Abstract
Aims
Ayahuasca is a traditional South American psychoactive beverage used in Amazonian shamanism, and in the religious ceremonies of Brazilian-based syncretic religious groups with followers in the US and several European countries. This tea contains measurable amounts of the psychotropic indole N,N-dimethyltryptamine (DMT), and β-carboline alkaloids with MAO-inhibiting properties. In a previous report we described a profile of stimulant and psychedelic effects for ayahuasca as measured by subjective report self-assessment instruments. In the present study the cerebral bioavailability and time-course of effects of ayahuasca were assessed in humans by means of topographic quantitative-electroencephalography (q-EEG), a noninvasive method measuring drug-induced variations in brain electrical activity.
Methods
Two doses (one low and one high) of encapsulated freeze-dried ayahuasca, equivalent to 0.6 and 0.85 mg DMT kg−1 body weight, were administered to 18 healthy volunteers with previous experience in psychedelic drug use in a double-blind crossover placebo-controlled clinical trial. Nineteen-lead recordings were undertaken from baseline to 8 h after administration. Subjective effects were measured by means of the Hallucinogen Rating Scale (HRS).
Results
Ayahuasca induced a pattern of psychoactive effects which resulted in significant dose-dependent increases in all subscales of the HRS, and in significant and dose-dependent modifications of brain electrical activity. Absolute power decreased in all frequency bands, most prominently in the theta band. Mean absolute power decreases (95% CI) at a representative lead (P3) 90 min after the high dose were −20.20±15.23 µV2 and −2.70±2.21 µV2 for total power and theta power, respectively. Relative power decreased in the delta (−1.20±1.31% after 120 min at P3) and theta (−3.30±2.59% after 120 min at P3) bands, and increased in the beta band, most prominently in the faster beta-3 (1.00±0.88% after 90 min at P3) and beta-4 (0.30±0.24% after 90 min at P3) subbands. Finally, an increase was also seen for the centroid of the total activity and its deviation. EEG modifications began as early as 15–30 min, reached a peak between 45 and 120 min and decreased thereafter to return to baseline levels at 4–6 h after administration.
Conclusions
The central effects of ayahuasca could be objectively measured by means of q-EEG, showing a time pattern which closely paralleled that of previously reported subjective effects. The modifications seen for the individual q-EEG variables were in line with those previously described for other serotonergic psychedelics and share some features with the profile of effects shown by pro-serotonergic and pro-dopaminergic drugs. The q-EEG profile supports the role of 5-HT2 and dopamine D2-receptor agonism in mediating the effects of ayahuasca on the central nervous system.
Keywords: ayahuasca, DMT, pharmaco-EEG, psychedelics, topography
Introduction
Ayahuasca is the Quechuan name for both the Amazon woody vine Banisteriopsis caapi (Malpighiaceae) and the sacred psychoactive beverage obtained from it. The beverage, also known by the names Yajé, Natema, Santo Daime and Vegetal, has been used throughout the Amazon Basin by shamans and healers since pre-Columbian times for medicinal purposes and as a means to contact the supernatural [1, 2]. More recently, syncretic religions combining the use of ayahuasca with Christian beliefs, particularly the Santo Daime and the União do Vegetal, have been established in Brazil, where they enjoy legal protection. Outside Brazil, smaller groups of followers have begun to consume the tea in the United States and in several European countries, including Germany, Great Britain, Holland, France and Spain [3]. Even though the number of users is still relatively small, adverse reactions associated with the simultaneous use of ayahuasca and other centrally active drugs have raised concern for public health [4], and extensive clinical data on its somatic, psychological and neurophysiological effects are warranted.
Banisteriopsis caapi, the basic ingredient of the beverage, is seldom found alone in ayahuasca. The tea is generally obtained by infusing the stems of the vine together with the leaves of other plants, namely Psychotria viridis (Rubiaceae) or Diplopterys cabrerana (Malpighiaceae) [5]. Chemical analyses have shown that B. caapi contains notable amounts of β-carboline alkaloids, mainly harmine and tetrahydroharmine (THH), followed by harmaline and trace amounts of harmol [5, 6]. P. viridis and D. cabrerana also contain indole alkaloids, mainly the potent short-acting psychedelic agent N,N-dimethyltryptamine (DMT) [5, 7].
