Abstract
Aims
The aim of this study was to investigate the effect of concomitant food intake on the bioavailability of two nifedipine containing modified release dosage forms for once daily administration. The clinical study was performed to investigate the in vivo relevance of pH-dependent differences in the in vitro release properties of the two dosage forms.
Methods
This was a randomized, open, 4-way crossover study in 24 healthy, male subjects. Following an overnight fast of 12 h single doses of Adalat® OROS or Slofedipine® XL were administered either in the fasted state or immediately after a high fat American breakfast. Nifedipine plasma concentrations in samples obtained until 48 h after drug administration were determined using a validated LC-MS/MS method. Calculation of pharmacokinetic parameters was conducted model-independently. The two dosage forms as well as the two administration conditions were compared by calculating point estimates and 90% confidence intervals for the relevant pharmacokinetic parameters. In vitro dissolution tests were performed using a paddle apparatus 3 acc. USP, a pharmacopoeial dissolution system consisting of reciprocating cylinders in flat-bottomed glass vessels, with various buffer systems covering the entire physiological pH-range of the gastrointestinal tract.
Results
After fasted administration the extent of bioavailability of nifedipine as characterized by AUC(0,∞) was slightly lower for Slofedipine® XL compared with Adalat® OROS with a point estimate of 82.3% primarily resulting from pronounced differences in nifedipine concentrations during the first 15 h after administration. Accordingly, maximum plasma concentrations were lower after administration of Slofedipine® XL compared with Adalat® OROS (point estimate: 84.3%). Under fed conditions the differences in bioavailability between the two products as characterized by the pharmacokinetic parameters AUC(0,tn) and Cmax were greater than after fasting conditions with point estimates of 69.6% and 81.0%, respectively. However, most striking was a pronounced delay in nifedipine absorption observed under fed conditions after administration of Slofedipine® XL which resulted in lag-times of more than 15 h in 15 out of 24 subjects. Owing to this lag-time under fed conditions the relative bioavailability of nifedipine from Slofedipine® XL compared with Adalat® OROS was only 28% over the intended dosing interval of 24 h.
Conclusions
In this study a dosage form-dependent food interaction was observed which, under fed conditions, resulted in pronounced differences in the relative bioavailability of nifedipine between Slofedipine® XL and Adalat® OROS over the intended dosing interval of 24 h. The delay in nifedipine absorption when Slofedipine® XL is administered after a high-fat breakfast may be explained by the formulation properties. Slofedipine® XL is an erosive tablet with an acid resistant coating whereas Adalat® OROS is designed with an osmotic push-pull system. Under fed conditions drug from the single unit enteric coated dosage form exhibits a delayed absorption probably due to an extensively prolonged gastric residence time which does not allow drug release, on the other hand the osmotically driven push-pull system is not sensitive to concomitant food intake. The observed phenomenon might be of therapeutic relevance. For example a change from taking Slofedipine® XL in the fed to the fasted state might result in increased systemic concentrations of nifedipine.
Keywords: bioavailability, bioequivalence, enteric coated dosage form, food effect, healthy volunteers, migrating motor complex, nifedipine, osmotic push-pull system
Introduction
Modified-release dosage forms are primarily developed to reduce dosing frequency which is more convenient for the patient and may therefore improve compliance in chronic treatment. Their use also decreases maximum drug concentrations for medications with concentration-related side-effects. However, such dosage forms are at risk of pharmacokinetic interactions affecting the absorption process especially in the case of concomitant food intake [1–3]. Such food effects are often not predictable from the in vitro characteristics of a dosage form, although several investigations have demonstrated a high probability of food interactions in formulations with pH-dependent release properties [4].
The osmotically driven oral therapeutic system (OROS) has been identified as being robust against potential food interactions, mainly because its release characteristics follow zero order kinetics for almost the entire release time and are independent of pH levels within the physiological range [5]. Such a system was developed for nifedipine (Adalat® OROS) in order to allow once-daily administration instead of a twice-daily dosage regimen achieved with conventional modified release nifedipine tablets.
