Introduction
Advances in catheter-based technology have put many innovative techniques at the disposal of the clinical investigator. Intravascular ultrasound (IVUS), in particular, has facilitated the more detailed functional and morphological assessment of the coronary circulation, and will be the major focus of this review.
Selective intracoronary drug infusion may be desirable for a number of reasons. When examining in vivo vascular responses, systemic drug administration causes concomitant effects on organs, such as the brain and kidney, and influences neurohumoral reflexes through changes in systemic haemodynamics. Intracoronary infusions have the advantage of assessing the heart and coronary circulation in relative isolation without invoking systemic effects. This is particularly important for the assessment of cardiac and coronary function which is heavily dependent on changes in the systemic vasculature and haemodynamics. In addition, relatively high doses can be administered locally which may be important for the desired physiological or therapeutic effect and may be further facilitated by the use of local drug delivery systems. Finally, combining intracoronary drug administration with coronary sinus catheterization and sampling can further extend the assessment of the coronary circulation to include additional aspects of cardiac metabolism and function.
In addition to providing functional information, IVUS provides an invasive method of assessing arterial structure and morphology. Detailed and high-resolution examination of the proximal coronary vasculature is possible with precise and accurate definition of tissue planes [1, 2]. This permits the quantitative assessment of atherosclerotic burden and composition as well as enabling the assessment of systemic therapeutic interventions targeted at reducing atherosclerosis such as lipid-lowering therapy [3, 4].
Methods
Instrumentation
Coronary arterial cannulation
As for diagnostic coronary angiography, femoral, brachial or radial artery approaches can be used for catheter insertion and drug administration. For practical reasons, the femoral route is usually the preferred site and may be associated with a lower rate of vascular complications. Since infusion protocols are often prolonged, a heparin bolus (50 units kg−1) is given prior to instrumentation of the coronary arteries.
The choice of guide catheter, guide wire and method of drug delivery system will depend upon the coronary artery anatomy and the requirements of the study protocol. Appropriate equipment selection is essential to ensure technical success of the procedure as this will heavily influence factors such as the stability of catheter position and the selectivity of potential infusions. Impaction of the guide catheter in the coronary ostium due to superselection, or the use of large guide catheters, should clearly be avoided as this will impair anterograde coronary blood flow. Administration of drugs through guide catheters with side-holes is equally inappropriate. In contrast, smaller guide catheters, particularly in the presence of imaging IVUS catheters, can hinder drug administration and require the use of higher pressures to administer bolus injections or continuous infusions.
Coronary sinus cannulation
Cannulation of the coronary sinus is most easily achieved by instrumentation from subclavian or jugular vein approaches. However, these approaches do incur a small risk of pneumothorax and femoral approaches are often more desirable. Cannulation of the coronary sinus from the femoral vein can be performed by using a preformed specific 6F catheter (modified Simmons Torcon NB catheter, HNB6.0-NT-100-PW-2S-112393-BH) [5] (Figure 1) or modifying a 6F Judkins left 5 catheter [6]. To avoid atrial blood mixing, the catheter needs to be advanced, sometimes with the assistance of a guide wire, deep into the ostium and beyond the posterior interventricular vein. Adequate positioning of the catheter can be ensured by determining coronary sinus blood oxygen saturations (SaO2 35–50%) although the latter may rise with increases in coronary blood flow [7, 8].
Figure 1.
Cannulation of the coronary sinus from the femoral vein using a modified Simmon's catheter.
Sampling blood from the coronary sinus is appropriate only during the evaluation of the left ventricle and, particularly, during left anterior descending artery infusions [8]. Moreover, because of extensive anastamoses within the venous system of the heart, any obstruction to venous flow will cause shunting of blood to alternative drainage systems. Thus, care must taken to ensure that catheters do not impede flow in the coronary sinus since this may cause diversion of significant quantities of blood into the anterior cardiac or Thebesian veins.
Materials and drugs
Local intra-arterial drug infusion
The administration of drugs directly into the coronary arteries can be performed via the instrumenting catheter as either a bolus or continuous infusion. Bolus injections are associated with an instantaneous increase in blood flow velocity, due to the mechanical ejection of fluid down the artery, and a subsequent additional rise or fall in blood flow velocity attributable to drug action (Figure 2). Prolonged injections or large volume boluses have the potential to obscure the second phase response because of superimposition of mechanical and pharmacological flow effects as well as causing shear stress and potentially inducing flow-associated dilatation. Bolus injections should therefore be kept to a minimal volume and must be compared with control saline injections.
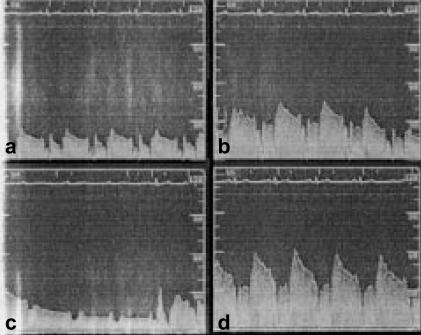
Figure 2.
Coronary flow velocity during bolus administration of acetylcholine (10−4 m) into the right coronary artery. (a). At baseline (time, −10 s) (b). During bolus injection (time, 0 s) (c). Ventricular standstill (time, 10 s), and (d). Hyperaemic response (time, 15 s).
Continuous infusions via the coronary guide catheter may not reliably permit selective intracoronary drug administration because of turbulence induced by blood ejection from the heart and the potential incomplete engagement with the coronary ostium. This may be a particular problem with concomitant instrumentation of the coronary vasculature, such as with IVUS catheters [9]. Therefore, continuous drug infusions should preferably be administered via a selective intracoronary catheter, such as a monorail infusion catheter [10]. Where a mechanical IVUS imaging catheter is being utilized, selective infusions can also be undertaken through the flush port of the imaging catheter [8, 11]. Consideration should also be given to the catheter dead space which can be considerable, especially with infusions via the guide catheter.
The functional assessment of endothelium-dependent vasomotion requires the administration of endothelium-dependent vasodilators, such as acetylcholine [12–15], bradykinin [15, 16] and substance P [7, 8, 14, 17], and endothelium-independent vasodilators, such as nitroprusside [8, 14, 18], papaverine [15, 18] and adenosine [19, 20]. Administration of acetylcholine and adenosine into the right coronary or dominant circumflex artery can result in atrioventricular block and transient ventricular standstill (Figure 2). If prolonged, this will confound the assessment of vasodilatation and flow responses, and continuous infusions of acetylcholine or adenosine into these arteries should be avoided.
When assessing the coronary vasomotor response by quantitative coronary angiography (QCA; see below), these concerns are further compounded by the necessity to aspirate the drug from the diagnostic or guide catheter before contrast injection [13, 14]. Moreover, many agents, especially endothelium-dependent vasodilators, have a near instantaneous onset and offset of action and performing QCA a minute after injection [16] is likely to result in misleading measurements. This is particularly the case where there is dissociation between the hyperaemic resistance vessel response and the subsequent epicardial flow associated vasodilatation.
Local intra-mural drug delivery to the coronary artery
Localized drug delivery to an isolated segment of the coronary artery can be achieved by several methods (Figure 3). The development of catheters that are able to isolate an arterial segment permits the brief passive exposure of the artery to very high concentrations of drug. In contrast, there are many angioplasty-based methods of actively instilling high local concentrations of drugs directly into the arterial wall. The former have the advantage of inducing less trauma to the vessel wall but at the expense of decreased efficiency of delivery. The local delivery of agents to the arterial wall may provide a novel approach in the prevention of angioplasty induced restenosis and thrombosis. Some of the balloon catheters described here are no longer commercially available but are discussed both for completeness and to illustrate the approaches that have been employed.
Figure 3.
Localized drug delivery systems: longitudinal (left) and cross-sectional (right) views. Dashed lines—arterial wall.
Passive
An arterial segment can be isolated using a catheter with a proximal and distal occluding balloon (Wolinsky™, USCI) and the intervening chamber can then be perfused with the agent under investigation [21, 22]. Alternatively a Dispatch™ catheter (SCIMED Life Systems) [23–26] may be used where a helical coil surrounding a polyurethane sheath is inflated within the arterial lumen and allows anterograde flow down the central lumen of the sheath. At the same time, this coil isolates a compartment between the sheath and the arterial wall which permits protracted drug administration for more than 1 h.
