Abstract
Aims
To investigate the effects of glutamate on insulin secretion and glucose tolerance in humans.
Methods
Monosodium (l)-glutamate (10 g) was given orally in a double-blind placebo-controlled cross-over study to 18 healthy volunteers, aged 19–28 years, with an oral (75 g) glucose load.
Results
The 75 min insulin response (AUC(0,75 min)), up to tmax of glutamate kinetics, was significantly correlated with the AUC(0,75 min) of glutamate concentrations (r = 0.485, P = 0.049). Glucose tolerance was not affected.
Conclusions
Oral (l)-glutamate enhances glucose-induced insulin secretion in healthy volunteers in a concentration-dependent manner.
Keywords: clinical investigation, glucose tolerance, glutamate, insulin secretion, insulin sensitivity
Introduction
Glutamate is the major excitatory neurotransmitter of the central nervous system. Nevertheless, the presence of ionotropic receptors of the AMPA subtype was demonstrated in rat islet α- and β-cells by immunocytochemistry [1].
(l)-Glutamate is known to stimulate insulin secretion in vitro, from isolated rat pancreas by acting on receptors of the AMPA subtype [2], and in vivo in normal rats [3] and in a rat model of type 2 diabetes [4], with glucose tolerance improvement.
The effects of glutamate on insulin secretion and glucose homeostasis in humans is unclear. In a noncontrolled study, Thomassen et al. [5] observed that intravenous monosodium glutamate stimulated insulin secretion. Oral administration of monosodium glutamate in fasting healthy subjects was reported to increase plasma insulin levels without altering glucose concentrations [6].
The aim of this study was to investigate the effects of monosodium (l)-glutamate on insulin secretion, glucose tolerance and insulin sensitivity during an oral glucose tolerance test in healthy volunteers.
Methods
Eighteen healthy volunteers, aged 19–28 years (mean 22 years) and weighing 52–81 kg (mean 68 kg), were included in a randomized double-blind placebo-controlled cross-over investigation. The protocol was approved by the institutional Ethics Committee of Montpellier and all subjects gave written informed consent to participate. One subject did not complete the study for personal reasons and was not included in the final analysis.
Subjects were assigned to take a single oral 10 g dose of monosodium (l)-glutamate (ORSAN S.A., Paris, France) or placebo, both packaged in identical capsules to mask the taste of glutamate and maintain the blind integrity. The two administrations were separated by a 7 day washout.
In the morning of each test-day, subjects were admitted to the Clinical Investigation Center, in fasting conditions for at least 12 h. Two baseline venous blood samples were taken at 15 min intervals. Then, the treatment (20 capsules of monosodium (l)-glutamate or 20 capsules of placebo) was administered simultaneously with an oral glucose load (75 g). Blood samples were taken 15, 30, 45, 60, 75, 90, 105 and 120 min after the start of the oral glucose tolerance test (OGTT).
Plasma glutamate concentrations were determined by high performance liquid chromatography [3]. Blood glucose concentrations were determined by the glucose oxidase method, insulin and glucagon by radioimmunoassay.
Insulin secretion was assessed as the area under the curve (AUC) of insulin concentrations during OGTT, glucose tolerance as the glucose AUC during OGTT and insulin sensitivity calculated as the composite insulin sensitivity index (ISI) according to Matsuda & DeFronzo [7].
Results are given as means ± standard error (s.e. mean). Where necessary, 95% confidence intervals on differences (95% CI) are given. AUC and ISI were compared with a paired t-test. Pearson correlation was performed between insulin response and glutamate concentrations, both expressed as AUC. The study power was set at 90%.
Results
Mean plasma glutamate concentrations showed no significant variation after ingestion of placebo: they ranged between 41.0 and 45.8 µmol l−1. Following administration of monosodium (l)-glutamate, there was a 15-min delayed and progressive increase in plasma glutamate levels, reaching a maximum (Cmax) of 79.6 ± 15.5 µmol l−1, 75 min (tmax) after ingestion. Individual values at tmax were highly variable and ranged between 21.3 and 288.9 µmol l−1.