Various nifedipine-containing modified-release dosage forms for once-daily administration based on differing galenic principles have been approved by health authorities in the European Union as generic alternatives to the OROS formulation. According to the European regulatory requirements such abbreviated approvals of modified-release formulations require the proof of comparable in vivo performance after single and multiple dose administration under both fasting and fed conditions [6]. These generic alternatives to the OROS formulation include Slofedipine® XL, a monolithic modified-release tablet with an erosive polymer matrix and dissolution properties of an enteric coated system. Investigation of the in vitro release characteristics of Slofedipine® XL before this in vivo study showed a dissolution behaviour which was highly dependent on both pH and other dissolution parameters such as agitation and osmolarity of the medium. Such instability of the dosage form might result in a formulation-dependent pharmacokinetic food interaction.
Therefore, the objective of this study was to compare the pharmacokinetics of nifedipine in healthy subjects after administration of Slofedipine® XL and Adalat® OROS in the fasted and fed state.
Methods
In vitro dissolution study
Both dosage forms were investigated under identical dissolution conditions. Since dissolution from the osmotically driven product was unaffected by all the test conditions the procedure was optimized with regard to the erosive tablet. Experiments were performed using an apparatus 3 acc. to USP, a dissolution system consisting of a set of cylinders reciprocating vertically in flat-bottomed glass-vessels. The apparatus was tested for system suitability according to the requirements of USP using USP drug release calibrator tablets. The cylinders were motor-driven in order to allow constant reciprocating rate, the vessels were partially immersed in a water bath holding the temperature at 37 ± 0.5° C during the test. The dissolution was carried out with an agitation of 20 dips min−1 (±5%), using 200 ml of the following buffers: 0.1 n HCl, acetate buffer pH 4.5, phosphate buffer pH 6.8 and phosphate buffer pH 8.0. 0.5% SDS were added to all dissolution media.
All investigations were performed under protection from daylight.
Clinical study
This was a randomized, open, 4-way crossover study in 24 healthy, male subjects. After an overnight fast of at least 12 h, each volunteer received single oral doses (60 mg nifedipine) of either Slofedipine® XL or Adalat® OROS either under fasting conditions or immediately after a high fat breakfast. Wash-out periods of at least 1 week between the treatments were maintained. Before the study, the general health status of the subjects was assessed from a clinical history, a physical examination including blood pressure and pulse rate measurements, a 12-lead ECG, routine haematology, clinical chemistry and urinalysis. Inclusion and exclusion criteria were specified to ensure the safety of the subjects and to optimize standardization of absorption conditions. Alcohol and drug tests were performed before dosing. The clinical study was conducted in accordance with ICH-GCP requirements and the current version of the Declaration of Helsinki. The study protocol together with the Informed Consent Form was reviewed and approved by the responsible Ethics Committee (Medical Chamber of Thuringia, Germany).
A total of 26 healthy male, Caucasian subjects, who had given written informed consent after comprehensive information about the risks and inconveniences they were exposed to, were initially enrolled in the study. Two subjects dropped out, but the reason for withdrawal was not related to the study drugs and both subjects were replaced. Twenty-five subjects received at least one study medication and were included in the safety analysis. The mean age of these 25 subjects was 25.1 years (range: 18–37 years), mean weight 76.3 kg (range: 66–99 kg) and mean height 180.9 cm (range: 167–193 cm). Mean BMI was calculated as 23.3 kg m−2 (range: 19.3–26.9 kg m−2).
The subjects were hospitalized for 12 h before and until 48 h after dosing. After an overnight fast the two formulations were given under standardized conditions either to fasting subjects or immediately after a high fat breakfast. A standardized meal was served to all subjects 4 h after dosing followed by standardized meals 7 and 11 h after dosing. Conditions were chosen in accordance with international requirements for food interaction studies [7]. Blood samples for the determination of nifedipine were collected at times 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 15, 24, 30, 36 and 48 h after dosing. Plasma was prepared under protection from daylight due to the photo-instability of nifedipine, deep frozen and stored below −20° C until analysis.
Vital signs, ECG and laboratory parameters were repeatedly determined during the hospitalization phase. Subjective well being was monitored by asking for adverse events in a nonleading manner and by documentation of spontaneously reported adverse events. These were classified according to their severity and potential relationship to the study drug. Any concomitant medication taken during the course of the study was documented.