Active
Suffusion of the coronary artery wall can be achieved using channelled (Channel™ balloon, SCIMED/Boston Scientific; Remedy catheter, SCIMED) [27, 28], gel coated (Hydro-Plus™, Boston Scientific) [29] or porous balloons (ACS, Santa Clara, CA) [30, 31]. The infusion times are short (20–60 s) and balloon inflation pressures low (2–6 atmospheres). Efficacy of drug delivery to the arterial wall can be enhanced by the use of iontophoretic (CorTrak Medical) [32, 33] or infiltrator catheters (Infiltrator Angioplasty Balloon Catheter, Interventional Technologies) [34, 35]. The latter uses microinjection from tri-axially arranged arrays of fine needles placed between the polyurethane pads of the angioplasty balloon catheter. Coated stents [36–38] can also provide an alternative method of delivering high local concentrations over a prolonged time period due to slow drug elution. Moreover, a combined approach is possible with the delivery of drug saturated microspheres through a porous balloon catheter [39, 40].
Procedures
Functional assessment of resistance arteries
There are several invasive methods of assessing coronary resistance vessels and blood flow. The angiogram-derived corrected TIMI frame count [41] has been shown to have clinical utility especially when assessing reperfusion after acute coronary thrombosis [42]. Although it is quantitative and can detect the presence of microvascular dysfunction [43], the corrected TIMI frame count does not directly measure absolute coronary blood flow. Other techniques are under development such as the use of the radiofrequency signal decorrelation rate from IVUS imaging catheters [44, 45]. However, the main invasive methods of assessing coronary resistance vessel function and blood flow responses are reverse thermodilution catheters and Doppler flow wires.
Reverse thermodilution catheter
Before the development of methods to measure directly coronary blood flow, the indirect approach of determining coronary sinus blood flow using a reverse thermodilution catheter was utilized [46]. Computerized integration of temporal changes in temperature permit assessments of blood flow and, with modifications, can provide continuous on-line measurements of coronary sinus flow [47]. Although not providing a measure of total or absolute coronary blood flow, it can provide a useful indicator of changes in coronary blood flow [48]. However, incomplete mixing of the blood may lead to errors [49] and this technique is not widely used for the precise determination of coronary blood flow.
Intravascular Doppler
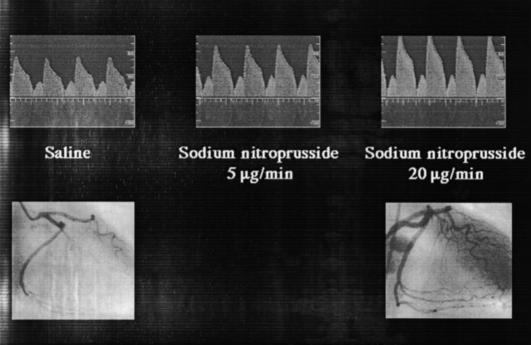
Intracoronary Doppler probes measure coronary blood flow velocity and can describe coronary resistance vessel function either as coronary flow reserve (see below and Appendix) or, when the luminal cross-sectional area is known, absolute coronary blood flow (Figure 4). Initial Doppler probes were mounted on the tip of 3F coronary catheters [50] that limited their widespread use. However, Doppler wires (Flowire™, Cardiometrics) are now available that have the same characteristics as conventional interventional guide wires (diameter 0.014 inch), but have a small piezoelectric cell (12.5 MHz) mounted on the tip which permits assessment of coronary flow velocity (Figure 4). This facilitates direct instrumentation of the proximal and distal coronary arteries. Complications are rare but do include bradycardia (right coronary artery; 1%), arterial spasm (1%) and the potential to cause dissection [51]. The Doppler signal is heavily dependent on the alignment of the wire (sample volume 10–15° to the longitudinal axis and 5 mm from the tip) and a stable coaxial position is essential to record high-fidelity measurements. Positional instability may be a particular problem with the steerable J-tipped wires that can more easily abut the arterial wall. Doppler wires with a wider arc sample volume are currently under development.
Figure 4.
Doppler wire measurement of coronary flow velocity (upper panel) and coronary angiograms (lower panel) during saline and sodium nitroprusside infusions.
Morphometric assessment of epicardial arteries
Quantitative coronary angiography
Because of the significant variability in the interpretation and analysis of coronary angiography [52, 53], computerized quantification of the coronary anatomy is essential (Figure 5). Quantitative coronary angiography uses contrast injection of the coronary arteries to outline the lumen and, using automated computerized edge detection algorithms, determines coronary arterial luminal diameter and indirectly estimates plaque burden. Care must be taken to visualize the artery without foreshortening or intrusion of overlapping branches. Since coronary stenoses are often eccentric and ellipsoidal, QCA should be performed in more than one orthogonal view. Furthermore, an assumption is made that the reference segments, against which the lesion is compared, are normal and this often contributes to the underestimation of lesion severity. Quantitative coronary angiography was used as one of the first methods of functionally assessing the conduit epicardial coronary arteries [7, 12], however, it is now more routinely used for assessing the minimal luminal area of coronary stenoses during coronary intervention. In combination with Doppler flow velocity measurements, QCA can facilitate the estimation of coronary blood flow by deriving the luminal cross-sectional area [15]. Derivation of the cross-sectional area is usually undertaken by assuming circular geometry, although when perpendicular views are obtained, ellipsoidal geometry may be more appropriate and provide a more accurate assessment (see Appendix).
Figure 5.
Quantitative coronary angiography: assessment of luminal diameter and ‘plaque load’.
Intravascular ultrasound
Ultrasound imaging catheters operate at 20–40 MHz and have either a solid state phased array (Five-64™, Endosonics) or a single rotating transducer (Ultracross™, Boston Scientific): the latter tend to produce superior images although solid state technology has improved. The probes are introduced into the coronary artery in the same manner as an angioplasty balloon under systemic heparinization (50 IU kg−1). Intravascular ultrasound catheters are very safe [54] but can induce coronary spasm and intracoronary nitrate (glyceryl trinitrate 100–200 µg) should be given before imaging. Some imaging catheters allow the guide wire to be retracted which thereby removes the artefactual acoustic shadow of the guide wire. An automated pull back device (0.25–1.0 mm s−1) is essential to assess systematically the coronary arteries particularly where volumetric measurements and three-dimensional reconstructions are being made. Changes in arterial dimensions occur during the cardiac cycle and ECG-gating is necessary, particularly for determination of arterial compliance and distensibility [55–57].
The orientation of the ultrasound image is not predetermined and therefore landmarks such as pericardial reflections, cardiac veins or side branches should be used as reference points. The centre of the image represents the catheter blank and if a guide wire is present, a small acoustic arc will be seen (Figure 6). The normal coronary arterial image gives the so-called ‘three-layered’ appearance representing the intima, the echolucent media and the adventitia. This three-layered appearance is not seen in young subjects because the initima is very thin (∼170 µm) and below the resolution of current devices. In addition, the inner border of the media (the internal elastic lamina) may be difficult to identify and the more echogenic interface between the adventitia and external elastic lamina is used to describe the plaque volume [58, 59].
Figure 6.
Intravascular ultrasound: image interpretation in a normal coronary artery.
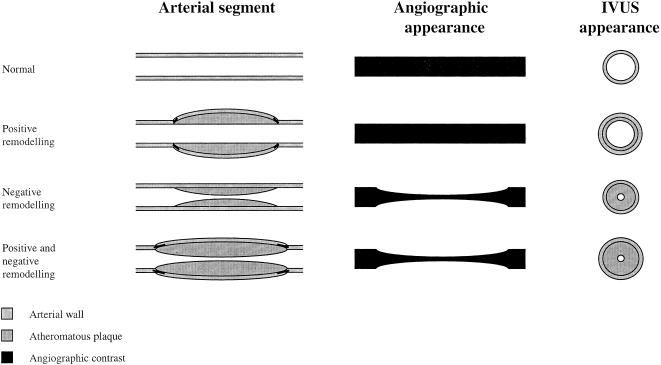
It is recognized that angiographically normal coronary arteries or arterial segments often contain a significant plaque load [55, 60–62] (Figure 7). This is due to adaptive or ‘Glagovian’ remodelling [63] where the artery expands to accommodate a large plaque burden [64]. Ultrasound imaging can also describe the plaque composition from soft fibrofatty plaques, through fibromuscular plaques to calcified hard plaques [55, 59, 65–68] and this may be enhanced by spectral analysis of the radiofrequency signal [69]. Three-dimensional reconstruction can be performed using computerized systems that use digitized ECG-gated images (Figure 8). Using a contour detection algorithm, plaque volume (volume of tissue lying between the intima–lumen interface and the external elastic lamina) can be automatically quantified [1], and in some cases, on-line [70]. This can provide an accurate index of plaque load within the coronary arteries.
Figure 7.
Glagovian arterial remodelling associated with atherosclerosis: angiographic and intravascular ultrasound (IVUS) views.
Figure 8.
Intravascular ultrasound: cross-sectional views (top left; arrow shows plaque) and three dimensional reconstruction (bottom right).
