Abstract
Aims
Bioavailability of orally administered drugs depends on several factors including active excretion, e.g. by P-glycoprotein (PGP), and presystemic metabolism, e.g. by cytochrome P450 3A (CYP3A), in both gastrointestinal tract and liver. Many drugs including saquinavir are substrates of both PGP and CYP3A. It was the aim of this study to test whether the extremely low bioavailability of saquinavir can be increased dose-dependently in vivo by cremophor EL, an ‘inactive’ pharmaceutic aid known to inhibit PGP in vitro.
Methods
In a randomized, placebo-controlled, double-blind, four phase cross-over design single doses of oral saquinavir (Invirase®, 600 mg, without food) were administered with increasing single doses of oral cremophor EL (up to 5000 mg) to eight healthy, male individuals. Saquinavir plasma concentrations were determined by LC/MS/MS up to 48 h after intake. Main outcome measures were area under the plasma concentration time curve (AUC), peak concentration (Cmax), time to reach Cmax (tmax) and terminal elimination half-life (t½).
Results
Cremophor EL dose-dependently increased Cmax, AUC(0,4 h), and AUC(0,∞) of saquinavir. As compared with placebo, the increment observed after 5000 mg cremophor EL was 13-fold for both Cmax and AUC(0,4 h) and 5-fold for AUC(0,∞). The terminal half-life and the time to reach Cmax (tmax) were unchanged.
Conclusions
Cremophor EL increased the systemic availability of saquinavir without affecting its elimination suggesting that cremophor EL is not devoid of pharmacological action and acts as a modulator of the absorption process, probably by inhibiting intestinal PGP.
Keywords: bioavailability, cremophor EL, P-glycoprotein, saquinavir
Introduction
Bioavailability of orally administered therapeutic agents depends on the extent of dissolution, absorption, and subsequent elimination processes such as active intestinal excretion and presystemic (first-pass) metabolism in both the gastrointestinal tract and liver. A relevant efflux pump is P-glycoprotein (PGP), which is expressed in high amounts in gut, renal tubule, biliary tract, and in the blood–brain barrier [1]. Important phase I enzymes, which may contribute to first-pass metabolism, belong to the cytochrome P450 3A subfamily (CYP3A). They represent 70% of the CYP content in the gastrointestinal tract [2] and are the major CYP isozymes in the liver [3].
Many drugs are substrates of both PGP and CYP3A [1], two systems that may critically limit bioavailability, as is the case for the HIV protease inhibitor saquinavir. Only about 0.7% of an oral dose will reach the systemic circulation when saquinavir is taken without food [4] and 4% when taken with food [5]. The plasma concentrations of such drugs may be markedly increased if coadministered with inhibitors of PGP and/or CYP3A. A pertinent example is the favourable interaction between saquinavir and ritonavir. In vitro, ritonavir acts as an inhibitor of both CYP3A [6] and PGP [7] and in HIV standard drug regimens in vivo, small doses of ritonavir may enhance the area under the plasma concentration-time curve (AUC) of saquinavir 50-fold [8].
A promising possibility to increase bioavailability of PGP substrates is the inhibition of intestinal PGP [9]. In contrast to inhibition of CYP3A this approach has the advantage of a substantially lower likelihood of changing the metabolic ratio between parent compound and metabolite(s). In addition, as opposed to systemic PGP-inhibition which may also modulate drug distribution into the CNS [10], it is not expected to increase the risk for adverse CNS effects.
Cremophor EL (polyoxyl 35 castor oil) is an ‘inactive’ pharmaceutic aid used as emulgator in oral, topical, and parenteral solutions. However, cremophor EL is not an inert additive because it has been shown to inhibit PGP in vitro [11, 12] and to increase the AUC of PGP-substrates when given intravenously [13]. So far, there is no published information available on intestinal PGP inhibition by oral cremophor EL and the corresponding dose–response relationship.
The aim of the current study was therefore to evaluate the influence of increasing oral doses of cremophor EL on the pharmacokinetics of a compound with extremely low oral bioavailability (saquinavir) in healthy individuals.
Methods
Study population
Eight healthy, nonsmoking male subjects participated after they had been fully informed about the study and given written informed consent. Before entering the study, the volunteers were ascertained to be healthy by medical history, physical examination, laboratory screening including haematological and biochemical blood tests, and a 12-lead electrocardiogram. Mean ± s.d. (range) of age and body weight were 29.3 ± 3.7 (25–37) years and 83.3 ± 16.2 (72–117) kg, respectively. None had received any other drug from 2 months before until the end of the study.