Analysis of nifedipine
Plasma samples were assayed using a LC-MS/MS method validated according to international requirements [8]. Frozen study plasma samples were thawed in a 20° C water bath. Internal standard nimodipine (equal to 20.01 ng) was added, followed by homogenization. Afterwards, a defined volume of 1 m sodium hydroxide was added, the mixture was shortly squirled, followed by organic extraction (n-pentane:ethyl acetate = 70:30 (v/v)). After centrifugation, the upper organic layer was recovered and evaporated to dryness under a stream of nitrogen (water bath, 25° C, 25 min). The dry residue was dissolved in the LC eluent (acetonitrile: H2O = 60:40 (v/v)), mixed, given in an ultrasonic bath, again mixed and centrifuged. Finally 50 µl of the cleared solution were used for subsequent LC-MS/MS-analysis, employing a cooled autosampler (approximately 8° C) with appropriate light protection.
The analytical column (Grom Sil120 Cyano 3 CP, 5 µm, 60×4.6 mm), mobile phase (acetonitrile:H2O/60:40 v/v), a flow rate of 1 ml min−1, pressure of 33 bar and an injection volume of 50 µl in a 100 µl sample loop were used for the chromatographic separation.
Mass spectrometric analysis was performed by means of atmospheric pressure chemical ionization (APcI) of analytes in the positive mode using multiple reaction monitoring (MRM) of the precursor/product ion transitions. Further instrumental conditions were a source temperature of 150° C, a probe temperature of 400° C, a drying gas flow of 350 l h−1 nitrogen and a sheath gas flow of 100 l h−1 nitrogen.
For increased sensitivity and enhanced selectivity, collision induced dissociation (CID) was employed with argon as collision gas for generation of product ions from precursor ions (CID-pressure 2.0 × 10−3 mbar).
The limit of quantification was 0.1 µg l−1. Quality control (QC) samples were analysed together with the study samples. Mean day-to-day coefficients of variation calculated from QC results during sample measurement were 7.7% (0.13 µg l−1, n = 98), 1.8% (8.84 µg l−1, n = 100) and 1.6% (88.4 µg l−1, n = 100). Accuracy was determined as mean deviations of −0.63% (0.13 µg l−1), −0.59% (8.84 µg l−1), and 1.6% (88.40 µg l−1), respectively.
Data analysis
The pharmacokinetic parameters of nifedipine were determined using model-independent methods (WinNonlin software, version 2.1). Statistical analyses were performed with SAS® for Windows 95/NT (Version 6.12, SAS Institute, Cary, North Carolina, USA). Plasma concentrations below the lower limit of quantification [LOQ] were set at half the limit of quantification. The mean concentrations at any individual time point were only calculated if at least 2/3 of the samples had been measured and the results were above the limit of quantification at that specific time point.
Cmax was read directly from the observed concentrations, actual tmax was determined from the observed concentrations as the blood sampling time corresponding to Cmax. Area under the plasma concentration vs time curve, AUC(0,tn), was calculated using the linear trapezoidal rule in the absorption phase and using the logarithmic trapezoidal rule in the terminal phase up to the time of the last quantifiable concentration. AUC(0,∞) was calculated as the sum of AUC(0,tn) and AUC extrapolated from the last measured value to infinity using the apparent terminal rate constant and AUC for the intended dosing interval of 24 h AUC(0,24 h) was also estimated. The apparent terminal half-life was calculated from nonlinear regression of a single (Ce-λzt) exponential function on data points visually assessed to be on the terminal log-linear phase of the (untransformed) plasma concentration vs time profile. The adjustments were performed by the method of least squares. The lag-time, tlag, was determined as the time span from dosing until the sampling point prior to the first quantifiable sample.
AUC(0,∞) and Cmax were compared using analysis of variance (anova) with sequence, subject (sequence), period and treatment effects. Point estimates and two-sided 90% confidence intervals for the ratios ‘test/reference’ following both fed and fasting conditions were calculated by re-transformation of the logarithmic data using the intraindividual standard deviation of the anova.
tmax and tlag were compared by nonparametric analysis using the SAS statistical package. 90% confidence intervals were calculated for median treatment differences.