Variations in methodology
Functional assessment
Quantiative coronary angiography, intravascular ultrasound and Doppler
Quantitative coronary angiography tends to underestimate the luminal area [71, 72] and functional severity of coronary stenoses [73] as well as necessitating the injection of vasoactive contrast agents that may cause alterations in coronary blood flow and confound measurements [74]. Moreover, drug infusion may need to be interrupted and the catheter aspirated before giving the contrast injection [14]. Although these limitations are not applicable to the combined use of IVUS and Doppler, the signal from the Doppler wire can cause some interference with the IVUS catheter and produces discrete and fleeting radial striae to appear on imaging: the so-called ‘star burst’ effect (Figure 9). This does not, however, cause significant image loss and measurements of coronary artery cross-sectional area are unaffected. The IVUS imaging catheter (cross-sectional area ∼0.8 mm2) can also cause significant obstruction to flow particularly in the presence of luminal stenoses of ≥70% and therefore heavily diseased arterial segments are not suited to this approach. Moreover, unlike QCA, IVUS provides a single cross-sectional image of the artery at a given time point, and although three-dimensional reconstructions of the artery can be performed, it does not permit an instantaneous assessment of the entire arterial tree. However, the combined use of IVUS and Doppler wire does facilitate the functional assessment of both conduit and resistance vessel vascular and endothelial function [8, 57, 75–78].
Figure 9.
Intravascular ultrasound: image distortion and interference.
Morphometric assessment
Quantiative coronary angiography and intravascular ultrasound
Quantitative coronary angiography has been widely used to determine the progression and regression of coronary plaque load following intervention [79, 80]. However, as already indicated, QCA has some inherent limitations and inaccuracies that permit only crude estimates of plaque load [81]. These inaccuracies occur because QCA only assesses the arterial lumen and the arterial wall is extrapolated from a reference segment and does not take account of ‘Glagovian’ remodelling [63] (Figure 7). Intravascular ultrasound is now the modality of choice for the precise determination of proximal atheromatous plaque volume. The latter also facilitates the accurate assessment of restenosis following percutaneous coronary intervention [38] although functional assessment using Doppler and pressure wires may also be of benefit.
Reproducibility
Doppler wire
Doppler wire measurements of coronary blood flow and velocity have been validated both in vitro and in vivo [82] and can provide an accurate assessment of coronary flow reserve [20, 83–86]. However, whilst short-term reproducibility is good [87], coronary flow velocity measurements have only modest long-term reproducibility because of its heavy dependence on arterial luminal area, heart rate and aortic pressure [88]. Although correction for these factors can increase the reproducibility [88], one further approach to increase the sensitivity and reproducibility has been to construct aortic pressure and coronary flow velocity plots during hyperaemic flow [89].
Quantiative coronary angiography, intravascular ultrasound and plaque volume
As already identified, QCA has many potential sources of error [81, 90] which may limit its use. Some of these drawbacks may be overcome by using improved and standardized methodologies [81, 90] that incorporate the assessment of QCA parameters by a central reference core laboratory. Such assessments are made off line in a standardized, blinded and prespecified manner [91]. This does not, however, remove significant margins of error that hamper the reproducibility of QCA [81, 90, 92] in the measurement of both minimal luminal diameter and plaque volume.
Intravascular ultrasound provides an accurate assessment of intracoronary plaque volume [1, 2, 58, 59, 70] that is very reproducible [2, 70, 93–95]. The determination of coronary artery cross-sectional area and plaque volume by IVUS is enhanced by ECG-gating of the images [2, 70]. Moreover, three-dimensional reconstructions and computerized edge detection algorithms facilitate measurements of plaque volume that differ little from those obtained by manual contour tracing and analysis [2]. Image analysis and plaque volume determinations can be hampered by large areas of heavy plaque calcification that cause acoustic shadows and mask the underlying vessel structure. The use of mechanical transducers may also cause image disruption called NURD, Non-Uniform Rotational Distortion, especially in tortuous vessels (Figure 9). Moreover, marked vessel curvature can also cause inaccuracies in volumetric measurements due to the effect of the inner curve being expanded and the outer curve compressed. Three-dimensional reconstructions from an automated pullback of an IVUS examination also assume a linear coaxial alignment.
Study of diseases and drugs
Coronary flow reserve
The measurement of coronary flow reserve has two main applications: assessment of the functional severity of coronary stenoses and determination of the function of the coronary microvasculature. Although the former has been largely superceded by the use of pressure wires and the measurement of fractional flow reserve, coronary flow reserve continues to be utilized for the assessment of the coronary microvasculature.
It has been appreciated for some time that, even in the absence of significant functional stenoses of the coronary arteries, the coronary flow reserve may be impaired due to structural abnormalities of the microvasculature, such as in patients with hypertension [96], diabetes mellitus [97], syndrome X [98], hyperlipidaemia [99] and aortic stenosis with left ventricular hypertrophy [100]. These changes are likely to represent a combination of factors that include structural abnormalities of the small vessels as well as physical extravascular forces, such as elevation of the ventricular end diastolic pressure [101].
Coronary flow reserve is measured in response to endothelium-independent microvascular vasodilators, such as adenosine, papaverine and sodium nitroprusside, although adenosine is the favoured coronary vasodilator since it produces near maximal reduction in coronary vascular resistance. However, comparisons of such responses with those obtained with endothelium-dependent vasodilators can provide a method of assessing endothelial function of the coronary resistance vessels.
Endothelial function
The determination of coronary blood flow responses to infusion of selective antagonists and inhibitors permits the assessment of the physiological and pathophysiological role of endogenous mediators, such as nitric oxide [14, 102] and bradykinin [103]. However, we will focus here on the assessment of endothelial function.
Epicardial coronary arteries
Initial methods of assessing coronary endothelial function have determined the epicardial conduit vessel responses to endothelium-dependent vasodilators, such as acetylcholine [12, 13], substance P [7, 8] or bradykinin [15, 16]. Acetylcholine causes paradoxical vasoconstriction in arterial segments affected by coronary atheroma [12] or, in the absence of overt coronary atheroma, in patients with risk factors for atheroma formation [104]. These abnormal vasomotor responses may be reversible following suitable pharmacological intervention [105]. In addition, hyperaemic endothelium-dependent flow-associated vasodilatation of the epicardial coronary arteries is also impaired in patients with atherosclerosis [18] and its associated risk factors [106]. However, the mechanical and pharmacological stimulation of the endothelium may result in differing degrees of apparent endothelial dysfunction according to the nature of the insult [10, 107, 108].
The TREND study [13] was one of the first studies to investigate the influence of intervention on coronary endothelial function. It was a multicentre double-blind randomized controlled trial that examined the effect of the angiotensin converting enzyme inhibitor, quinapril (40 mg daily), on acetylcholine induced changes in epicardial coronary diameter. A hundred and five patients with coronary artery disease were assessed at baseline and following 6 months of quinapril or placebo therapy. The investigators found that quinapril therapy was associated with a significant improvement in endothelial function as demonstrated by the abolition of acetylcholine induced vasoconstriction.
Coronary resistance arteries
To date, coronary resistive vessel endothelial function has been assessed by determining responses in coronary blood flow, using QCA and Doppler wire, to infusions of endothelium-dependent vasodilators. Such an approach also permits the simultaneous assessment of epicardial coronary endothelial function through the measurement of epicardial coronary artery diameter.
This methodology has been used successfully to demonstrate the improvement of endothelial dysfunction in response to acute drug administration, such as with oestrogen [109], l-arginine [110, 111], vitamin C [106] and reduced glutathione [112]. Long-term studies are lacking but in a small poorly controlled study, 6 months of lipid-lowering therapy was associated with improvements in coronary microvascular endothelial function [113].
Release and extraction across the coronary circulation
Coronary blood flow measurements combined with coronary sinus and arterial blood sampling permit the assessment of the release or extraction of factors across the coronary circulation and left ventricle (see Appendix). Measures of both basal and stimulated, release and extraction can be undertaken in this manner [8, 15, 48, 114], and can also be used to assess the metabolic response of the myocardium to various interventions [115, 116].
This technique has been used to assess other aspects of endothelial function and is exemplified by studies that have measured the release of endothelium-derived tissue plasminogen activator in response to substance P [8] and bradykinin [15] infusions. This work has established a link between impaired tissue plasminogen activator release and atherosclerosis and some of its risk factors [8]. Moreover, angiotensin converting enzyme inhibition is associated with a marked potentiation of bradykinin induced tissue plasminogen activator release in the coronary circulation [15].
Atherogenesis and plaque regression
The assessment of atheromatous plaque progression and regression has been applied to studies that have investigated the effects of lipid lowering therapy and transplant vasculopathy. The former has been under investigation for over 10 years, predated the availability of IVUS and has relied heavily on QCA to provide an index of coronary atheromatous load. In contrast, transplant vasculopathy is often angiographically silent in the early phases of development and necessitates the use of IVUS to delineate the consequences and severity of the disease process.