Study design
The study was approved by the Ethics Committee of the Medical Faculty of the University of Heidelberg and was conducted at the Department of Internal Medicine VI, Clinical Pharmacology and Pharmacoepidemiology in accordance with the Declaration of Helsinki and subsequent amendments. The study was performed according to a randomized, placebo-controlled, double-blind, four phase cross-over design. Each volunteer participated in four individual sessions separated by at least 1 week. Food and beverages containing alcohol or methylxanthines were not allowed from 12 h before drug administration until the last blood sample was drawn. Volunteers fasted from 12 h before drug administration until 4 h after drug intake. Standardized liquid food (Fresubin®, a standard diet for enteral nutrition) was served 4 and 8 h after the study drug, which was administered with 200 ml of mineral water around 08 00 h and consisted of 600 mg saquinavir with 0, 100, 1000, or 5000 mg cremophor EL.
Blood sampling
Blood was collected through an intravenous catheter into heparinized tubes. Blood samples (7.5 ml) were obtained immediately before and 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, 12, 24, and 48 h after dosing. The samples were immediately put on ice and centrifuged (3000 g for 15 min) within 30 min. Plasma samples were stored at −20° C until analysis.
Determination of saquinavir plasma concentrations
Saquinavir plasma concentrations were determined by LC/MS/MS (electrospray ionization, multiple reaction monitoring) after alkaline liquid/liquid-extraction with acetic acid ethylester. For calibration deuterated saquinavir was used. The lower limit of quantification was 72 ng l−1 (0.107 nmol l−1) with a batch-to-batch accuracy of 110 ± 10% (n = 10 batches with two values per batch). The prestudy validation revealed a mean precision of 10.0% (range −2.8% to +19.8%, n = 18 determinations) [14]. Validation of the method using quality control samples at three different concentrations (double determination per batch) was performed according to the recommendation of the FDA [15].
Determination of C3435T polymorphism of PGP
C3435T polymorphism of the human multidrug-resistance-1 (MDR1) gene which encodes PGP was determined as described by Nauck et al. [16].
Materials
Invirase® (containing 200 mg saquinavir) was obtained from Hoffmann La Roche Ltd (Grenzach-Wyhlen, Germany), cremophor EL from BASF via P.F.V. GmbH (Burgbernheim, Germany), Fresubin® from Fresenius Ltd (Bad Homburg, Germany). All reagents and solvents used for the chromatographic, spectroscopic, and sample procedures were of analytical or higher quality and were purchased from Fluka (Neu-Ulm, Germany), E. Merck (Darmstadt, Germany) and Promochem (Wesel, Germany). Pure standards of saquinavir (Ro 31–8959/008) and deuterated saquinavir (internal standard, Ro 31–8959/048) were generous gifts from Roche (Roche Products Limited, Hertfordshire, England) and were used for analytical purposes only. The study drug was prepared by the hospital pharmacy by mixing the content of Invirase® capsules with the appropriate amount of cremophor EL and dispensing it into hard gelatine capsules. In order to assure that all volunteers received the same number of capsules, identically looking lactose capsules were prepared.
Pharmacokinetic calculations
The peak plasma concentration before lunch (Cmax) and the time to reach Cmax (tmax) were obtained directly from the raw data. The terminal plasma half-life (t½) was calculated as t½ = ln 2/λz where λz represents the slope of the terminal part of the plasma concentration-time curve obtained by linear regression after semilogarithmic transformation. The area under the plasma concentration-time curve from hour 0 to infinity (AUC(0,∞) was calculated as
where t is the time of the last blood sample and Ct the corresponding plasma concentration. Partial AUC(0,t) was calculated by the trapezoidal rule. All calculations were performed with WinNonlin Professional (Version 3.1, Pharsight Corporation, Mountain View, CA).
Statistics
The results are expressed as mean values ± s.d., except in figures in which, for clarity, mean values ± s.e.mean are given. The different treatments were compared by repeated measures anova followed by the Tukey HSD test. The effect of cremophor on the occurrence of a second postprandial peak exceeding the peak concentrations in the fasted state was analysed by the chi-square test. A P value ≤0.05 was considered to be statistically significant.
Results
The study drugs (600 mg saquinavir and varying doses of cremophor EL p.o.), which each volunteer received four times, were well tolerated. Three volunteers reported mild headache on two occasions (once without cremophor, once with 100 mg, twice with 1000 mg and 5000 mg, respectively), one individual complained of moderate headache on one study day (600 mg cremophor), and one volunteer had diarrhoea on one study day (100 mg cremophor). All adverse events disappeared spontaneously and were considered as possibly related to study participation by the responsible physicians. Only diarrhoea was considered as possibly related to the study drug.