Results
In vitro dissolution studies
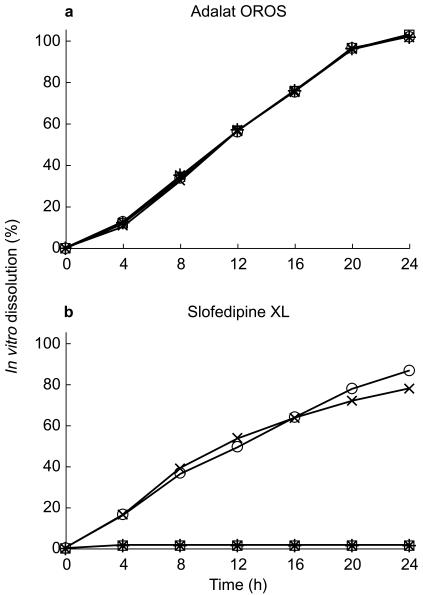
The mean profiles obtained from in vitro dissolution tests performed with both dosage forms are presented in Figures 1 and 2. Adalat® OROS proved to be unaffected by the pH of the media whereas Slofedipine® XL exhibited clearly pH-dependent dissolution. Dissolved drug was not detected in 0.1 N HCl and in acetate buffer pH 4.8, but the dissolution profile of Slofedipine® XL was comparable to Adalat® OROS at pH 6.8. However dissolution decreased again when tested at pH 8.0. Additional investigations (data not shown) have shown that the dissolution behaviour of Slofedipine® XL but not Adalat® OROS also depended on agitation and osmolarity.
Figure 1.
Mean in vitro dissolution of nifedipine (n = 6) from a) Adalat® OROS and b) Slofedipine® XL in different media (⋆ 0.1 m HCl, □ acetate buffer pH 4.5, ○ phosphate buffer pH 6.8, × phosphate buffer pH 8.0).
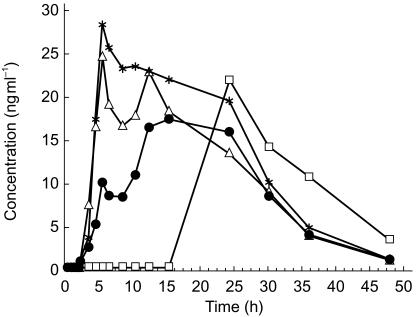
Figure 2.
Geometric mean plasma concentration vs time curves (n = 24) of nifedipine determined after oral administration of Adalat® OROS and Slofedipine® XL under fasting conditions and after a high fat breakfast (▵ Adalat® OROS, fasted administration; ⋆ Adalat® OROS, fed administration; • Slofedipine® XL, fasted administration; □ Slofedipine® XL, fed administration).
Nifedipine pharmacokinetics
Mean nifedipine plasma concentration vs time profiles of the formulations are shown in Figure 2, the pharmacokinetic measurements are presented in Table 1 and statistical evaluations are summarized in Table 2.
Table 1.
Pharmacokinetic parameters of nifedipine after administration of Adalat® OROS and Slofedipine® XL to fasted and fed healthy volunteers.
| Fasted administration | Fed administration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adalat OROS® 60 mg nifedipine | Slofedipine® XL 60 mg nifedipine | Adalat OROS® 60 mg nifedipine | Slofedipine® XL 60 mg nifedipine | |||||||||
| Variable (unit) | n | geometric mean (s.d.) | range | n | geometric mean (s.d.) | range | n | geometric mean (s.d.) | range | n | geometric mean (s.d.) | range |
| Cmax (µg l−1) | 24 | 33.9 (1.50) | 13.7–72.9 | 24 | 28.6 (1.89) | 4.94–87.6 | 24 | 37.6 (1.42) | 24.2–80.8 | 24 | 30.4 (1.76) | 6.61–79.3 |
| AUC(0,∞) (µg l−1 h) | 24 | 612.8 (1.81) | 171.0–1566.7 | 23 | 516.0 (2.38) | 26.5–1701.0 | 23 | 715.9 (1.69) | 315.7–1967.5 | 16 | 453.6 (2.01) | 84.4–1580.9 |
| AUC(0,tn) (µg l−1 h) | 24 | 588.8 (1.75) | 170.0–1519.8 | 24 | 498.8 (2.32) | 25.8–1632.0 | 24 | 701.7 (1.65) | 314.9–1899.2 | 24 | 499.4 (1.93) | 80.2–1791.3 |
| AUC(0,24 h) (µg l−1 h) | 24 | 406.5 (1.59) | 161.4–1112.5 | 24 | 323.5 (2.14) | 25.8–1048.0 | 24 | 488.8 (1.54) | 303.7–1341.0 | 24 | 137.1 (1.95) | 46.1–373.6 |
| t1/2 (h) | 24 | 5.75 (1.47) | 3.35–13.4 | 23 | 5.50 (1.62) | 2.40–14.60 | 23 | 5.49 (1.45) | 3.19–13.1 | 16 | 5.56 (1.43) | 3.20–14.80 |
| tmax (h)a | 24 | 10.0 | 5.00–30.00 | 24 | 15.0 | 5.00–36.00 | 24 | 10.0 | 5.00–36.00 | 24 | 24.1 | 10.00–36.00 |
| tlag (h)a | 24 | 1.00 | 0.50–1.50 | 24 | 1.00 | 0.00–3.00 | 24 | 1.50 | 0.50–3.00 | 24 | 15.0 | 4.00–15.10 |
Note: = Median and ranges.
