Abstract
Aims
The aim of this study was to assess the efficacy of dextromethorphan and ketamine relative to placebo on the acute nociceptive threshold and wind-up of second pain response in healthy male volunteers.
Methods
The trial was a randomized, double-blind, placebo-controlled, three period crossover, double dummy design in 12 healthy male volunteers. During each of the three periods (which were separated by a 1 week washout period) each volunteer received either a single oral dose of 0.7 mg kg−1 dextromethorphan and placebo to ketamine, or placebo to dextromethorphan followed by a single intravenous injection of 0.375 mg kg−1 ketamine, or placebo to both dextromethorphan and ketamine. The trial did not schedule administration of both ketamine and dextromethorphan together. Acute nociceptive thresholds and wind-up of second pain were measured in the skin of the thenar eminence of the ventral surfaces of the right and left hands, using a SOMEDIC™ thermotest apparatus, before and at the estimated tmax for dextromethorphan (i.e. 2.15 h). Blood pressure and heart rate were also monitored before dosing and after the dosing regimen.
Results
Neither dextromethorphan nor ketamine had any significant effect on acute nociceptive thresholds on either hand (P>0.05). Moreover, dextromethorphan was without any significant effect (P>0.05) on the wind-up of the second pain response on either hand. The lsmean number of stimuli tolerated vs placebo (95% confidence intervals of the difference in number of stimuli in parentheses) were 15.84 vs 16.48 (−5.52, 4.24) and 11.75 vs 15.25 (−11.89, 4.90) for left- and right-hand, respectively, following dextromethorphan administration. In contrast ketamine produced significant reductions in wind-up to second pain in both the left and right hands (P=0.0002 and 0.0386, respectively). The lsmean numbers of stimuli tolerated vs placebo (95% confidence intervals of the difference in number of stimuli in parentheses) were 28.41 vs 16.48 (6.60, 17.25) and 25.00 vs 15.25 (0.58, 18.93) for left- and right-hand, respectively.
Conclusions
Wind-up of second pain induced by noxious heat is sensitive to intervention by ketamine, which is known to block the NMDA receptor. These data infer that the wind-up phenomenon evoked by noxious heat involves the activation of NMDA receptors. This volunteer model of pain may have utility in the evaluation of agents that modulate their antinociceptive actions via NMDA mechanisms.
Keywords: antinociception, dextromethorphan, ketamine, NMDA-receptors, noxious heat
Introduction
A single noxious heat stimulus can evoke two successive distinct pain sensations known as ‘first’ and ‘second’ pain. The first, often described as ‘sharp’ or ‘pricking’ is well localized, occurs within about 0.4 s of the heat stimulus and is short in duration. It is followed approximately 1 s later by a second pain which is less well localized, of longer duration, being described as ‘burning’ ‘throbbing’ or ‘swelling’ [1].
Repeated noxious heat stimulations reduce the intensity of the first pain response whenever the interstimulus interval is 80 s or less and when the same area of skin is stimulated. Second pain perceptions, however, increase or ‘wind-up’ in intensity whenever the interstimulus interval is 3 s or less [2]. This ‘wind-up’ phenomenon is thought to involve sensitization of neurones in the dorsal horn of the spinal cord [3, 4], which may contribute to some of the chronic pain states such as neuropathic pain [5]. Further, evidence suggests that N-methyl-d-aspartate (NMDA)-mediated spinal reflexes are intimately involved as the pharmacological basis for this ‘wind-up’ process as demonstrated in animal models [5, 6] and human subjects [7–9]. These findings have led to the proposal that NMDA-receptor antagonists may act as antinociceptive agents [10] and, in support of this hypothesis, it has been shown preclinically that extracellular recordings taken from neurones within the dorsal horn show a cessation in wind-up activity following administration of NMDA antagonists [11, 12].
Ketamine and dextromethorphan (and dextrorphan, the active metabolite of dextromethorphan) are agents purported to possess NMDA-receptor antagonist properties [13] and have been shown to reduce thermal hyperalgesia in animal models of neuopathic pain [14, 15]. This trial was designed to further investigate the role of these putative NMDA-modulators (using ketamine and dextromethorphan) in the ‘wind-up’ phenomenon in man and to assess the utility of this volunteer pain model for the identification of potential therapeutic agents for those pain conditions thought to involve ‘wind-up’ as a mechanism.