Lipid lowering therapy and plaque regression
The FATS [79] and Lifestyle Heart [80] trials were amongst the first trials to show that aggressive lipid lowering therapy and lifestyle modifications could halt the progression, and even induce regression, of coronary atherosclerosis. There have been several subsequent studies to demonstrate that the progression of coronary atherosclerosis can be reduced in a broad spectrum of patients using lipid-lowering therapy [117, 118]. These studies have relied on the assessment of coronary atheroma using QCA and therefore provide only a crude estimate of plaque load.
Plaque regression can be accurately defined by IVUS. In the first long-term study, Takagi and colleagues were able to demonstrate plaque regression with pravastatin over a 3 year treatment period [3]. Plaque composition is also important and IVUS can detect differences in plaque density. Coronary plaques prone to rupture and thrombosis tend to be large eccentric lesions with echolucent zones indicative of a lipid-rich core [119, 120]. Schartl and colleagues [121] compared the effects of 12 months aggressive lipid lowering therapy with standard lipid lowering therapy in 131 patients undergoing a percutaneous coronary intervention. Whilst no effect was seen on plaque volume, there was a significant change in the apparent composition of the atherosclerotic plaque. Aggressive LDL cholesterol reduction was associated with an increase echogenicity of the atherosclerotic plaque indicating a reduction in lipid content and greater plaque stability.
Transplant vasculopathy
The development of accelerated atherosclerosis in the coronary arteries following cardiac transplantation, so-called transplant vasculopathy, is a major cause of long-term morbidity and mortality. Angiographically evident coronary artery disease occurs in nearly a half of transplant recipients within 5 years [122]. However, using IVUS, heterogenous intimal thickening can be detected in the majority of patients within the first year of transplantation [77, 123, 124]. Endothelial vasomotor responses are heterogenous and, unlike those in patients with coronary artery disease [125], do not appear to be correlate with the degree of intimal thickening [77, 108, 126]. This is likely to be due to the differing aetiological and pathogenetic process involved in the formation of coronary atherosclerosis and immunologically mediated transplant vasculopathy. Acute l-arginine administration does appear to improve the endothelial dysfunction associated with transplant vasculopathy although this is particularly evident in vessels with normal wall morphology [111].
Restenosis
Because of the problems of restenosis following angioplasty and intracoronary stenting, there has been a great deal of interest in the use of IVUS to quantify restenotic plaque volume and neointimal hyperplasia [127]. This has also facilitated the assessment of strategies to prevent neointimal hyperplasia and restenosis including systemic therapeutic interventions [128] as well as local drug and gene transfer delivery systems [129, 130].
The ERASER study [131] was a double-blind randomized controlled trial of the effect of antiplatelet therapy with abciximab on the occurrence of in-stent restenosis at 6 months. IVUS examination identified no significant difference in plaque volume within the stented segments, suggesting that procedural use of abciximab did not prevent the subsequent development of in-stent restenosis. There are several ongoing trials, such as the Reversal of Atherosclerosis with Lipitor (REVERSAL) [4] and NOrvasc for Regression of Manifest Atherosclerotic Lesions (NORMALISE) trials [132], that are comparing the impact of various cardiovascular therapies on IVUS determined plaque volume.
One preliminary study, ITALICS [128], has attempted to address the issue of local antimitotic gene therapy as a strategy of preventing restenosis. In this double-blind randomized controlled trial of 90 patients who received intracoronary stent implantation, a delivery catheter was positioned across the stent and 10 mg of antisense oligoDNA or placebo vehicle was locally administered. The antisense DNA was directed against the c-myc proto-oncogene which is a transcription factor for cyclin D, a key mediator of cellular mitosis. The investigations reported no significant differences in restenosis plaque volume. It is likely, however, that local drug delivery systems will be superseded by drug-eluting stents that have shown early promise [38].
Conclusion and future directions
The clinical researcher now has a range of invasive and noninvasive techniques with which to assess and investigate the functional and haemodynamic responses of the coronary circulation. The invasive assessment of coronary vasomotion and blood flow has progressed from the imprecise measures of coronary sinus blood flow to the more direct assessment of blood flow using IVUS and Doppler.
These ultrasound techniques are likely to be increasingly utilized, especially in the expanding field of applied molecular biology, and where novel antiatherogenic interventions are being evaluated. Future trials will provide more accurate and detailed information in relation to plaque remodelling and regression, and perhaps give insights into how the composition of the plaques may be modified by therapeutic interventions.
The determination of plaque characteristics, and in particular areas of plaque disruption and inflammation, will be critical in understanding the pathogenesis and resolution of acute coronary syndromes, as well as potentially guiding therapeutic interventions. Many in vivo techniques are being developed to identify vulnerable or inflamed plaques as well as providing detailed information about plaque composition [133]. However, future developments in IVUS may be able to provide more detailed information on the state of vascular structure and function. Such approaches may include the use of high-resolution IVUS in combination with antibody labelled echocontrast microspheres [134]. Antibody binding to selected antigens would have the potential to provide information regarding the surface expression of specific vascular cell proteins and receptors in vivo. This would provide the exciting prospect of relating the structural characteristics of the endothelium and vascular wall to its in vivo function.
Despite the use of higher frequency IVUS transducers, the spatial resolution and physical properties of ultrasound imaging have their limitations. It is likely that advances in magnetic resonance imaging will complement, or even displace, the use of IVUS. Both noninvasive [135] and invasive [136] magnetic resonance imaging approaches have the potential to improve dramatically tissue resolution and characterization, and it may become the imaging modality of choice for the assessment of vascular structure and function.
Acknowledgments
Dr David Newby and Professor Keith Fox are currently conducting research in this area supported by grants from the British Heart Foundation and the Scottish Office. We are grateful to Dr Neal Uren for his assistance in the preparation of this review.
Appendix
Mathematical calculations
Coronary blood flow
Reverse thermodilution catheter
where V = volume (ml) of indicator infused over time, t (min); Tb = temperature of blood; Ti = temperature of indicator; Tm = temperature of the mixture of blood and indicator; k = a constant that is dependent on the density and specific heat capacity of the indicator and blood [46]. The density and specific heat capacity of blood is constant for haematocrits between 0.30 and 0.60 and therefore varies mainly depending on the indicator used: for 0.9% saline, k = 1.19 and 5% dextrose, k = 1.17.
Quantiative coronary angiography and Doppler
Assuming circular geometry:
where d = coronary arterial diameter (mm) and APV = average peak velocity (cm/s). With a parabolic velocity profile, mean blood flow velocity equates to half the APV [82].
Assuming elliptical geometry [18]:
 |
where d1 and d2 = coronary arterial diameters (mm) in two perpendicular views.
Intravascular ultrasound and Doppler
Assuming a parabolic velocity profile [82]:
where CSA = coronary arterial cross-sectional area (mm2) and APV = average peak velocity (cm/s).
Estimated net release/extraction from the coronary circulation
Assuming that the coronary sinus drains exclusively from the left anterior descending coronary artery territory [8, 15]:
 |
where CBF = left anterior descending coronary artery blood flow (ml min−1), CSConc = plasma coronary sinus concentration (AU/ml), ArtConc = plasma arterial/aortic concentration (AU/ml), and Hct = haematocrit.