Table 2.
Point estimates and 90% confidence intervals for the comparison of pharmacokinetic parameters evaluated for Adalat® OROS and Slofedipine® XL after administration to fed and fasted healthy volunteers.
| Variable (unit) | Slofedipine® XL – Fasting vs Adalat® OROS – Fasting | Slofedipine® XL – Non-fasting vs Adalat® OROS – Non-fasting | Adalat® OROS – Non-fasting vs Adalat® OROS – Fasting | Slofedipine® XL – Non-fasting vs Slofedipine® XL – Fasting | |
|---|---|---|---|---|---|
| Cmax (µg l−1) | PE | 84.3 | 81.0 | 110.8 | 106.4 |
| 90% CI | 70.9, 100.2 | 65.8, 99.6a | 93.2, 131.7 | 89.5, 126.5 | |
| AUC(0,∞) (µg l−1 h) | PE | 82.3 | 69.6 | 116.4 | 98.4 |
| 90% CI | 65.8, 103.0 | 53.7, 90.2 | 93.0, 145.7 | 75.9, 127.7 | |
| AUC(0,tn)a (µg l−1 h) | PE | 84.7 | 71.2 | 119.2 | 100.1 |
| 95% CI | 66.0, 108.7 | 55.5, 91.3 | 92.9, 152.9 | 78.0, 128.4 | |
| AUC(0,24 h)a (µg l−1 h) | PE | 79.6 | 28.0 | 120.2 | 42.4 |
| 95% CI | 61.1, 103.7 | 21.5, 36.5 | 92.3, 156.7 | 32.5, 55.2 | |
| tmax (h)b | PE | 4.50 | 15.0 | 0.00 | 9.49 |
| 95% CI | 0.98, 8.51 | 10.5, 19.0 | −4.62, 5.50 | 4.98, 13.0 | |
| tlag (h)b | PE | 0.38 | 10.4 | 0.48 | 9.75 |
| 95% CI | 0.00, 0.56 | 8.29, 13.5 | 0.01, 0.75 | 8.50, 13.5 |
Note: =95% confidence intervals
=Non-parametric analysis; PE=Point Estimate; CI=Confidence Interval.
Plasma concentration vs time profiles of the two dosage forms differ significantly in fasted subjects, in particular during the first 15 h after drug administration. In this period plasma concentrations measured after Adalat® OROS were significantly higher compared to those determined after Slofedipine® XL. After 15 h both mean profiles are almost identical. Geometric mean AUC(0,∞) (613 µg l−1 h for Adalat® OROS, 516 µg l−1 h for Slofedipine® XL) and Cmax (34 µg l−1 for Adalat® OROS, 29 µg l−1 for Slofedipine® XL) differed by approximately 18% and 16%, respectively. The 90% confidence intervals for the ratio of geometric means were 66, 103% for AUC(0,∞) and 71, 100% for Cmax.
After administration to fed subjects differences between plasma profiles of the two formulations were even more significant than after fasted administration primarily due to a pronounced lag-time after intake of Slofedipine® XL. In 15 out of 24 subjects nifedipine concentrations remained below the lower limit of quantification for at least 15 h after administration.