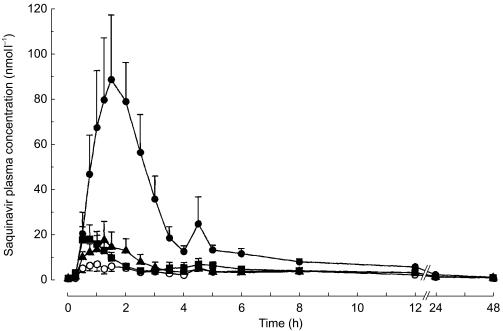
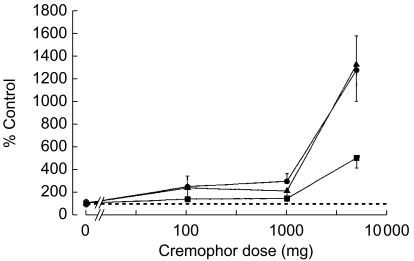
After performance of two pilot studies with two volunteers each, which were performed to determine appropriate oral doses of Cremophor EL (data not shown), doses were set at 100, 1000 and 5000 mg in this study. When combined with cremophor EL peak concentrations (Cmax), AUC(0,4 h), and AUC(0,∞) of saquinavir dose-dependently increased. As compared with placebo, the increment observed after 5000 mg cremophor EL was 5-fold for AUC(0,∞), and 13-fold for both Cmax and AUC(0,4 h). Cmax and AUC(0,4 h) were highly correlated (linear regression AUC(0,4 h) = 1.51 × Cmax−2.36 (r = 0.977)). Terminal half-life and tmax were unchanged (Table 1, Figures 1 and 2).
Table 1.
Pharmacokinetic characteristics of a single dose of saquinavir (Invirase®, 600 mg orally) after administration with increasing single oral doses of cremophor EL in eight healthy individuals.
| 0 mg | 100 mg | 1000 mg | 5000 mg | |
|---|---|---|---|---|
| Cmax | 8.8±8.5 | 21.7±23.3 | 25.8±17.0 | 112.7±74.7* |
| (nmol l−1) | (0.8–23.3) | (3.1–72.2) | (3.3–48.9) | (24.7–210.1) |
| tmax | 1.9±0.7 | 1.3±0.5 | 1.2±0.9 | 1.8±0.5 |
| (h) | (1.0–3.0) | (0.5–2.0) | (0.5–3.5) | (1.0–2.5) |
| t½ | 12.4±4.7 | 10.0±1.7 | 10.1±1.5 | 10.5±1.8 |
| (h) | (8.1–21.2) | (7.6–12.6) | (8.0–12.5) | (8.3–13.9) |
| AUC(0,4 h) | 13.9±11.7 | 32.7±37.1 | 28.9±18.8 | 184.5±128.4* |
| (nmol l−1 h) | (1.6–32.9) | (3.4–109.8) | (5.5–54.1) | (41.9–423.9) |
| AUC(0,∞) | 66.3±27.5 | 90.7±45.5 | 95.1±49.1 | 332.3±167.3* |
| (nmol l−1 h) | (23.5–101.1) | (32.1–182.8) | (48.3–198.4) | (169.1–682.6) |
Data are given as mean±s.d. (range).
P < 0.01 vs placebo.
Figure 1.
Average plasma concentration – time curves of a single dose of saquinavir (Invirase®, 600 mg orally) after administration with increasing single oral doses of cremophor EL (○ mg; ▴ 100 mg; ▪ 1000 mg; • 5000 mg) in eight healthy volunteers. Data are presented as mean ± s.e.mean.
Figure 2.
Mean values of Cmax (•), AUC(0,4 h) (▴), and AUC(0,∞) (▪) of a single dose of saquinavir (Invirase®, 600 mg p.o.) after administration with increasing single oral doses of cremophor EL in eight healthy volunteers. Data are presented as mean ± s.e.mean.
In most saquinavir plasma concentration-time curves (i.e. 27 out of 32) a second peak after 4 h was observed, reflecting the known influence of food on absorption [17]. The second peak was higher than the peak before lunch in 3 of 8 curves without but in only 2 of 19 with cremophor EL (P = 0.10).
In order to compare genotype with phenotype, the C3435T polymorphism of the MDR1 gene which encodes PGP was determined. Of the eight volunteers, one volunteer was C/C, five were C/T, and two T/T. No apparent association with the phenotype could be found with regard to Cmax, AUC(0,4 h) or AUC(0,∞) after placebo or 5000 mg cremophor EL or the ratio of 5000 mg to placebo of these parameters (data not shown). Due to the small number of subjects no statistical evaluation was performed.