Methods
Twelve, healthy, male volunteers (age range 21–50 years) were recruited from the Zeneca Pharmaceuticals healthy volunteer panel to take part in this trial. Health was confirmed on the basis of a normal medical examination and history, including clinical chemistry, haematology and urinalysis, respiratory function tests (FEV1 and FVC), 12-lead ECG and 24 h ambulatory cardiac monitoring. A negative test for hepatitis B surface antigen was also a prerequisite for this study. As part of the inclusion criteria, all volunteers were required to attend three separate screening sessions to familiarize themselves with the heat-pain apparatus.
All volunteers gave fully informed written consent prior to participation in the study, which was approved by the independent Zeneca Pharmaceuticals Research Ethics Committee. This trial conformed to the International Association for the Study of Pain ethical guidelines for research involving human subjects [16].
Study design
The study was double-blind, placebo-controlled, randomized three-period crossover design. During each of the three periods, which were separated by 1 week's washout, each volunteer received the following treatments:
single oral dose of 0.7 mg kg−1 dextromethorphan followed 1 h 40 min later by a single intravenous injection of 0.375 mg kg−1 ketamine;
single oral dose of placebo to dextromethorphan followed 1 h 40 min later by a single intravenous injection of 0.375 mg kg−1 ketamine;
single oral dose of placebo to dextromethorphan followed 1 h 40 min later by a single intravenous injection of placebo to ketamine
The trial did not assess the effect of combining dextromethorphan and ketamine together.
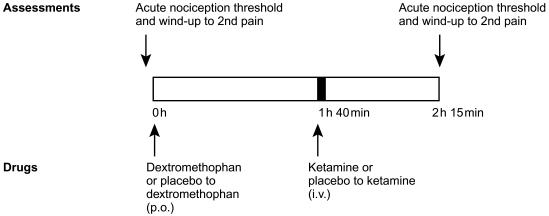
Heat pain thresholds and wind-up of second pain were measured in the skin of the thenar eminence of the ventral surfaces of the right and left hands, using a SOMEDIC™ thermotest apparatus, before and at the estimated tmax for dextromethorphan (i.e. 2 h 15 min) as illustrated in Figure 1. Blood pressure and heart rate were also monitored before dosing and after the dosing regimen. Volunteers underwent a medical examination within 14 days of completion of the trial.
Figure 1.
Schema representing the timing of administration of dextromethorphan (or placebo to dextromethorphan) and ketamine (or placebo to ketamine) with respect to the heat pain and wind-up of second pain assessments.
Heat-pain stimulation
The SOMEDIC™ Thermotest apparatus (Somedic AS, Stockholm, Sweden) was used to deliver quantifiable and reproducible heat impulses via a thermode to the thenar eminence of the ventral surface of the hand. The thermode temperature could be varied between 10 and 52° C and cools or warms depending on the direction of current applied.
Determination of the acute nociceptive threshold
The acute nociception threshold was determined by placing the thermode (set at 40° C) on the skin of the thenar eminence of the ventral surface of the volunteer's left hand. The temperature of the thermode was then increased at a rate of 0.5° C s−1 until the volunteer felt that the quality of the perceived sensation altered from warm to painful (typically between 45° C and 50° C [17]. At this point, the rise in temperature was terminated by the volunteers pressing the hand-held button. The temperature attained was recorded by the SOMEDIC™ Thermotest apparatus and the thermode temperature returned to 40° C. This procedure was repeated on three further occasions, the acute nociception threshold being the mean of the last two responses calculated by the SOMEDIC™ Thermotest apparatus. The entire process was then repeated using the volunteer's right hand.
The procedure to determine acute nociceptive threshold was then repeated for both hands but with the thermode set at 3° C below the acute nociceptive thresholds initially determined for both hands. The acute nociceptive thresholds determined on this second occasion were recorded as the definitive values of this parameter.
This procedure to determine acute nociceptive threshold was performed in three separate screening sessions (each 1 week apart) prior to entry into the drug phase of the trial (only those volunteers that demonstrated acute nociceptive thresholds of ≤48.5° C, were progressed further). During the trial, on each trial day, the procedure was performed prior to administration of drug treatment and at 2 h 15 min after dextromethorphan or placebo to dextromethorphan.