Because of this pronounced lag-time observed after administration of Slofedipine® XL to fed subjects and the resulting delayed absorption of nifedipine, AUC with extrapolation to infinity could not be calculated appropriately in most of the volunteers. Thus, AUC(0,tn) was estimated for this treatment instead. The pharmacokinetic parameters showed differences between both dosage forms. AUC(0,tn) (702 µg l−1 h for Adalat® OROS, 499 µg l−1 h for Slofedipine® XL) differed by 29% and Cmax (38 µg l−1 for Adalat® OROS, 30 µg l−1 for Slofedipine® XL) by 19%. Furthermore, differences between tmax (medians of 10 h, range 5–36 h for Adalat® OROS, and of 24.1 h, 10–36 h for Slofedipine® XL) were larger. The 90% confidence intervals for the geometric means were 56, 91% for AUC(0,tn) and 66, 100% for Cmax.
A comparison of the geometric means of pharmacokinetic parameters for fed compared to fasted subjects showed only minor differences for Adalat® OROS (point estimates and 90% confidence intervals for the mean ratios of geometric means of AUC(0,∞) = 116% (93, 146%) and Cmax = 111% (93, 132%)).
In contrast, nifedipine concentration-time profiles following Slofedipine® XL were completely different under fed and fasting conditions. However, the geometric means for AUC(0,tn) (499 vs 499 µg l−1 h) and Cmax (28.6 vs 30.4 µg l−1) were similar. Clearly, AUC(0,24 h) reflects better the observed differences in pharmacokinetics between fed (geometric mean 137 µg l−1 h) and fasting (geometric mean 324 µg l−1 h) subjects after administration of Slofedipine® XL, respectively.
No serious or life-threatening events were observed, all adverse events ranging from mild to moderate in intensity. Furthermore, none of the changes in clinical laboratory parameters determined within the course of the study was considered clinically relevant.
Discussion
According to the CPMP Note for Guidance [6] bioequivalence assessment is required for the approval of generic modified-release oral dosage forms such as Slofedipine® XL, to ensure appropriate efficacy and safety of the generic product. In this context data from both single and multiple dose administration as well as that to fasted and fed subjects are needed. The generic product Slofedipine® XL and the reference preparation Adalat® OROS were expected to exhibit comparable in vivo pharmacokinetics after single doses given orally both under fasted and fed conditions.
In vitro dissolution tests revealed significant differences between Slofedipine® XL and Adalat® OROS primarily with regard to pH dependency. While drug release was almost independent of the pH in the case of Adalat® OROS, a pronounced effect of pH of the dissolution media was detected for Slofedipine® XL. Thus, the erosive tablet Slofedipine® XL behaved in vitro like an enteric coated dosage form with nearly no drug release in acidic media. Such dissolution characteristics increase the risk of drug–food interaction in vivo, as food intake greatly influences gastric transit times. After the intake of a meal, indigestible solid particles are known to remain in the stomach until phase III of the migrating motor complex [9]. The duration of this process usually depends on the diameter of the dosage form, the frequency of food intake, as well as on the composition and caloric density of the meal [10, 11]. Published data show that single unit dosage forms with a diameter of 11 × 6 mm may remain in the stomach for about 10 h or even more when coadministered with a high fat breakfast followed by several meals over the course of the day. In contrast after administration to fasting subjects such dosage forms pass the pylorus in less than 1 h. This phenomenon can be explained by the closed pylorus during the filled status of the stomach, so that dosage forms with a diameter of more than 10 mm cannot pass through. Frequent intake of meals results in a further delay of the interdigestive migrating motor complex, so that a dosage form that is taken after breakfast may be passed on to the duodenum only late at night.
The results of the in vivo study showed moderate differences in rate and extent of nifedipine relative bioavailability between Slofedipine® XL and Adalat OROS® under fasting conditions. Owing to a slightly delayed absorption from Slofedipine® XL in comparison with Adalat OROS® and a reduction in extent of nifedipine bioavailability bioequivalence between both formulations was not quite reached. However, under fed conditions the dissolution characteristics of Slofedipine® XL appeared to change completely. The most striking observation was that during the first 15 h after dosing with Slofedipine® XL virtually no drug absorption could be detected in the majority of subjects. Thus, the results of this in vivo study are compatible with the in vitro dissolution data. Since the formulation properties of Slofedipine® XL prevent dissolution or disintegration of the tablet in the acidic medium of the stomach, the pronounced lag-time can plausibly be explained by a prolonged gastric transit time.