This combination of B. caapi and P. viridis in a single oral preparation is a remarkable achievement of empirical ethnopharmacological knowledge, as psychoactivity arises from combining the pharmacodynamic actions of the β-carbolines and of DMT. Similarly to other indole and phenethylamine psychedelics such as LSD and mescaline [8], DMT shows affinity for the 5-HT2A/2C receptor sites in the central nervous system (CNS), where it displays agonist activity [9]. However, unlike most psychedelics, DMT is a priori only active when parenterally administered, because the oral ingestion of the drug alone leads to its metabolic breakdown by the enzyme monoamine oxidase (MAO) [10]. Interestingly, harmine and harmaline, and to a lesser extent THH, are potent MAO inhibitors [6]. Thus, it is widely accepted that the MAO-inhibiting action of the β-carbolines present in the tea allows the viable access of DMT to the systemic circulation and the CNS. In addition to facilitating a direct agonist action of DMT at the 5-HT2A/2C sites, the MAO-inhibiting properties of the β-carbolines may contribute to the overall effects of ayahuasca, firstly, by prolonging the effects of DMT due to its decreased metabolism, and secondly, by simultaneously enhancing the levels of endogenous catecholamines and serotonin [11].
In a previous study conducted to characterize the tolerability and psychological effect profile of ayahuasca [12], this tea was found to induce a pattern of psychostimulant and psychedelic effects, which qualitatively resembled those of other classical serotonergic agents, such as psilocybin, and parenteral DMT [13, 14]. Ayahuasca was able to induce dose-dependent perceptual, cognitive and affective modifications, with a milder intensity and longer duration than those previously described for intravenous DMT [14], but with an overall duration shorter than that of better characterized psychedelics such as LSD or mescaline [15].
The aim of the present study was to assess the central actions of ayahuasca by means of quantitative-electroencephalography (q-EEG), an objective noninvasive method used to evaluate drug effects on the CNS with high temporal resolution [16]. We intended thus to demonstrate its cerebral bioavailability and subsequent psychoactivity by means other than subjective self-report instruments, and implementing a double-blind randomised placebo-controlled design. Recordings of brain electrical activity were carried out before and at different time points after the administration of two different doses of encapsulated freeze-dried ayahuasca to a group of healthy volunteers with previous experience in the use of psychedelics.
Methods
Volunteers
Eighteen healthy volunteers (15 males and three females) with no current or previous history of neurological or psychiatric disorder and no family history of Axis-I psychiatric disorder in first degree relatives were included in the study. Eligibility criteria included prior experience with psychedelic drugs at least on five occasions without sequelae derived therefrom. The volunteers were given a structured psychiatric interview (DSM-III-R) and completed the trait-anxiety scale from the State-Trait Anxiety Inventory [17]. Exclusion criteria included a present or past history of Axis-I disorders and alcohol or other substance dependence, and high scores on trait anxiety. Volunteers were given a complete physical examination that included medical history, laboratory tests, ECG and urinalysis. All volunteers gave their written informed consent to participate. Mean age was 25.7 years (range: 19–38), mean weight 66.47 kg (range: 50.7–79.5) and mean height 175.11 cm (range: 158–188). In addition to their prior intake of psychedelics, all volunteers had previous experience with cannabis and cocaine. Although prior exposure to ayahuasca was not required for participation, two of the volunteers had ingested this tea before inclusion. The study was conducted in accordance with the Declarations of Helsinki and Tokyo concerning experimentation on humans, and was approved by the hospital's ethics committee and the Spanish Ministry of Health. The volunteers received detailed information on the nature of ayahuasca, the general psychological effects of psychedelics and their possible adverse effects, as reported in the psychiatric literature.