References
- 1.von Birgelen C, van der Lugt A, Nicosia A, et al. Computerised assessment of coronary lumen and atherosclerotic plaque dimensions in three-dimensional intravascular ultrasound correlated with histomorphometry. Am J Cardiol. 1996;78:1202–1209. doi: 10.1016/s0002-9149(96)00596-6. [DOI] [PubMed] [Google Scholar]
- 2.von Birgelen C, de Vrey EA, Mintz GS, et al. ECG-gated three-dimensional intravascular ultrasound feasibility and reproducibility of the automated analysis of coronary artery lumen and atherosclerotic plaque dimensions in humans. Circulation. 1997;96:2944–2952. doi: 10.1161/01.cir.96.9.2944. [DOI] [PubMed] [Google Scholar]
- 3.Takagi T, Yoshida K, Akasaka T, Hozumi T, Morioka S, Yoshikawa J. Intravascular ultrasound analysis of reduction in progression of coronary narrowing by treatment with pravastatin. Am J Cardiol. 1997;79:1673–1676. doi: 10.1016/s0002-9149(97)00221-x. [DOI] [PubMed] [Google Scholar]
- 4.Nissen SE. Rationale for a postintervention continuum of care: insights from intravascular ultrasound. Am J Cardiol. 2000;86(Suppl):12H–17H. doi: 10.1016/s0002-9149(00)01095-x. [DOI] [PubMed] [Google Scholar]
- 5.Winters KJ, Lasala JM, Eisenberg PR, Smith SC, Sewall DJ, Shelton ME. Modified heparin-bonded catheter for cannulation of the coronary sinus from the femoral vein. Cathet Cardiovasc Diagn. 1996;39:433–437. doi: 10.1002/(SICI)1097-0304(199612)39:4<433::AID-CCD25>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Katritsis D, Webb-Peploe MW. Cannulation of the coronary sinus via the femoral vein – a new technique. Clin Cardiol. 1997;20:446–448. doi: 10.1002/clc.4960200508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crossman DC, Larkin SW, Fuller RW, Davies GJ, Maseri A. Substance P dilates epicardial coronary arteries and increases coronary blood flow in humans. Circulation. 1989;80:475–484. doi: 10.1161/01.cir.80.3.475. [DOI] [PubMed] [Google Scholar]
- 8.Newby DE, MacLeod AL, Uren NG, et al. Impaired coronary tissue plasminigen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation. 2001;103:1936–1941. doi: 10.1161/01.cir.103.15.1936. [DOI] [PubMed] [Google Scholar]
- 9.Newby DE. Intracoronary infusions and the assessment of coronary blood flow in clinical studies. Heart. 2000;84:118–120. doi: 10.1136/heart.84.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton JL, Davison CA, Kuhner PA, Torossov MT, Strogatz DS, Carr AA. Heterogenous vasomotor responses of coronary conduit and resistance vessels in hypertension. J Am Coll Cardiol. 1998;31:374–382. doi: 10.1016/s0735-1097(97)00505-6. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzacher SP, Uren NG, Ward MR, et al. Determinants of coronary remodeling in transplant coronary disease. Circulation. 2000;101:1384–1389. doi: 10.1161/01.cir.101.12.1384. [DOI] [PubMed] [Google Scholar]
- 12.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic human coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 13.Mancini GBJ, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial dysfunction in patients with coronary heart disease. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 14.Quyyumi AA, Mulcahy D, Andrews NP, Husain S, Panza JA, Cannon RO. Coronary vascular nitric oxide activity in hypertension and hypercholesterolaemia. Circulation. 1997;95:104–110. doi: 10.1161/01.cir.95.1.104. [DOI] [PubMed] [Google Scholar]
- 15.Minai K, Matsumoto T, Horie H, et al. Bradykinin stimulates the release of tissue plasminogen activator in human coronary circulation: effects of angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2001;37:1565–1570. doi: 10.1016/s0735-1097(01)01202-5. [DOI] [PubMed] [Google Scholar]
- 16.Kuga T, Egashira K, Mohri M, et al. Bradykinin-induced vasodilatation is impaired at the atherosclerotic site but is preserved at the spastic site of human coronary arteries in vivo. Circulation. 1995;92:183–189. doi: 10.1161/01.cir.92.2.183. [DOI] [PubMed] [Google Scholar]
- 17.Okumura K, Yasue H, Ishizaka H, Ogawa H, Fujii H, Yoshimura M. Endothelium-dependent dilator response to substance P in patients with coronary spastic angina. J Am Coll Cardiol. 1992;20:838–844. doi: 10.1016/0735-1097(92)90181-l. [DOI] [PubMed] [Google Scholar]
- 18.Nabel EG, Selwyn AP, Ganz P. Large coronary arteries in humans are responsive to changing blood flow: an endothelium-dependent mechanism that fails in patients with atherosclerosis. J Am Coll Cardiol. 1990;16:349–356. doi: 10.1016/0735-1097(90)90584-c. [DOI] [PubMed] [Google Scholar]
- 19.Cox DA, Vita JA, Treasure CB, et al. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 20.Heller LI, Cates C, Popma J, et al. Intracoronary Doppler assessment of moderate coronary artery disease. Circulation. 1997;96:484–490. doi: 10.1161/01.cir.96.2.484. [DOI] [PubMed] [Google Scholar]
- 21.Nabel EG, Plautz G, Boyce FM, Stanley JC, Nabel GJ. Recombinant gene expression in vivo within endothelial cells of the artery wall. Science. 1989;244:1342–1344. doi: 10.1126/science.2499928. [DOI] [PubMed] [Google Scholar]
- 22.Nabel EG, Plautz G, Nabel GJ. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990;249:1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- 23.Camenzind E, Kint PP, Di Mario C, et al. Intracoronary heparin delivery in humans. Circulation. 1995;92:2463–2472. doi: 10.1161/01.cir.92.9.2463. [DOI] [PubMed] [Google Scholar]
- 24.Baumbach A, Oberhoff M, Bohnet A, et al. Efficacy of low-molecular-weight heparin delivery with the Dispatch catheter following balloon angioplasty in the rabbit iliac artery. Cathet Cardiovasc Diagn. 1997;41:303–307. doi: 10.1002/(sici)1097-0304(199707)41:3<303::aid-ccd11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Glazier JJ, Kiernan FJ, Bauer HH, et al. Treatment of thrombotic saphenous vein bypass grafts using local urokinase infusion therapy with the Dispatch catheter. Cathet Cardiovasc Diagn. 1997;41:261–267. doi: 10.1002/(sici)1097-0304(199707)41:3<261::aid-ccd6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Fram DB, Mitchel JF, Azrin MA, Chow MSS, Waters DD, McKay RG. Local delivery of heparin to balloon angioplasty sites with a new angiotherapy catheter. Cathet Cardiovasc Diagn. 1997;41:275–286. doi: 10.1002/(sici)1097-0304(199707)41:3<275::aid-ccd8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell JF, Barry JJ, Bow LM, Alberghini TA, Abbas SA, McKay RG. Local urokinase delivery with the channel balloon. Cathet Cardiovasc Diagn. 1997;41:254–260. doi: 10.1002/(sici)1097-0304(199707)41:3<254::aid-ccd5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Atalar E, Li D, et al. Magnetic resonance imaging permits in vivo monitoring of catheter-based vascular gene delivery. Circulation. 2001;104:1588–1590. doi: 10.1161/hc3901.097505. [DOI] [PubMed] [Google Scholar]
- 29.Azrin MA, Mitchel JF, Bow LM, et al. Local delivery of C-myb antisense oligonucleotides during balloon angioplasty. Cathet Cardiovasc Diagn. 1997;41:232–240. doi: 10.1002/(sici)1097-0304(199707)41:3<232::aid-ccd2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Herdeg C, Oberhoff M, Baumbach A, et al. Local drug delivery with porous balloons in the rabbit. Cathet Cardiovasc Diagn. 1997;41:308–314. doi: 10.1002/(sici)1097-0304(199707)41:3<308::aid-ccd12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Robinson KA, Chronos NAF, Schieffer E, et al. Endoluminal local delivery of PCN/cdc2 antisense oligonucleotides by porous balloon catheter does not affect neointima formation or vessel size in the pig coronary artery model of postangioplasty restenosis. Cathet Cardiovasc Diagn. 1997;41:348–353. doi: 10.1002/(sici)1097-0304(199707)41:3<348::aid-ccd17>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Robinson KA, Chronos NAF, Schieffer E, et al. Pharmacokinetics and tissue localisation of antisense oligonucleotides in balloon-injured pig coronary arteries after local delivery with an iontophoretic balloon catheter. Cathet Cardiovasc Diagn. 1997;41:354–359. doi: 10.1002/(sici)1097-0304(199707)41:3<354::aid-ccd18>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell JF, Azrin MA, Fram DB, Bow LM, McKay RG. Localised delivery of heparin to angioplasty sites with iontophoresis. Cathet Cardiovasc Diagn. 1997;41:315–323. doi: 10.1002/(sici)1097-0304(199707)41:3<315::aid-ccd13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 34.Barath P, Popov A, Dillehay GL, Matos G, McKiernan T. Infiltrator angioplasty balloon catheter. Cathet Cardiovasc Diagn. 1997;41:333–341. doi: 10.1002/(sici)1097-0304(199707)41:3<333::aid-ccd15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Pavlides GS, Barath P, Maginas A, Vasilikos V, Cokkinos DV, O'Neill WW. Intramural drug delivery by direct injection within the arterial wall. Cathet Cardiovasc Diagn. 1997;41:287–292. doi: 10.1002/(sici)1097-0304(199707)41:3<287::aid-ccd9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Lambert TL, Dev V, Rechavia E, et al. Localised arterial wall drug delivery from a polymer coated removable metallic stent: kinetics, distribution and bioactivity of forskolin. Circulation. 1994;90:1003–1011. doi: 10.1161/01.cir.90.2.1003. [DOI] [PubMed] [Google Scholar]