Discussion
In the current investigation the pharmacokinetic characteristics of saquinavir administered without cremophor were roughly comparable with those observed by Kupferschmidt et al. [4] who also administered saquinavir to fasted volunteers. Food was avoided during the presumed drug absorption phase as it is known to increase saquinavir bioavailability profoundly [17] and was expected to render data interpretation more difficult.
Compared with placebo, administration of cremophor EL dose-dependently increased the systemic availability of saquinavir. The extent of the increase of Cmax was close to the fold increase of AUC(0,4 h) which represents the early phase of the plasma concentration-time profile not influenced by food. The relationship between dose and effect (Cmax, AUC) showed no plateau at high doses suggesting that the maximum increase of Cmax and AUC was not reached with 5000 mg cremophor. However, we refrained from administering higher doses to avoid the occurrence of diarrhoea that is a common effect of castor oil and polyethylene glycol [18], which are expected to be formed by intestinal hydrolysis of cremophor, and thus might influence the intraluminal availability of saquinavir for absorption in the small intestine.
As compared with placebo AUC(0,∞) was increased 1.4 fold by 100 mg and 1000 mg cremophor EL and 5-fold by 5000 mg. Compared with the effect of an opulent breakfast on saquinavir pharmacokinetics, the magnitude of the effect of 5000 mg cremophor EL was roughly similar [4, 5]. Whether the effect of cremophor EL is less pronounced, additive or even synergistic, when saquinavir is taken with food, as recommended by the manufacturer cannot be answered and remains to be studied.
Cremophor EL increased Cmax without affecting the elimination half-life, at least at the single dose-regimen used in our study. This suggests that cremophor primarily acted as a modulator of the absorption process. While this study clearly showed a substantial effect on saquinavir bioavailability it is not suitable to elucidate the mechanism of the interaction. Surfactants like cremophor EL are classically used to increase solubilization or facilitate paracellular movement across the gastrointestinal mucosa [19]. In addition or alternatively to increase absorption for physicochemical reasons, Cremophor EL may inhibit PGP and/or CYP3A in the gastrointestinal tract. Cremophor EL has been shown to inhibit PGP in vitro [11, 12]. Thus, at least part of the increase in bioavailability can be explained by inhibiting intestinal PGP. This is in line with results in PGP deficient mice, which after oral administration of saquinavir showed 5-fold higher plasma concentrations as compared with wild type mice [20]. To the best of our knowledge nothing is known about the inhibiting potency of cremophor EL on CYP3A. However, Wandel et al. [21] have shown that the related cremophor RH40, which contains hydrogenated castor oil instead of nonhydrogenated castor oil, inhibited CYP3A in vitro with a 10-fold lower potency than PGP (IC50 values of 0.3% v/v and 0.03% v/v, respectively) rendering it possible, that part of our interaction might also be mediated by CYP3A.
Cremophor EL is included in several infusion solutions, e.g. Sandimmun® (cyclosporin) or Taxol® (paclitaxel). At the recommended dosage about 6.5 g day−1 cremophor EL is infused in the case of Sandimmun® and 20 g in the case of Taxol® [22]. The dose of the different cremophor products included in oral dosage forms is usually not disclosed by the manufacturers. To the best of our knowledge, they may contain up to several hundred milligrams per tablet and are unlikely to contain higher amounts because of the relatively large volume. At this dose, cremophor EL may increase the bioavailability of saquinavir to a clinically relevant extent as shown by AUC(0,∞). It is therefore possible that the amount of cremophor included in commercially available oral preparations will increase the bioavailability of concomitantly administered PGP-substrates when given simultaneously.
In conclusion, we have shown that a single oral administration of the pharmaceutic aid cremophor EL dose-dependently increases the bioavailability of single doses of oral saquinavir (Invirase®), a PGP and CYP3A substrate with extremely low bioavailability. Cremophor EL probably acts by inhibiting intestinal PGP. Because the increase in bioavailability is likely to occur with oral cremophor doses used in clinical settings, cremophor has to be considered as a pharmacologically active pharmaceutic aid.
Acknowledgments
This study was supported by BMBF grant #01EC9902 from the German Federal Ministry for Education and Research.
We are grateful to A. Deschlmayr, M. Longo and K. Riedel for their excellent technical assistance. We thank B. Tubach for organizing parts of the study, Dr J. Weiss for determination of the MDR-1 polymorphism and Y. Tayrouz for occasional blood collections. Pure standards of saquinavir (Ro 31–8959/008) and deuterated saquinavir (internal standard, Ro 31–8959/048) were generous gifts from Roche (Roche Products Limited, Hertfordshire, England).