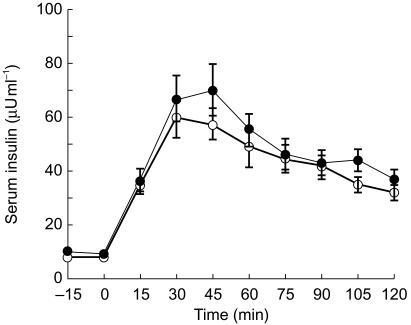
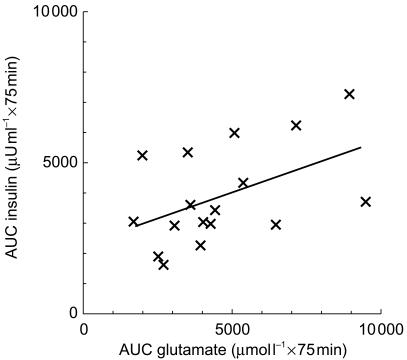
The increase in serum insulin concentrations (Figure 1) after glucose absorption tended to be higher, although not significantly, after glutamate administration than after placebo. However, insulin AUC from 0 to the tmax of glutamate concentrations (AUC(0,75 min)) were significantly correlated (r = 0.485, P = 0.049, n = 17) with the AUC(0,75 min) of glutamate concentrations (Figure 2). Moreover, in the subgroup of subjects with higher glutamate bioavailability, defined as a glutamate AUC from 0 to 120 min (AUC(0,120 min)) after glutamate administration ≥ + 34% over AUC(0,120 min) after placebo (median increase, n = 9), significantly higher insulin response (+30.6%, 95% CI: 189, 2892 µU ml−1 × 120 min) was reached vs placebo (P = 0.03); no significant response was observed when glutamate bioavailability was < + 34% vs placebo (n = 8).
Figure 1.
Time course of serum insulin after monosodium (l)-glutamate or placebo administration at 0 min during OGTT (75 g glucose load at 0 min). • monosodium (l)-glutamate (10 g); ○ placebo.
Figure 2.
Correlation of insulin response with the AUC(0, 75 min) of plasma glutamate concentrations during 75 g OGTT (r = 0.485, P = 0.049, n = 17).
Monosodium (l)-glutamate did not affect glucose tolerance, even in the subgroup of subjects with a higher glutamate bioavailability: 696 ± 29 vs 688 ± 34 mmol l −1 × 120 min, n = 9.
An impairment of the whole-body composite ISI was observed (−18.0% vs placebo, 95% CI: −2.419, −0.183, n=17, P=0.01).
Two subjects reported some minor adverse effects after glutamate, consisting of dizziness, weakness and gastric discomfort with nausea for one of them, and headache for the other one. Dizziness and headache disappeared before the end of the day while weakness and digestive discomfort lasted till the next morning.
Discussion
(l)-glutamate is widely distributed and commonly consumed; it is used in some countries as a flavour enhancer in foods. In this study, healthy subjects were exposed to circulating levels of glutamate exceeding basal concentrations, following an oral dose of approximately 150 mg kg−1 monosodium (l)-glutamate. The mean plasma levels of endogenous glutamate were stable and consistent with those previously reported in man [6, 8]. Exogenous glutamate was ingested in capsules and absorption was 15 min delayed, with peak levels at 75 min. When given orally in a solution form at equivalent dose [6], an immediate increase in plasma glutamate concentrations was reported, with peak levels occurring at 60 min. Glutamate absorption from capsules also appeared to be incomplete considering the plasma concentrations achieved for a given dose, in comparison with previously reported data following intravenous injection [5] or oral administration of a solution form without glucose coadministration [6]. Nevertheless, this study shows for the first time a positive correlation between glutamate concentrations, in the micromolar range, and insulin response to a glucose challenge in humans. This significant relationship is consistent with the involvement of glutamate receptors mediating insulin-releasing action of that compound. The plasma glutamate concentrations that effectively enhanced insulin secretion are comparable to those reported to occur in man after ingestion of a meal [8]. Thus, during food intake, glutamate may participate in the insulin response to nutrients.