Determination of wind-up of second pain
Repeated noxious heat stimulations (from 3° C below to 3° C above the acute nociceptive threshold for that site for 0.5 s at 0.33 Hz), up to a maximum of 50, were administered to each hand, in turn. On each occasion, volunteers were asked to lift the thermode away from their skin when the augmentation of successive noxious heat pulses had resulted in their pain tolerance threshold. In no event was the number of stimuli allowed to exceed 50. The number of heat-stimuli tolerated by each hand was recorded.
This procedure to determine wind-up of second pain was performed in three separate screening sessions (each 1 week apart) prior to entry into the drug phase of the trial (only those volunteers who experienced wind-up of second pain were progressed further). During the trial, on each trial day, the procedure was performed prior to administration of drug treatment and at 2 h 15 min after dosing with dextromethorphan or placebo to dextromethorphan.
Pharmacokinetic assessments
Venous blood (20 ml) was collected before dosing and at 2 h 15 min after dosing with dextromethorphan or placebo to dextromethorphan. Plasma samples were then analysed by high performance liquid chromatography for concentrations of ketamine, dextromethorphan and dextorphan. The limit of quantification for these assays was 1 ng ml−1.
Safety assessments
On each trial day a pulse-oximeter was used to continuously display the oxygen saturation of the volunteers over a 35 min period from the start of infusion of ketamine or placebo to ketamine. Supine blood pressure and pulse rate was measured on each trial day after the volunteer had been lying for 10 min, before dosing, at minute intervals for the 5 min of ketamine or placebo to ketamine infusion thereafter at 4 and 6 h postdosing. 12-lead ECGs were also obtained on each trial day after 10 min supine.
Drug supply
Ketamine was supplied as Ketalar™ 50 mg ml−1 in 10 ml vials from Parke-Davis Research Laoratories, UK. Placebo to ketamine was 0.9% (w/v) saline (50 ml) from Baxter Healthcare Ltd, UK. Dextromethorphan was supplied as Robbitussin™ Dry Cough Mixture 7.5 mg/5 ml (100 ml) from Whitehall Laboratories Ltd, UK and placebo to dextromethorphan was supplied as Paediatric Simple Linctus (100 ml) from Thornton and Ross Ltd, UK.
Data analysis
The effects of dextromethorphan and ketamine relative to placebo on the acute nociceptive threshold and wind-up of second pain were assessed by using an analysis of variance (anova) model, with factors fitted for the effects of volunteers, periods and treatments. The results of the anova were presented in terms of least squares means (lsmeans) and treatment effect (lsmean of dextromethorphan/ketamine minus lsmean of placebo). 95% confidence intervals and associated P values were also presented for the treatment effect. In all analyses, the assumptions of normality and constant variance were investigated using residual plots. The distribution of residuals was sufficiently normal to allow a valid anova.
Results
One volunteer was withdrawn from the trial as an error in the application of his randomized treatment on the second trial day led to him receiving both ketamine and dextromethorphan on the same day. Several protocol deviations (Table 1) occurred during the conduct of this trial which led to some of the data being excluded from the statistical analyses. Hence, the ‘n’ sizes range between 9 and 11.
Table 1.
Deviations that led to exclusion of data from the statistical analyses.
| Deviation | Volunteer number | Treatment | Timepoint |
|---|---|---|---|
| Dosing error – volunteer received the infusion prepared for volunteer 0004. Hence, volunteer received both de xtromethorphan and ketamineon the same trial day | 0001 | Dextromethorphan | NA |
| Different thermode used, which was | 0001 | Dextromethorphan | 2 h 15 min |
| spontaneously reported (4 out of 6 of | 0002 | Ketamine | Pre-dose and 2 h 15 min |
| the affected volunteers) to feel less hot | 0003 | Placebo | Pre-dose and 2 h 15 min |
| than the original thermode used when | 0004 | Ketamine | Pre-dose and 2 h 15 min |
| determining their heat pain thresholds | 0005 | Dextromethorphan | Pre-dose |
| and wind up | 0006 | Placebo | Pre-dose |
Variability of the model
In a pilot study, which preceded this current investigation, formal statistical analysis of the variability of this model was fully investigated in 12 subjects. The within-subject variances (standard deviation2) on 2 study days, 1 week apart, for the two parameters used as endpoints in the current study (heat threshold and number of stimuli tolerated) are shown in Table 2.