References
- 1.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Ann Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 2.Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 4.Kupferschmidt HH, Fattinger KE, Ha HR, Follath F, Krähenbühl S. Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br J Clin Pharmacol. 1998;45:355–359. doi: 10.1046/j.1365-2125.1998.t01-1-00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noble S, Faulds D. Saquinavir. A review of its pharmacology and clinical potential in the management of HIV infection. Drugs. 1996;52:93–112. doi: 10.2165/00003495-199652010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44:190–194. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CG, Gottesman MM, Cardarelli CO, et al. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37:3594–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 8.Hsu A, Granneman GR, Cao G, et al. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob Agents Chemother. 1998;42:2784–2791. doi: 10.1128/aac.42.11.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wacher VJ, Silverman JA, Zhang Y, Benet LZ. Role of P-glycoprotein and cytochrome P450, 3A in limiting oral absorption of peptides and peptidomimetics. J Pharm Sci. 1998;87:1322–1330. doi: 10.1021/js980082d. [DOI] [PubMed] [Google Scholar]
- 10.Sadeque AJ, Wandel C, He H, Shah S, Wood AJ. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther. 2000;68:231–237. doi: 10.1067/mcp.2000.109156. [DOI] [PubMed] [Google Scholar]
- 11.Woodcock DM, Jefferson S, Linsenmeyer ME, et al. Reversal of the multidrug resistance phenotype with Cremophor EL, a common vehicle for water-insoluble vitamins and drugs. Cancer Res. 1990;50:4199–4203. [PubMed] [Google Scholar]
- 12.Cornaire G, Woodley JF, Saivin S, et al. Effect of polyoxyl 35 castor oil and polysorbate 80 on the intestinal absorption of digoxin in vitro. Arzneimittel-Forschung. 2000;50:576–579. doi: 10.1055/s-0031-1300252. [DOI] [PubMed] [Google Scholar]
- 13.Webster LK, Cosson EJ, Stokes KH, Millward MJ. Effect of the paclitaxel vehicle, Cremophor EL, on the pharmacokinetics of doxorubicin and doxorubicinol in mice. Br J Cancer. 1996;73:522–524. doi: 10.1038/bjc.1996.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burhenne J, Borwitzky H, Martin-Facklam M, Ding R, Mikus G, Haefeli WE. Determination of saquinavir in human plasma by LC/MS/MS. Eur J Clin Pharmacol. 2000;56:A25. [Google Scholar]
- 15.Shah VP, Midha KK, Dighe S, et al. Analytical methods validation. Bioavailability, bioequivalence and pharmacokinetic studies. Conference report. J Pharm Sci. 1992;81:309–312. [Google Scholar]
- 16.Nauck M, Stein U, von Karger S, März W, Wieland H. Rapid Detection of the C3435T polymorphism of multidrug resistance gene 1 using fluorogenic hybridization probes. Clin Chem. 2000;46:1996–1997. [PubMed] [Google Scholar]
- 17.Muirhead GJ, Shaw T, Williams PEO, Madigan MJ, Mitchell AM, Houston AC. Pharmacokinetics of the HIV-proteinase inhibitor, Ro 31–8959, after single and multiple oral doses in healthy volunteers. Br J Clin Pharmacol. 1992;34:170P–171P. [Google Scholar]
- 18. Martindale prepared by the editorial staff at the Royal Pharmaceutical Society of Great Britain. Micromedex, CD-ROM, 108, 2001.
- 19.Amidon GE, Higuchi WI, Ho NF. Theoretical and experimental studies of transport of micelle-solubilized solutes. J Pharm Sci. 1982;71:77–84. doi: 10.1002/jps.2600710120. [DOI] [PubMed] [Google Scholar]
- 20.Washington CB, Wiltshire HR, Man M, et al. The disposition of saquinavir in normal and P-glycoprotein deficient mice, rats, and in cultured cells. Drug Metab Dispos. 2000;28:1058–1062. [PubMed] [Google Scholar]
- 21.Wandel C, Kim RB, Wood AJJ, Stein CM. ‘Inactive’ ingedients may alter bioavailability of drugs through effects on p-glycoprotein (PGP) and CYP3A. Clin Pharmacol Ther. 2001;69:P71. [Google Scholar]
- 22.Medscape DrugInfo. http://promini.medscape.com/drugdb/search.asp, accessed on April 17, 2001.