Glutamate did not lower blood glucose despite its insulinotropic action, which may be related to the impairment of the whole body insulin sensitivity index. This lack of effect cannot be attributed to a stimulating action of glutamate on glucagon secretion, as previously reported in rat [9], since no significant effect of glutamate on plasma glucagon levels was observed (not shown). It might be due to an increase in catecholamine secretion, as previously shown in animal model [10] and as suggested by a tendency to transient increase in systolic blood pressure in our conditions (not shown). A well-adapted regulation of glucose homeostasis in subjects who had normal glucose tolerance may also explain why a moderate insulin response to glutamate was insufficient to lower blood glucose.
We conclude that oral glutamate is able to amplify glucose-induced insulin secretion in a concentration-dependent manner in humans, at concentrations in the low micromolar range.
Acknowledgments
The authors thank A. Bonardet (Department of Biochemistry), B. Bories-Azeau (Clinical Investigation Center), F. Costa (Clinical Investigation Center) and A. M. Puech-Cathala (Department of Nuclear Medicine) for expert technical and logistic assistance, and P. Guion from ORSAN S.A. for supplying monosodium (l)-glutamate.
This study was supported by a grant from the University Hospital of Montpellier, France (n°7555, 1997).
References
- 1.Weaver CD, Yao TL, Powers AC, Verdoorn TA. Differential expression of glutamate receptor subtypes in rat pancreatic cells. J Biol Chem. 1996;271:12977–12984. doi: 10.1074/jbc.271.22.12977. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand G, Gross R, Puech R, Loubatières-Mariani MM, Bockaert J. Evidence for a glutamate receptor of the AMPA subtype which mediates insulin release from rat perfused pancreas. Br J Pharmacol. 1992;106:354–359. doi: 10.1111/j.1476-5381.1992.tb14340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand G, Puech R, Loubatières-Mariani MM, Bockaert J. Glutamate stimulates insulin secretion and improves glucose tolerance in rats. Endocrinol Metab. 1995;32:E551–E556. doi: 10.1152/ajpendo.1995.269.3.E551. Am J Physiol, 269. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand G, Ravier M, Puech R, Loubatières-Mariani MM, Bockaert J. Effects of glutamate on glucose tolerance and insulin secretion in a rat model of type II diabetes. Diabetologia. 1997;40:A132. [Google Scholar]
- 5.Thomassen A, Nielsen TT, Bagger JP, Henningsen P. Effects of intravenous glutamate on substrate availability and utilization across the human heart and leg. Metabolism. 1991;40:378–384. doi: 10.1016/0026-0495(91)90148-p. [DOI] [PubMed] [Google Scholar]
- 6.Fernstrom JD, Cameron JL, Fernstrom MH, McConaha C, Weltzin TE, Kaye WH. Short-term neuroendocrine effects of a large oral dose of monosodium glutamate in fasting male subjects. J Clin Endocrinol Metab. 1996;81:184–191. doi: 10.1210/jcem.81.1.8550750. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, De Fronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 8.Stegink LD, Filer LJ, Brummel MC, et al. Plasma amino acid concentrations and amino acid ratios in normal adults and adults heterozygous for phenylketonuria ingesting a hamburger and milk shake meal. Am J Clin Nutr. 1991;53:670–675. doi: 10.1093/ajcn/53.3.670. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand G, Gross R, Puech R, Loubatières-Mariani MM, Bockaert J. Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. Eur J Pharmacol. 1993;237:45–50. doi: 10.1016/0014-2999(93)90091-u. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez MP, Herrero MT, Vincente S, Oset-Gasque MJ. Effect of glutamate receptor agonists on catecholamine secretion in bovine chromaffin cells. Neuroendocrinology. 1998;67:181–189. doi: 10.1159/000054313. [DOI] [PubMed] [Google Scholar]