Table 2.
Variance (standard deviation2) of heat pain threshold and number of stimuli tolerated.
| Study day | |||
|---|---|---|---|
| Site and time of assessment | 1 | 2 | |
| Heat pain threshold (°C) | |||
| Left hand | 0 and 2 h | 0.18 | 0.33 |
| 0 and 6 h | 0.27 | 0.20 | |
| Right hand | 0 and 2 h | 0.18 | 0.20 |
| 0 and 6 h | 0.55 | 0.24 | |
| Number of stimuli tolerated | |||
| Left hand | 0 and 2 h | 11.9 | 4.6 |
| 0 and 6 h | 15.1 | 3.3 | |
| Right hand | 0 and 2 h | 12.6 | 6.8 |
| 0 and 6 h | 6.5 | 2.6 | |
Acute nociceptive threshold
Neither ketamine nor dextromethorphan produced any significant change (P>0.05) in the heat pain threshold of either the right or left hands or the mean value of both hands combined (Tables 3 and 4).
Table 3.
Analysis of acute nociceptive threshold (°C) – effect of dextromethorphan.
| Dextromethorphan | Placebo | Treatment | |||||
|---|---|---|---|---|---|---|---|
| n | lsmean | n | lsmean | effect | 95% CI | P value | |
| Left hand | 11 | 47.50 | 10 | 47.46 | 0.04 | −0.41, 0.50 | 0.8480 |
| Right hand | 11 | 47.54 | 10 | 47.62 | −0.08 | −0.56, 0.41 | 0.7315 |
| Mean hands | 11 | 47.52 | 10 | 47.54 | −0.02 | −0.40, 0.36 | 0.9165 |
Table 4.
Analysis of acute nociceptive threshold (°C) – effect of ketamine.
| Ketamine | Placebo | Treatment | |||||
|---|---|---|---|---|---|---|---|
| n | lsmean | n | lsmean | effect | 95% CI | P value | |
| Left hand | 9 | 47.64 | 10 | 47.46 | 0.18 | −0.32, 0.67 | 0.4606 |
| Right hand | 9 | 47.90 | 10 | 47.62 | 0.28 | −0.25, 0.81 | 0.2708 |
| Mean hands | 9 | 47.77 | 10 | 47.54 | 0.23 | −0.18, 0.65 | 0.2542 |
Wind-up of second pain
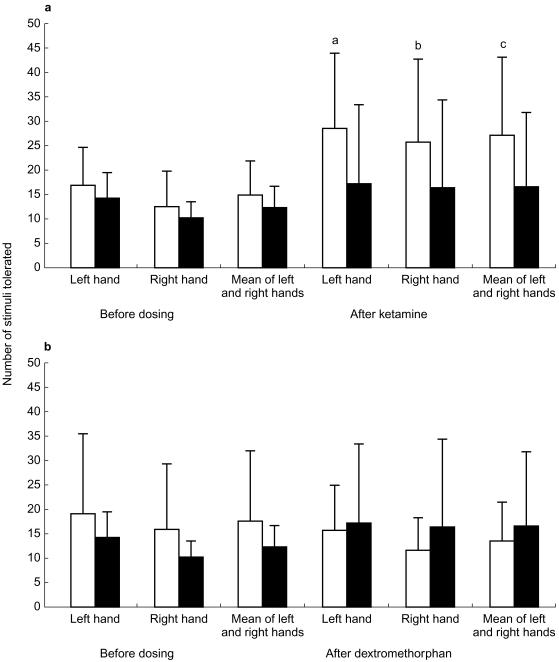
Dextromethorphan produced no significant effect (P>0.05) on the number of stimuli tolerated on either the left or right hand or the mean value of both hands combined (Table 5). In contrast, ketamine produced a highly significant increase in the number of stimuli tolerated by the left hand, right hand or the mean of both hands combined (P=0.0002, 0.0386 and 0.0009, respectively; Figure 2 and Table 6).
Table 5.