- 37.Serruys PW, Emannuelsson H, van der Giessen W, et al. Heparin-coated Palmaz-Schatz stents in human coronary arteries. Early outcome of the Benestent-II Pilot Study. Circulation. 1996;93:412–422. doi: 10.1161/01.cir.93.3.412. [DOI] [PubMed] [Google Scholar]
- 38.Honda Y, Grube E, de La Fuente LM, Yock PG, Stertzer SH, Fitzgerald PJ. Novel drug-delivery stent: intravascular ultrasound observations from the first human experience with the QP2-eluting polymer stent system. Circulation. 2001;104:380–383. doi: 10.1161/hc2901.094149. [DOI] [PubMed] [Google Scholar]
- 39.Guzman LA, Labhasetwar V, Song C, et al. Local intraluminal infusion of biodegradeable polymeric nanoparticles: a novel approach for prolonged drug delivery after balloon angioplasty. Circulation. 1996;94:1441–1448. doi: 10.1161/01.cir.94.6.1441. [DOI] [PubMed] [Google Scholar]
- 40.Dev V, Eigler N, Fishbein MC, et al. Sustained local drug delivery to the arterial wall via biodegradable microspheres. Cathet Cardiovasc Diagn. 1997;41:324–332. doi: 10.1002/(sici)1097-0304(199707)41:3<324::aid-ccd14>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.Gibson CM, Murphy S, Menown IBA, et al. Determinants of coronary blood flow after thrombolytic administration. J Am Coll Cardiol. 1999;34:1403–1412. doi: 10.1016/s0735-1097(99)00397-6. [DOI] [PubMed] [Google Scholar]
- 42.Gibson CM, Cannon CP, Murphy SA, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–130. doi: 10.1161/01.cir.101.2.125. [DOI] [PubMed] [Google Scholar]
- 43.Goel PK, Gupta SK, Agarwal A, Kapoor A. Slow coronary flow: a distinct angiographic subgroup in syndrome X. Angiology. 2001;52:507–514. doi: 10.1177/000331970105200801. [DOI] [PubMed] [Google Scholar]
- 44.Carlier SG, Cespedes EI, Li W, et al. Blood flow assessment with intravascular ultrasound catheters: the ideal tool for simultaneous assessment of the coronary haemodynamics and vessel wall? Semin Interv Cardiol. 1998;3:21–29. [PubMed] [Google Scholar]
- 45.van der Steen AF, Cespedes EI, Carlier SG, et al. Flow estimation using an intravascular imaging catheter. Ultrasonics. 2000;38:363–368. doi: 10.1016/s0041-624x(99)00213-9. [DOI] [PubMed] [Google Scholar]
- 46.Ganz W, Tamura K, Marcus HS, Donoso R, Yoshida S, Swan HJC. Measurement of coronary sinus blood flow by continuous thermodilution in man. Circulation. 1971;44:181–195. doi: 10.1161/01.cir.44.2.181. [DOI] [PubMed] [Google Scholar]
- 47.Hayes PC, Terrace D, Peaston I, Bouchier IAD, Redhead D, Brash HM. Computerised system for the continuous measurement of azygos venous blood flow. Gut. 1992;33:372–374. doi: 10.1136/gut.33.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pernow J, Kaijser L, Lundberg JM, Ahlborg G. Comparable potent coronary vasoconstrictor effects of endothelin-1 and big endothelin-1 in humans. Circulation. 1996;94:2077–2082. doi: 10.1161/01.cir.94.9.2077. [DOI] [PubMed] [Google Scholar]
- 49.Hirzel HO, Amende I, Schriber K, Rhiner A, Krayenb¸hl HP, Rutishauser W. Evaluation of the thermodilution technique for measuring coronary sinus blood flow. Pfļ Gers Arch. 1971;330:257–264. doi: 10.1007/BF00588616. [DOI] [PubMed] [Google Scholar]
- 50.Wilson RF. Assessment of the human coronary circulation using a Doppler catheter. Am J Cardiol. 1991;67:44D–56D. doi: 10.1016/s0002-9149(05)80007-4. [DOI] [PubMed] [Google Scholar]
- 51.Qian J, Ge J, Haude M, et al. Safety of intracoronary Doppler flow measurement. Am Heart J. 2000;140:502–510. doi: 10.1067/mhj.2000.109221. [DOI] [PubMed] [Google Scholar]
- 52.De Rouen TA, Murray JA, Owen W. Variability in the analysis of coronary angiograms. Circulation. 1977;55:324–328. doi: 10.1161/01.cir.55.2.324. [DOI] [PubMed] [Google Scholar]
- 53.White CW, Wright CB, Doty DB, et al. Does visual interpretation of the coronary angiogram predict the physiologic importance of a coronary stenosis? N Engl J Med. 1984;310:819–824. doi: 10.1056/NEJM198403293101304. [DOI] [PubMed] [Google Scholar]
- 54.Haussmann D, Erbel R, Alibelli-Chemarin MJ, et al. The safety of intracoronary ultrasound: a multicentre survey of 2207 examinations. Circulation. 1995;91:623–630. doi: 10.1161/01.cir.91.3.623. [DOI] [PubMed] [Google Scholar]
- 55.Alfonso F, Macaya C, Goicolea J, et al. Intravascular ultrasound imaging of angiographically normal coronary artery segments in patients with coronary artery disease. Am Heart J. 1994;127:536–544. doi: 10.1016/0002-8703(94)90660-2. [DOI] [PubMed] [Google Scholar]
- 56.Reddy KG, Suneja R, Nair RN, Dhawale P, Hodgson JM. Measurement by intravascular ultrasound of in vivo arterial distensibility within atherosclerotic lesions. Am J Cardiol. 1993;72:1232–1237. doi: 10.1016/0002-9149(93)90289-o. [DOI] [PubMed] [Google Scholar]
- 57.Sudhir K, MacGregor JS, Barbant SD, et al. Assessment of coronary conductance and resistance vessel reactivity in response to nitroglycerin, ergonovine and adenosine: in vivo studies with simultaneous intravascular two-dimensional and Doppler ultrasound. J Am Coll Cardiol. 1993;21:1261–1268. doi: 10.1016/0735-1097(93)90255-y. [DOI] [PubMed] [Google Scholar]
- 58.Kearney PP, Starkey IR, Sutherland GR. Intracoronary ultrasound: current state of the art. Br Heart J. 1995;73(Suppl 2):16–25. doi: 10.1136/hrt.73.5_suppl_2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Mario C, Görge G, Peters R, et al. Clinical application and image interpretation in intracoronary ultrasound. Eur Heart J. 1998;19:207–229. doi: 10.1053/euhj.1996.0433. [DOI] [PubMed] [Google Scholar]
- 60.Ge J, Erbel R, Gerber T, et al. Intravascular ultrasound imaging of angiographically normal coronary arteries: a prospective study in vivo. Br Heart J. 1994;71:572–578. doi: 10.1136/hrt.71.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgart D, Liu F, Haude M, Görge G, Ge J, Erbel R. Acute plaque rupture and myocardial stunning in patient with normal coronary arteriography. Lancet. 1995;346:193–194. [PubMed] [Google Scholar]
- 62.Erbel R, Ge J, Bokisch A, et al. Value of intracoronary ultrasound and Doppler in the differentiation of angiographically normal coronary arteries: a prospective study in patients with angina pectoris. Eur Heart J. 1996;17:880–889. doi: 10.1093/oxfordjournals.eurheartj.a014969. [DOI] [PubMed] [Google Scholar]
- 63.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 64.Ge J, Erbel R, Zamorano J, et al. Coronary artery remodelling in atherosclerotic disease: an intravascular ultrasonic study in vivo. Coronary Artery Dis. 1993;4:981–986. doi: 10.1097/00019501-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Friedrich GJ, Moes NY, Muhlberger VA, et al. Detection of intralesional calcium by intracoronary ultrasound depends on the histological pattern. Am Heart J. 1994;128:435–441. doi: 10.1016/0002-8703(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 66.Kearney PP, Erbel R, Rupprecht HJ, et al. Differences in the morphology of unstable and stable coronary lesions and their impact on the mechanisms of angioplasty. Eur Heart J. 1996;17:721–730. doi: 10.1093/oxfordjournals.eurheartj.a014939. [DOI] [PubMed] [Google Scholar]
- 67.Schermund A, Baumgart D, Görge G, et al. Coronary artery calcium in acute coronary syndromes. Circulation. 1997;96:1461–1467. doi: 10.1161/01.cir.96.5.1461. [DOI] [PubMed] [Google Scholar]
