | 653.5 | 1590.5 | 383.0 | 184.8 |
| s.e. mean | 0.8 | 84.4 | 234.3 | 60.3 | 8.4 |
| n | 3 | 3 | 3 | 3 | 3 |
| % CV | 0.32 | 0.22 | 0.25 | 0.27 | 0.07 |
| High | 5.9 | 754.9 | 2057.7 | 503.4 | 195.2 |
| Low | 3.22 | 719.7 | 1324.5 | 315.2 | 168.0 |
| 1250 µg dose | |||||
| Mean | 12.8 | 863.5 | 1515.0 | 266.9 | 143.4 |
| s.e. mean | 2.3 | 137.5 | 212.9 | 43.2 | 7.7 |
| n | 3 | 3 | 3 | 3 | 3 |
| % CV | 0.32 | 0.27 | 0.24 | 0.28 | 0.09 |
| High | 17.3 | 1135.5 | 1807.5 | 353.0 | 151.5 |
| Low | 12 | 691.5 | 1100.8 | 216.8 | 127.8 |
| 2000 µg dose | |||||
| Mean | 8.8 | 1352.1 | 1554.3 | 375.3 | 172.3 |
| s.e. mean | 2.8 | 195.4 | 260.6 | 70.3 | 9.0 |
| n | 3 | 3 | 3 | 3 | 3 |
| % CV | 0.55 | 0.25 | 0.29 | 0.32 | 0.09 |
| High | 14.5 | 1587.3 | 2074.2 | 515.1 | 187.8 |
| Low | 5.09 | 1505.0 | 1259.9 | 291.9 | 156.4 |
| Dose group | |||||
| 600 µg | 4.31 | 269 | 2285 | 251 | 164 |
| 750 µg | 6.10 | 485 | 1724 | 289 | 180 |
| 1000 µg | 4.32 | 654 | 1591 | 383 | 185 |
| 1250 µg | 12.82 | 864 | 1515 | 267 | 143 |
| 2000 µg | 8.88 | 1352 | 1554 | 375 | 172 |
Adverse events
All symptoms or signs reported spontaneously or in response to questioning were recorded as adverse events.
No severe adverse events were encountered at any time. The majority of symptoms were noted during the 24 h close observation period following dosing. The only symptom arising outwith this period occurred in Subject 602 (recipient of 1000 µg CNS 5161), who described mild throat discomfort for 2 days after dosing. This symptom resolved fully.
Minor symptoms fell into three general categories: nonspecific general effects, cardiovascular effects and sensory disturbance. The time to onset and duration of these symptoms are given in Table 3.
Table 3.
Adverse events.
| Effect | Number of subjects | Mean onset time (min) | Range (min) | Mean duration (min) | Range (min) | Severity |
|---|---|---|---|---|---|---|
| Lightheadedness | 10 | 55 | 40–85 | 44 | 3–120 | Mild |
| Paraesthesia | 13 | 80 | 28–90 | 29 | 1–60 | Mild |
| Headache | 4 | 230 | 60–340 | 45 | 25–60 | Mild |
| Drowsiness | 4 | 150 | 120–240 | 26 | 10–40 | Mild |
| Flushing | 3 | 60 | 35–85 | 6 | 1–11 | Mild |
| Derealisation | 2 | 55 | 22–80 | 55 | 50–60 | Mild |
The sensory symptoms involved the peripheries and perioral area. They were universally mild, comprising dysaesthesia or numbness without any associated objective sensory deficit. All sensory symptoms resolved fully within 2 h.
The nonspecific effects comprised sensations of lightheadedness, derealization, drowsiness, flushing and dizziness, not associated with any objective abnormal neurological or cardiovascular findings on examination. These all resolved fully within 1 h.
No associated postural changes in blood pressure were observed. At no time were true vertiginous symptoms observed. Symptoms in the lower dosage groups (panels 1, 2, and 3) were particularly ill-defined and occurred with similar frequency in control and treated subjects.
The more marked fluctuations in blood pressure witnessed in the higher dose groups were recorded as adverse events. The time course of the haemodynamic effects is shown in Figure 2.
Discussion
CNS 5161 was well-tolerated when given in intravenous doses up to 2 mg. No ECG abnormalities were observed, nor were there any significant changes in PR QRS or QTc intervals. Side-effects were moderate and well-tolerated by all subjects. Plasma drug concentration in the higher dose groups transiently exceeded postulated neuroprotective levels with no significant adverse effects. More prolonged maintenance of plasma concentration at the neuroprotective threshold is likely to be required in the context of human stroke.