Analysis of number of stimuli tolerated – effect of dextromethorphan.
| Dextromethorphan | Placebo | Treatment | |||||
|---|---|---|---|---|---|---|---|
| n | lsmean | n | lsmean | effect | 95% CI | P value | |
| Left hand | 11 | 15.84 | 10 | 16.48 | −0.64 | −5.52, 4.24 | 0.7834 |
| Right hand | 11 | 11.75 | 10 | 15.25 | −3.50 | −11.89, 4.90 | 0.3885 |
| Mean hands | 11 | 13.80 | 10 | 15.87 | −2.07 | −7.21, 3.07 | 0.4043 |
Figure 2.
a) Effects of ketamine (□) compared with placebo (▪) on number of heat stimuli tolerated on either the right or left hand or the mean of both hands combined. Values shown are the lsmeans (±s.d.) obtained from 9 (ketamine) or 10 (placebo) volunteers.a P=0.0002;b P=0.035;c P=0.0009. b) Effects of dextromethorphan (□) compared with placebo (▪) on number of heat stimuli tolerated on either the right or left hand or the mean of both hands combined. Values shown are the lsmeans (±s.d.) obtained from 11 (dextromethorphan) or 10 (placebo) volunteers. None of the values was significantly different from placebo (P>0.05).
Table 6.
Analysis of number of stimuli tolerated – effect of ketamine.
| Ketamine | Placebo | Treatment | |||||
|---|---|---|---|---|---|---|---|
| n | lsmean | n | lsmean | effect | 95% CI | P value | |
| Left hand | 9 | 28.41 | 10 | 16.48 | 11.92 | 6.60, 17.25 | 0.0002 |
| Right hand | 9 | 25.00 | 10 | 15.25 | 9.76 | 0.58, 18.93 | 0.0386 |
| Mean hands | 9 | 26.71 | 10 | 15.87 | 10.84 | 5.22, 16.45 | 0.0009 |
Adverse events
There were no serious adverse events and only minor adverse events were reported. Three of the volunteers dosed with dextromethorphan reported three adverse events of headache, somnolence and right shoulder pain. All volunteers dosed with ketamine reported a total of 35 adverse events. The commonest of these events accounting for 33 out of the 35 reported were paresthesia (10 events in 10 volunteers), alterations in mood (either euphoria [6 events in 6 volunteers] or dysphoria [4 events in 4 volunteers]), dizziness (5 events in 5 volunteers), somnolence/asthenia/clouding of consciousness (6 events in 4 volunteers) and nystagmus (2 events in 2 volunteers). Typically, these adverse events occurred within minutes of commencing the ketamine infusion, and ceased within minutes of terminating the ketamine infusion.
Plasma concentrations of ketamine, dextromethorphan and dextrorphan
Geometric mean (minimum and maximum) plasma concentrations of ketamine, dextromethorphan and dextrorphan at 2 h 15 min after dosing with dextromethorphan were 72.25 (53.02, 100.04), 2.60 (not quantifiable, 26.62) and 711.87 (157.48, 1235.44) ng ml−1, respectively (conversion multiplication factors to µm are 0.0036, 0.0037 and 0.0039, respectively).
Discussion
The model utilized for the current study is a reliable and robust model with clear evidence of low variance using a design that allows subjects to be employed as the own controls by crossing over the periods of treatment (Table 2). When within subject variance is assessed by taking the mean variance of both hands for the heat pain threshold and number of stimuli tolerated, these values are 0.18 and 6.89, respectively. In terms of identifying an effect, these data relate to 12 subjects having a greater than 90% chance of detecting a true difference of ±4 in the number of stimuli tolerated and a true difference of 0.65° C in heat pain threshold taking the mean of both hands, between dextromethorphan and placebo and between ketamine and placebo using a two-sided test.
Unfortunately, we have not included any measures of variability to assess the effect of ‘handedness’ in the current study, and are not able to comment on how hand-dominance would impact on the findings.