- 68.Hodgson J McB, Reddy D, Suneja R, Nair R, Lesnefsky E, Sheehan H. Intracoronary ultrasound imaging: correlation of plaque morphology with angiography, clinical syndrome and procedural results in patients undergoing coronary angioplasty. J Am Coll Cardiol. 1993;21:35–44. doi: 10.1016/0735-1097(93)90714-c. [DOI] [PubMed] [Google Scholar]
- 69.Moore MP, Spencer T, Salter DM, et al. Characterization of coronary atherosclerotic morphology by spectral analysis of radiofrequency signal: in vitro intravascular ultrasound study with histological and radiological validation. Heart. 1998;79:459–467. doi: 10.1136/hrt.79.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Birgelen C, Mintz GS, Nicosia A, et al. Electrocardiogram-gated intravascular ultrasound image acquisition after coronary stent deployment facilitates on-line three-dimensional reconstruction and automated lumen quantification. J Am Coll Cardiol. 1997;30:436–443. doi: 10.1016/s0735-1097(97)00154-x. [DOI] [PubMed] [Google Scholar]
- 71.De Scheerder I, De Man F, Herregods MC, et al. Intravascular ultrasound versus angiography for measurement of luminal diameters in normal and diseased coronary arteries. Am Heart J. 1994;127:243–251. doi: 10.1016/0002-8703(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 72.Moussa I, Moses J, De Gregorio J, et al. Effects of arterial remodelling on the degree of discrepancy between quantitative coronary angiography and ultrasound imaging in determining true vessel dimensions. J Am Coll Cardiol. 1999;33:1159–1189. (Abstract) [Google Scholar]
- 73.Tron C, Kern MJ, Donohue TJ, et al. Comparison of quantitative angiographically derived and measured translesion pressure and flow velocity in coronary artery disease. Am J Cardiol. 1995;71:111–117. doi: 10.1016/s0002-9149(00)80057-0. [DOI] [PubMed] [Google Scholar]
- 74.Hodgson J McB, Mancini GBJ, Legrand V, Vogel RA. Characterization of changes in coronary blood flow during the first six seconds after intracoronary contrast injection. Invest Radiol. 1985;20:246–252. doi: 10.1097/00004424-198505000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Hollenberg SM, Tamburro P, Johnson MR, et al. Simultaneous intracoronary ultrasound and Doppler flow studies distinguish flow-mediated from receptor-mediated endothelial responses. Cathet Cardiovasc Diagn. 1999;46:282–288. doi: 10.1002/(SICI)1522-726X(199903)46:3<282::AID-CCD5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 76.Sakamura I, Makita Y, Hirasawa K, et al. Assessment of flow-dependent dilatation in the human conductance coronary artery using a simultaneous intravascular two-dimensional and Doppler ultrasound system. Japan J Intervent Cardiol. 1995;10(Suppl 2):11–14. [Google Scholar]
- 77.Caracciolo EA, Wolford TL, Underwood RD, et al. Influence of intimal thickening on coronary blood flow responses in orthotopic heart transplant recipients. Circulation. 1995;92(Suppl II):82–90. doi: 10.1161/01.cir.92.9.182. [DOI] [PubMed] [Google Scholar]
- 78.Isner JM, Kaufman J, Rosenfield K, et al. Combined physiologic and anatomic assessment of percutaneous revascularisation using a Doppler guidewire and ultrasound catheter. Am J Cardiol. 1993;71:70D–86D. doi: 10.1016/0002-9149(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 79.Brown G, Albers J, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 80.Ornisch D, Brown SE, Schweritz LW, et al. Can life style changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336:129–133. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]
- 81.De Feyter PJ, Serruys PW, Davies MJ, Richardson P, Lubsen J, Oliver MJ. Quantitative coronary angiography to measure progression and regression of coronary atherosclerosis. Circulation. 1991;84:412–423. doi: 10.1161/01.cir.84.1.412. [DOI] [PubMed] [Google Scholar]
- 82.Doucette JW, Corl PD, Payne HM, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 83.Ofili EO, Kern MJ, Labowitz AJ, St Vrain JA, Segal J, Aguirre FV. Analysis of coronary blood flow velocity dynamics in angiographically normal and stenosed arteries before and after endolumen enlargement by angioplasty. J Am Coll Cardiol. 1993;21:308–316. doi: 10.1016/0735-1097(93)90668-q. [DOI] [PubMed] [Google Scholar]
- 84.Miller DD, Donohue TJ, Younis LT, et al. Correlation of pharmacological Tc-sestamibi myocardial perfusion imaging with poststenotic coronary flow reserve in patients with angiographically intermediate coronary artery stenoses. Circulation. 1994;89:2150–2160. doi: 10.1161/01.cir.89.5.2150. [DOI] [PubMed] [Google Scholar]
- 85.Joye JD, Schulman DS, Lasorda D, et al. Intracoronary Doppler guide wire versus stress single-photon emission computed tomographic thallium-201 imaging in assessment of intermediate coronary stenoses. J Am Coll Cardiol. 1994;24:940–947. doi: 10.1016/0735-1097(94)90853-2. [DOI] [PubMed] [Google Scholar]
- 86.Dib N, Bajwa T, Shalev Y, Nesto R, Schmidt DH. Validation of Doppler Flowire for measurement of coronary flow reserve in humans. Cathet Cardiovasc Diagn. 1998;45:382–385. doi: 10.1002/(sici)1097-0304(199812)45:4<382::aid-ccd6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 87.De Bruyne B, Bartunek J, Sys SU, Pijls NHJ, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Circulation. 1996;94:1842–1849. doi: 10.1161/01.cir.94.8.1842. [DOI] [PubMed] [Google Scholar]
- 88.Di Mario C, Gil R, Serruys PW. Long-term reproducibility of coronary flow velocity measurements in patients with coronary artery disease. Am J Cardiol. 1993;75:1177–1180. doi: 10.1016/s0002-9149(99)80755-3. [DOI] [PubMed] [Google Scholar]
- 89.Cleary RM, Moore NB, DeBoe SF, Mancini GBJ. Sensitivity and reproducibility of the instantaneous hyperemic flow versus pressure slope index compared to coronary flow reserve for the assessment of stenosis severity. Am Heart J. 1993;126:57–65. doi: 10.1016/s0002-8703(07)80010-x. [DOI] [PubMed] [Google Scholar]
- 90.Reiber JHC, Serruys PW, Kooijman CJ, et al. Assessment of short-, medium- and long-term variations in arterial dimesions from computer-assisted quantification of coronary cineangiograms. Circulation. 1985;71:280–288. doi: 10.1161/01.cir.71.2.280. [DOI] [PubMed] [Google Scholar]
- 91.Mancini GBJ, Simon SB, McGillem MJ, Lefree MT, Freeman HZ, Vogel RA. Automated quantitative coronary arteriography: morphologic and physiologic validation in vivo of a rapid digital angiographic method. Circulation. 1987;75:452–460. doi: 10.1161/01.cir.75.2.452. [DOI] [PubMed] [Google Scholar]
- 92.Rothwell PM, Gibson RJ, Villagra R, Sellar R, Warlow CP. The effect of angiographic technique and image quality on the reproducibility of measurement of carotid stenosis and assessment of plaque surface morphology. Clin Radiol. 1998;53:439–443. doi: 10.1016/s0009-9260(98)80273-0. [DOI] [PubMed] [Google Scholar]
- 93.Hausmann D, Sudhir K, Mullen WL, et al. Contrast-enhanced intravascular ultrasound: validation of a new technique for delineation of the vessel wall boundary. J Am Coll Cardiol. 1994;23:981–987. doi: 10.1016/0735-1097(94)90647-5. [DOI] [PubMed] [Google Scholar]
- 94.Foster GP, Mittleman MA, Koch M, Abela G, Zarich SW. Variability in the measurement of intracoronary ultrasound images: implications for the identification of atherosclerotic plaque regression. Clin Cardiol. 1997;20:11–15. doi: 10.1002/clc.4960200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dhawale PJ, Wilson DL, Hodgson JM. Volumetric intracoronary ultrasound: methods and validation. Cathet Cardiovasc Diagn. 1994;33:296–307. doi: 10.1002/ccd.1810330403. [DOI] [PubMed] [Google Scholar]
- 96.Schwartzkopff B, Motz W, Frenzel H, Vogt M, Knauer S, Strauer BE. Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation. 1993;88:993–1003. doi: 10.1161/01.cir.88.3.993. [DOI] [PubMed] [Google Scholar]
- 97.Nahser PJ, Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995;91:635–640. doi: 10.1161/01.cir.91.3.635. [DOI] [PubMed] [Google Scholar]
- 98.Camici PG, Gistri R, Lorenzoni R, et al. Coronary reserve and exercise ECG in patients with chest pain and normal coronary angiograms. Circulation. 1992;86:179–186. doi: 10.1161/01.cir.86.1.179. [DOI] [PubMed] [Google Scholar]