In contrast, drug release from Adalat® OROS is unaffected by acidic media and consequently, dissolved nifedipine may leave the stomach even if the pylorus is closed. Thus, the relative bioavailability of nifedipine formulation in this way is not influenced by food.
In conclusion, the results of this in vivo study showed that after single dose administration pronounced differences in relative bioavailability of nifedipine from Slofedipine® XL could be detected when changing from fasted to fed administration. This phenomenon is formulation-dependent, as for Adalat OROS® no such food effect could be observed. The in vitro data allow the assumption that pH-dependent dissolution properties of Slofedipine® XL together with unpredictable gastric-transit times of the single unit dosage form are the underlying mechanisms of the delayed absorption. In patients, an increase in intraindividual variability together with the risk of additively increased maximum concentrations may occur when changing from fed to fasted administration. However, further investigations would be necessary to test this hypothesis, as results from a single dose study are difficult to extrapolate to those when the drug is given chronically.
Acknowledgments
The authors would like to acknowledge the support and co-operation of Bayer AG, Wuppertal, in this project. Furthermore, the authors would like to thank Sabine Krautwald and Gisela Zenker for excellent technical assistance during conduct of the clinical study.
References
- 1.Karim A, Burns T, Wearly L, Streicher J, Palmer M. Food-induced changes in theophylline absorption from controlled-release formulations. Part I. Substantial increased and decreased absorption with Uniphyl tablets and Theo-Dur Sprinkle. Clin Pharmacol Ther. 1985;38:77–83. doi: 10.1038/clpt.1985.138. [DOI] [PubMed] [Google Scholar]
- 2.Karim A, Burns T, Janky D, Hurwitz A. Food-induced changes in theophylline absorption from controlled-release formulations. Part II. Importance of meal composition and dosing time relative to meal intake in assessing changes in absorption. Clin Pharmacol Ther. 1985;38:642–647. doi: 10.1038/clpt.1985.238. [DOI] [PubMed] [Google Scholar]
- 3.Waldman SA, Morganroth J. Effect of food on the bioequivalence of different verapamil sustained-release formulations. J Clin Pharmacol. 1995;35:163–169. doi: 10.1002/j.1552-4604.1995.tb05006.x. [DOI] [PubMed] [Google Scholar]
- 4.Blume HH, Schug BS, Potthast H. Influence of food on the bioavailability of controlled/modified release products. In: Kuhlmann J, Weihrauch TR, editors. Clinical Pharmacology 12, Food–Drug Interactions. München, Bern, Wien, New York: Zuckschwerdt Verlag; 1996. pp. 25–32. [Google Scholar]
- 5.Modi NB, Wang B, Hu WT, Gupta SK. Effect of food on the pharmacokinetics of osmotic controlled-release methylphenidate HCl in healthy subjects. Biopharm Drug Dispos. 2000;21:23–31. doi: 10.1002/1099-081x(200001)21:1<23::aid-bdd212>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Committee for Proprietary Medicinal Products, the European Agency for the Evaluation of Medicinal Products; 2000. Note for Guidance on Modified Release Oral and Transdermal Dosage Forms: Section II (Pharmacokinetic and Clinical Evaluation) [Google Scholar]
- 7.US Department of Health and Human Services, Food and Drug Administration, CDER; 1997. Draft Guidance for Industry. Food-Effect Bioavailability and Bioequivalence Studies. [Google Scholar]
- 8.Shah VP, Midha KK, Dighe S, et al. Analytical methods validation. bioavailability, bioequivalence, and pharmacokinetic studies. J Pharm Sci. 1992;81:309–312. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- 9.Szurszewski JH. A migrating electric complex of the canine small intestine. Am J Physiol. 1969;217:1757–1763. doi: 10.1152/ajplegacy.1969.217.6.1757. [DOI] [PubMed] [Google Scholar]
- 10.Ewe K, Press AG, Bollen S, Schuhn I. Gastric emptying of indigestible tablets in relation to composition and time of ingestion of meals studied by metal detector. Digest Dis Sci. 1991;36:146–152. doi: 10.1007/BF01300748. [DOI] [PubMed] [Google Scholar]
- 11.Davis SS, Hardy JG, Fara JW. The transit of pharmaceutical dosage forms through the small intestine. Gut. 1986;27:886–892. doi: 10.1136/gut.27.8.886. [DOI] [PMC free article] [PubMed] [Google Scholar]