There is now much evidence that NMDA receptors play a key role in chronic pain states and hyperalgesia [10, 18] and agents such as ketamine [19] and dextromethorphan [8, 20, 21] are finding use for this indication. This current study has shown that ketamine, but not dextromethorphan (60 mg), can interfere with the mechanisms responsible for the wind-up associated with ‘second-pain’ induced by heat. There was no effect with either ketamine or dextromethorphan on the acute nociceptive threshold, indicating that the acute nociceptive mechanisms were not influenced by the NMDA-modulators ketamine and dextromethorphan. Moreover in terms of ensuring the ‘blindness’ of the treatments as both ketamine and dextromethorphan can produce unwanted central nervous system side-effects, it was interesting to note that although these effects were apparent and that subjects may have realized that they had taken something other than a placebo, the fact that acute nociceptive threshold was unaffected (Tables 3 and 4) suggests that psycho-suggestive effects were not influencing the reaction of the subjects to the pain stimulus. In terms of absolute blinding of the placebo to dextromethorphan, this was not possible as the latter substance was not identical in terms of its constituents compared with Robbitussin™ Dry Cough Mixture, however, the taste and colour of the materials in both preparations were very similar. Moreover, as the subjects had at least 1 week interval between taking either the Paediatric Simple Linctus or Robbitussin™ Dry Cough Mixture, this made the likelihood of a difference being noticed very unlikely.
The second pain elicited by repeated heat stimuli evokes sensitization of neurones in the dorsal horn of the spinal cord [3, 4]. Animal studies have shown that NMDA receptors in the dorsal horn play an important role in the generation of central sensitization [5, 6]. The repeated stimulation of c-fibres by noxious heat leads to a progressive increase in the firing rate of the dorsal horn neurones. This phenomenon can be prevented in animals by the administration of NMDA receptor antagonists [11, 12]. Ketamine is classed as a noncompetitive NMDA antagonist [22] that has been shown in man to eliminate secondary hyperalgesia induced by noxious heat, to both brush and punctate stimuli, when administered at a low dose (0.15 mg kg−1 iv over 10 min [23]). The dose of ketamine used in the present study (0.375 mg kg−1) was somewhat higher than that employed by Warnecke et al. [23], however, ketamine clearly prevented the pain phenomenon associated with wind-up without affecting acute nociceptive perception per se as determined by heat pain thresholds. Moreover, the side-effect profile with ketamine in the current study was very similar to that reported by Warnecke et al. [23] where a lower dose of ketamine was employed. Park et al. [9] used similar doses to that used in the current study and were able to demonstrate a reduction in pin-prick hyperalgesia and ongoing pain following intradermal injections of capsaicin in healthy volunteers. The dose employed in this study is similar to those utilized in the clinical relief of acute postoperative pain [24–26] and we achieved plasma concentrations approximating those associated with clinically meaningful pain relief [27], but not associated with CNS-adverse effects [28].
Dextromethorphan has been cited as an NMDA antagonist [29, 30] but also has been shown to possess affinity for serotonin receptors at relatively high concentrations compared with its affinity for NMDA receptors (e.g. half-maximum inhibitory concentration to inhibit effects at the serotonin 1A-receptor was 14.3 µm [31] vs between 2 and 0.698 µm to half-maximally block cloned rat NMDA-receptors [32]). Moreover, the effects of dextromethorphan on centrally located serotonin receptors may be to produce an indirect modulation of NMDA receptors [33].
Dextromethorphan, in contrast to ketamine, was without significant antinociceptive activity in this current study, despite using doses which are in the high range of antitussive doses [43]. There is evidence that the agent produces antinociceptive activity in both animal [14], and human-pain models [20] although its efficacy in the clinic is less well defined [34]. Indeed, doses in excess of 300 mg dextromethorphan have been reported to be necessary before meaningful clinical analgesia is noted [35, 36]. However, at doses between 300 and 600 mg, side-effects then need to be managed appropriately and, further, these dose-levels equate to approximately 4–17 µm plasma dextrorphan [32] which may be high enough to induce non-NMDA-mediated effects. Clearly, titration to reduce the side-effect liability and any lack of selectivity, is required. The maximum well-tolerated single dose of dextromethorphan of approximately 60 mg [37–41] was the dose employed in this study. Whilst this dose was similar to that used by Price et al. [20] who demonstrated attenuation of temporal summation to second pain with 30 and 45 mg dextromethorphan, doses of 40–80 mg have not been found to be associated with meaningful pain relief [42]. We were not able to demonstrate any analgesic effect at these doses in the volunteer model of pain. Clearly, it would be interesting to further investigate the effects of 300 mg dextromethorphan in this pain model following a period of dose titration, however, it would then need caution to fully explain any positive findings.