- 99.Yokoyama I, Ohtake T, Momomura SI, Nishikawa J, Sasaki Y, Omata M. Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis. Circulation. 1996;94:3232–3238. doi: 10.1161/01.cir.94.12.3232. [DOI] [PubMed] [Google Scholar]
- 100.Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL. Decreased coronary reserve. a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med. 1982;307:1362–1367. doi: 10.1056/NEJM198211253072202. [DOI] [PubMed] [Google Scholar]
- 101.Bache RJ. Vasodilator reserve. a functional assessment of coronary health. Circulation. 1998;98:1257–1360. doi: 10.1161/01.cir.98.13.1257. [DOI] [PubMed] [Google Scholar]
- 102.Quyyumi AA, Dakak N, Andrews NP, et al. Nitric oxide activity in the human coronary circulation: impact of risk factors for coronary atherosclerosis. J Clin Invest. 1995;95:1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Groves P, Kurz S, Just H, Drexler H. Role of endogenous bradykinin in human coronary vasomotor control. Circulation. 1995;92:3424–3430. doi: 10.1161/01.cir.92.12.3424. [DOI] [PubMed] [Google Scholar]
- 104.Vita JA, Treasure CB, Nabel EG, et al. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 105.Leung WH, Lau CP, Wong CK. Beneficial effect of cholesterol lowering therapy on coronary endothelium-dependent vasorelaxation in hypercholesterolaemic patients. Lancet. 1993;341:1496–1500. doi: 10.1016/0140-6736(93)90634-s. [DOI] [PubMed] [Google Scholar]
- 106.Solzbach U, Hornig B, Jeserich M, Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation. 1997;96:1513–1519. doi: 10.1161/01.cir.96.5.1513. [DOI] [PubMed] [Google Scholar]
- 107.Zeiher AM, Drexler H. Wollschläger H, Just H. Modulation of coronary vasomotor tone in humans. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 108.Hollenberg SM, Tamburro P, Klein LW, et al. Discordant epicardial and microvascular endothelial responses in heart transplant recipients early after transplantation. J Heart Lung Transplant. 1998;17:487–494. [PubMed] [Google Scholar]
- 109.Gilligan DM, Quyyumi AA, Cannon RO. Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- 110.Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by l-arginine. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- 111.Drexler H, Fischell TA, Pinto FJ, et al. Effect of l-arginine on coronary endothelial function in cardiac transplant recipients. Circulation. 1994;89:1615–1623. doi: 10.1161/01.cir.89.4.1615. [DOI] [PubMed] [Google Scholar]
- 112.Kugiyama K, Ohgushi M, Motoyama T, et al. Intracoronary infusion of reduced glutathione improves endothelial vasomotor responses to acetylcholine in human coronary circulation. Circulation. 1998;97:2299–2301. doi: 10.1161/01.cir.97.23.2299. [DOI] [PubMed] [Google Scholar]
- 113.Egashira K, Hirooka Y, Kai H, et al. Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in patients with hypercholesterolemia. Circulation. 1994;89:2519–2524. doi: 10.1161/01.cir.89.6.2519. [DOI] [PubMed] [Google Scholar]
- 114.Hirata Y, Serizawa T, Kohmoto O, et al. Estimation of the secretion rate of atrial natriuretic peptide from the coronary sinus in coronary artery disease. Am J Cardiol. 1988;62:56–58. doi: 10.1016/0002-9149(88)91364-1. [DOI] [PubMed] [Google Scholar]
- 115.Montalescot G, Drobinski G, Maclouf J, et al. Evaluation of thromboxane production and complement activation during myocardial ischemia in patients with angina pectoris. Circulation. 1991;84:2054–2062. doi: 10.1161/01.cir.84.5.2054. [DOI] [PubMed] [Google Scholar]
- 116.Camici PG, Marraccini P, Lorenzoni R, et al. Coronary hemodynamics and myocardial metabolism in patients with syndrome X: response to pacing stress. J Am Coll Cardiol. 1991;17:1461–1470. doi: 10.1016/0735-1097(91)90632-j. [DOI] [PubMed] [Google Scholar]
- 117.Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS) Circulation. 1995;91:2528–2540. doi: 10.1161/01.cir.91.10.2528. [DOI] [PubMed] [Google Scholar]
- 118.Herd JA, Ballantyne CM, Farmer JA, et al. Effects of fluvastatin on coronary atherosclerosis in patients with mild to moderate cholesterol elevations (Lipoprotein and Coronary Atherosclerosis Study [LCAS]) Am J Cardiol. 1997;80:278–286. doi: 10.1016/s0002-9149(97)00346-9. [DOI] [PubMed] [Google Scholar]
- 119.Grínholdt MLM. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Circulation. 1999;19:2–13. doi: 10.1161/01.atv.19.1.2. [DOI] [PubMed] [Google Scholar]
- 120.Yamagashi M, Terashima M, Awano K, et al. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol. 2000;35:106–111. doi: 10.1016/s0735-1097(99)00533-1. [DOI] [PubMed] [Google Scholar]
- 121.Schartl M, Bocksch W, Koschyk DH, et al. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque Volume and composition in patients with coronary artery disease. Circulation. 2001;104:387–392. doi: 10.1161/hc2901.093188. [DOI] [PubMed] [Google Scholar]
- 122.Gao SZ, Alderman EL, Schroeder JS, et al. Accelerated coronary vascular disease in the heart transplant patient: coronary angiographic findings. J Am Coll Cardiol. 1988;12:334–340. doi: 10.1016/0735-1097(88)90402-0. [DOI] [PubMed] [Google Scholar]
- 123.Klauss V, Mudra H, Uberfuhr P, Theisen K. Intraindividual variability of cardiac allograft vasculopathy as assessed by intravascular ultrasound. Am J Cardiol. 1995;76:463–466. doi: 10.1016/s0002-9149(99)80131-3. [DOI] [PubMed] [Google Scholar]
- 124.Richenbacher PR, Pinto FJ, Chenzbraun A, et al. Incidence and severity of transplant coronary artery disease early and up to 15 years after transplantation as detected by intravascular ultrasound. J Am Coll Cardiol. 1995;25:171–177. doi: 10.1016/0735-1097(94)00323-i. [DOI] [PubMed] [Google Scholar]
- 125.Zeiher AM, Schachinger V, Hohnloser SH, Saurbier B, Just H. Coronary atherosclerotic wall thickening and vascular reactivity in humans. Circulation. 1994;89:2525–2532. doi: 10.1161/01.cir.89.6.2525. [DOI] [PubMed] [Google Scholar]
- 126.Anderson TJ, Meredith IT, Uehata A, et al. Functional significance of intimal thickening as detected by intravascular ultrasound early and late after cardiac transplantation. Circulation. 1993;88:1093–1100. doi: 10.1161/01.cir.88.3.1093. [DOI] [PubMed] [Google Scholar]
- 127.Costa MA, Kozuma K, Gaster AL, et al. Three dimensional intravascular ultrasonic assessment of the local mechanism of restenosis after balloon angioplasty. Heart. 2001;85:73–79. doi: 10.1136/heart.85.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kutryk MJ, Foley DP, van den Brand M, et al. Local intracoronary administration of antisense digonucleotide against c-myc for the prevention of in-stent restonosis. J Am Coll Cardiol. 2002;39:281–287. doi: 10.1016/s0735-1097(01)01741-7. [DOI] [PubMed] [Google Scholar]
- 129.Brieger D, Topol E. Local drug delivery systems and prevention of restenosis. Cardiovasc Res. 1997;35:405–413. doi: 10.1016/s0008-6363(97)00155-7. [DOI] [PubMed] [Google Scholar]
- 130.Feldman LJ, Steg G. Optimal techniques for arterial gene transfer. Cardiovasc Res. 1997;35:391–404. doi: 10.1016/s0008-6363(97)00148-x. [DOI] [PubMed] [Google Scholar]
- 131.The ERASER Investigators. Acute platelet inhibition with abciximab does not reduce in-stent restenosis (ERASER Study) Circulation. 1999;100:799–806. doi: 10.1161/01.cir.100.8.799. [DOI] [PubMed] [Google Scholar]
- 132.Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation. 2001;103:604–616. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 133.Naghavi M, Madjid M, Khan MR, Mohammadi RM, Willerson JT, Casscells SW. New developments in the detection of vulnerable plaque. Curr Atheroscler Rep. 2001;3:125–135. doi: 10.1007/s11883-001-0048-1. [DOI] [PubMed] [Google Scholar]
- 134.Villanueva FS, Jankowski RJ, Klibanov S, et al. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998;98:1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- 135.Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation. 2000;102:2582–2587. doi: 10.1161/01.cir.102.21.2582. [DOI] [PubMed] [Google Scholar]
- 136.Matschl V, Heverhagen JT, Kalinowski M, et al. In vivo evaluation of an intravascular receiver coil for MRI at 1.0 Tesla. Vasa. 2001;30:9–13. doi: 10.1024/0301-1526.30.1.9. [DOI] [PubMed] [Google Scholar]