Certain pharmacodynamic effects were drug-related. It is likely that the minor sensory disturbances, drowsiness and flushing are also attributable to dosing. The aetiology of less specific complaints such as headache and dizziness is less clear, as they are frequently encountered in normal subjects who are required to fast and maintain bed rest for prolonged periods. The transient sensations of derealization experienced by some subjects were mild and self-limiting. They do not represent a significant psychoactive effect of CNS 5161 within the dose range studied.
Prominent cardiovascular effects were seen at doses of 1 mg and above. A 15–20% increase in mean arterial pressure was apparent, maximal 45–105 min after cessation of the infusion but present for approximately 6 h. Cardiovascular effects appear to be dose-related within the dosage range studied. The haemodynamic effects observed were well-tolerated by healthy volunteers, however, similar increases in blood pressure may be less well-tolerated by elderly patients with stroke.
Higher concentrations were associated with increased pharmacological effects, both on cardiovascular variables and on reports of adverse events. The timing of these effects was such that plasma concentrations are not directly related to the pharmacodynamic effects.
The pharmacokinetics are linear within the dose range studied. The low correlation between body weight and clearance or volume of distribution suggests that dosage adjustment for body weight is unlikely to be necessary.
The small number of subjects reported at each dose in this study means that statistical analysis of effects between doses is inappropriate. The study has provided a valuable guide to the effects at various doses of CNS 5161, but variability in the pharmacokinetics and similar or greater variability in the pharmacodynamics can be anticipated. A larger number of subjects must be studied to determine with confidence the effects of any individual dose.
By undertaking preliminary studies in normal subjects, information is obtained quickly and reliably about the disposition and pharmacological effects of a drug. The effects of age, disease, drug interactions and other factors have not yet been examined, and no information on therapeutic efficacy has been collected. Whilst the results of this study are encouraging for continued clinical development of CNS 5161, a cautious approach to the use of similar doses in patients will be necessary.
Conclusions
CNS 5161 has been shown to have a potent neuroprotective effect against acute focal brain ischaemia in adult rats and against hypoxic/ischaemic brain damage in neonatal rats. On the basis of its effects in animal models of stroke it is a potential agent for the treatment of acute focal cerebral ischaemia in humans. It appears to be safe and well-tolerated when administered to healthy young males, with minor side-effects which are dose-dependent. The maximum tolerated dose was not identified, as neuroprotective plasma concentrations were transiently achieved with relatively minor haemodynamic and adverse effects. It is likely that the haemodynamic effects may be dose-limiting given the mild and subjective nature of the other side-effects. The cardiovascular effects were predicted from animal models however, their mechanistic basis is unclear. Similar haemodynamic effects were seen with the related compound aptiganel (CNS 1102). These effects require further investigation, especially with regard to the cerebral circulation.
Acknowledgments
This study was sponsored by Cambridge NeuroScience, Inc. (now CeNeS Pharmaceuticals, Inc.).
References
- 1.Choi D. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985;58:293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 2.Graham S. Changes in extracellular amino acid neurotransmitters produced by focal cerebral ischaemia. Neurosci Lett. 1990;110:124–130. doi: 10.1016/0304-3940(90)90799-f. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste H. Calcium accumulation of glutamate receptor activation is involved in hippocampal cell damage after ischaemia. Acta Neurol Scand. 1988;78:529–536. doi: 10.1111/j.1600-0404.1988.tb03697.x. [DOI] [PubMed] [Google Scholar]
- 4.Hu L-Y. Synthesis and pharmacological evaluation of N-Methylguanidines as potent NMDA-receptor ion channel blockers. J Med Chem. 1997;40:4281–4289. doi: 10.1021/jm970459c. [DOI] [PubMed] [Google Scholar]
- 5.Amitay O. In vitro potency of novel NMDA antagonists predicts in vivo protection against seizures and neurotoxicity. Behav Pharm. 1995;6:615. [Google Scholar]
- 6.Wang S, Zhou D, Hu L, Durant G, Fischer JB, Holt WF. CNS 5161 protects against brain damage from hypoxic-ischaemia in neonatal rats. Soc for Neuroscience. 1997;23:2433. (946.1) [Google Scholar]
- 7.Zhou D, Wang S, Hu L, et al. Neuroprotective effect of CNS 5161, a potent NMDA ion-channel antagonist, after focal cerebral ischaemia in rats. Soc for Neuroscience. 1997;23:2433. (946.2) [Google Scholar]