Nevertheless, we consider this model to be a well-tolerated and useful model for profiling putative NMDA antagonists in early phase clinical development.
References
- 1.Cross SA. Pathophysiology of pain. Mayo Clinic Proc. 1994;69:375–383. doi: 10.1016/s0025-6196(12)62225-3. [DOI] [PubMed] [Google Scholar]
- 2.Price DD, Wu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 3.LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia; psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 4.LaMotte RH, Lundberg LER, Torebjörk HE. Pain hyperalgesia and activity in nociceptive C units in humans after intradermal capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf CJ, Thompson SWN. The induction and maintainence of central sensitisation is dependent on N-methyl-d-aspartic acid receptor activity; implication for treatment of post-injury pain hypersenstivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 6.Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-d-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- 7.Guirimand F, Dupont X, Brasseur L, Chauvin M, Bouhassira D. The effects of ketamine on the the temporal summation (wind up) of the R (III) nociceptive flexion reflex and pain in humans. Anesthes Analges. 2000;90:408–414. doi: 10.1097/00000539-200002000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Ilkjaer S, Petersen KL, Bennum J, Wernberg M, Dahl JB. Effect of systemic N-methyl-d-aspartate receptor antagonist (ketamine) on primary and secondary hyperalgesia in humans. Br J Anaesth. 1996;76:829–834. doi: 10.1093/bja/76.6.829. [DOI] [PubMed] [Google Scholar]
- 9.Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pin-prick hyperalgesia and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–172. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- 10.Dickenson AHA. cure for wind up: NMDA receptor antagonists as potential analgesics. Trends Pharmacol Sci. 1990;11:307–330. doi: 10.1016/0165-6147(90)90228-z. [DOI] [PubMed] [Google Scholar]
- 11.Davies SN, Lodge D. Evidence for involvement of N-methyl-d-aspartate receptors in ‘wind-up’ of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- 12.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency-dependent potentiation of deep rat dorsal horn nociceptive neurones following C-fibre stimulation. Neuropharmacol. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 13.Dickenson AH, Sullivan AF. Combination therapy in analgesia; seeking synergy. Curr Opin Anaesthiol. 1993;6:861–865. [Google Scholar]
- 14.Mao J, Price DD, Hayes RL, Lu J, Mayer DJ, Frenck H. Intrathecal treatment with dextrorphan or ketamine reduces pain-related behaviours in a rat model of peripheral mononeuropathy. Brain Res. 1993;605:164–168. doi: 10.1016/0006-8993(93)91368-3. [DOI] [PubMed] [Google Scholar]
- 15.Tai M, Bennett G. Dextrorphan relieves neuropathic heat-induced hyperalgesia in the rat. Neurosci Lett. 1993;151:107–110. doi: 10.1016/0304-3940(93)90058-s. [DOI] [PubMed] [Google Scholar]
- 16.Charlton E. Ethical guidelines for pain research in humans. Pain. 1995;63:277–278. doi: 10.1016/0304-3959(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 17.Meh D, Denislic M. Quantitative assessment of thermal and pain sensitivity. J Neurol Sci. 1994;127:164–169. doi: 10.1016/0022-510x(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 18.Coderre TJ. The role of excitatory amino acid receptors and intracellular messengers in persistent nociception after tissue injury in rats. Mol Neurobiol. 1993;7:229–246. doi: 10.1007/BF02769177. [DOI] [PubMed] [Google Scholar]
- 19.Eide PK, Jørum E, Stubhaug A, Bremnes J, Breivik H. Relief of post-herpetic neuralgia with the N-methyl-d-aspartate acid receptor antagonist ketamine: a doubleblind, cross-over comparison with morphine and placebo. Pain. 1994;58:347–354. doi: 10.1016/0304-3959(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 20.Price DD, Mao J, Frenck H, Mayer DJ, The N. -methyl-d-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 21.Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 22.Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- 23.Warnecke T, Stubhaug A, Jørum E. Ketamine, an NMDA. Receptor antagonist, suppresses spatial and temporal properties of burn-induced secondary hyperalgesia in ma: a double blind, cross-over comparison with morphine and placebo. Pain. 1997;72:99–106. doi: 10.1016/s0304-3959(97)00006-7. [DOI] [PubMed] [Google Scholar]
- 24.Maurset A, Skoglund LA, Hustveit O, Øye I. Comparison of ketamine and pethidine in experimental and post-operative pain. Pain. 1989;36:37–41. doi: 10.1016/0304-3959(89)90109-7. [DOI] [PubMed] [Google Scholar]
- 25.Kohrs R, Durieux ME. Ketamine. Teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 26.Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82:111–125. doi: 10.1016/S0304-3959(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 27.Clements JA, Nimmo WS. Pharmacokinetics and analgesic effects of ketamine in man. Br J Anaesth. 1981;53:27–30. doi: 10.1093/bja/53.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Hartvig P, Valtysson J, Linder K-J, et al. Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther. 1995;58:165–173. doi: 10.1016/0009-9236(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 29.Sang CN. NMDA-receptor antagonists in neuropathic pain: experimental methods to clinical trials. J Pain Symptom Manage. 2000;19:S21–S25. doi: 10.1016/s0885-3924(99)00125-6. [DOI] [PubMed] [Google Scholar]
- 30.Bennett G. Update on the neurophysiology of pain transmission and modulation: focus on the NMDA-receptor. J Pain Sympt Manage. 2000;19:S2–S6. doi: 10.1016/s0885-3924(99)00120-7. [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi H, Kuwano K, Takahama K. Inhibition of the 5 HT (1A) receptor-mediated inwardly rectifying K (+) current by dextromethorphan in rat dorsal raphe neurones. Neuropharmacol. 2000;39:2302–2308. doi: 10.1016/s0028-3908(00)00092-7. [DOI] [PubMed] [Google Scholar]
- 32.Fisher K, Coderre TJ, Hagen NA. Targetting the N-methyl-D-aspartate receptor for chronic pain management. pre-clinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20:358–373. doi: 10.1016/s0885-3924(00)00213-x. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Park IS, Park WK. NMDA receptor antagonists enhance 5-HT2 receptor-mediated behaviour, head-twitch response in mice. Life Sci. 1998;63:2305–2311. doi: 10.1016/s0024-3205(98)00519-0. [DOI] [PubMed] [Google Scholar]
- 34.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 35.Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48:1212–1218. doi: 10.1212/wnl.48.5.1212. [DOI] [PubMed] [Google Scholar]
- 36.Gilron I, Booher SL, Rowan MS, Smoller MS, Max MBA. Randomized, controlled trial of high-dose dextromethorphan in facial neuralgias. Neurology. 2000;55:964–971. doi: 10.1212/wnl.55.7.964. [DOI] [PubMed] [Google Scholar]
- 37.Silvasti M, Karttunen P, Tukiainen H, Kokkonen P, Hänninen U, Nykänen S. Pharmacokinetics of dextromethorphan. A single-dose comparison of three preparations in human volunteers. Int J Clin Pharmacol Ther Toxicol. 1987;25:493–497. [PubMed] [Google Scholar]
- 38.Silvasti M, Karttunen P, Happonen P, Mykkänen M, Romppanen T, Tukiainen H. Pharmacokinetic comparison of dextromethorphan-salbutamol combination tablet and a plain dextromethorphan tablet. Int J Clin Pharmacol Ther Toxicol. 1990;28:268–272. [PubMed] [Google Scholar]
- 39.Walker FO, Hunt VP. An open trial of dextromethorphan in Huntingdon's disease. Clin Neuropharmacol. 1989;12:322–330. doi: 10.1097/00002826-198908000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Vetticaden ST, Cabana BE, Prasad VK, et al. Phenotypic differences in dextromethorphan metabolism. Pharm Res. 1989;6:9–13. doi: 10.1023/a:1015835215945. [DOI] [PubMed] [Google Scholar]
- 41.Bem JL, Peck R. Dextromethorphan. An overview of safety issues. Drug Safety. 1992;7:190–199. doi: 10.2165/00002018-199207030-00004. [DOI] [PubMed] [Google Scholar]
- 42.McQuay HJ, Carroll D, Jadad AR, et al. Dextromethorphan for the treatment of neuropathic pain: a double-blind randomised controlled crossover trial with integral n-of-1 design. Pain. 1994;59:127–133. doi: 10.1016/0304-3959(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 43.ABPI. Compendium of data sheets and summaries of product characteristics.