Drug
The ayahuasca doses administered to the volunteers in the present study as the low and the high dose were the equivalent to 0.6 and 0.85 mg DMT kg−1 body weight. These doses were chosen based on tolerability and subjective effect data gathered in a previous study [12]. The ayahuasca was not administered in its original liquid form, but as a liophilizate. The DMT contents in the liophilizate had been determined by h.p.l.c., as described by Callaway and coworkers [18], and the β-carboline constituents following a modification of the method described therein. The concentrations found were: 8.33 mg DMT, 14.13 mg harmine, 0.96 mg harmaline and 11.36 mg THH per gram of freeze-dried material. These alkaloid contents corresponded to the following concentrations in the original tea: DMT 0.53 mg ml−1, harmine 0.90 mg ml−1, harmaline 0.06 mg ml−1 and THH 0.72 mg ml−1. The calculated individual dose for each volunteer was administered by combining 00 gelatin capsules containing 0.5 g, 0.25 g or 0.125 g of freeze-dried ayahuasca and placebo capsules containing 0.75 g lactose. Placebo capsules were added when necessary, so that all volunteers took the same number of capsules on each experimental day.
Study design and experimental procedure
The volunteers participated in four experimental sessions. Volunteers were informed that they would randomly receive on each experimental day a single oral dose of encapsulated freeze-dried ayahuasca (one low and one high dose), a placebo and a random repetition of one of the three mentioned treatments. In actual fact they all received a placebo on the first experimental day in a single-blind fashion, followed by one of the three treatments from days 2 to 4 in a double-blind balanced fashion, according to a randomization table. The first nonrandomized placebo was administered in order to familiarize the volunteers with the experimental setting and to minimize the stress associated with the experimental interventions. Two weeks prior to the beginning of the experimental sessions, volunteers were requested to abstain from any medication or illicit drug until the completion of the study. Volunteers also abstained from alcohol, tobacco and caffeinated drinks 24 h prior to each experimental day. Urinalysis for illicit drug use was performed for each experimental session and was found negative for amphetamines, cocaine, opioids, benzodiazepines and alcohol. A 7 day washout period was established between experimental days.
On each experimental day participants arrived in the laboratory in the morning under fasting conditions. EEG electrodes were placed on the scalp and treatment capsules were administered at approximately 10.00 h with 250 ml tap water. EEG recordings were obtained at baseline and at regular intervals after treatment administration. The experimental sessions were undertaken in a quiet and dimly lit room with the volunteers seated in a reclining chair. The experimenter remained outside the room during the EEG recordings. At 4 h after administration of the capsules, when the most prominent subjective effects associated with the drug had disappeared, the volunteers answered subjective effect questionnaires, and had a meal. The last recording was performed at 8 h and volunteers were discharged approximately 9 h after drug administration.
Measurements
EEG acquisition and analysis
EEG recordings were obtained through 19 electrodes placed on the scalp according to the international 10/20 system on the following locations: Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1 and O2, referenced to averaged mastoids by means of a Neuroscan SYNAMPS amplifier. Additionally, vertical and horizontal electrooculograms (EOG) were recorded. Vigilance controlled EEG (V-EEG) for 3 min with eyes closed was recorded at −15 (PRE-1), baseline (PRE-2), +15, +30, +45, +60, +90, +120, +150, +180, +210, +240, +360 and +480 min from drug administration. During the V-EEG recordings, the experimenter tried to keep the volunteers alert; as soon as drowsiness patterns appeared in the EEG they were aroused by acoustic stimulation. The EEG signal was recorded using high-pass and low-pass filters of 0.3 Hz and 30 Hz, respectively, and digitized online with a sampling frequency of 100 Hz.
A two-step artefact processing procedure was used. It included ocular artifact minimization based on regression analysis in the time domain, as described by Semlitsch et al. [19], and automatic artifact rejection based on a time and frequency domain approach as described by Anderer et al. [20]. Subsequently, validity of the artifact processing procedure was visually inspected [21].
After recomputation to average reference, spectral analysis was performed for artefact-free 5 s epochs, resulting in a frequency resolution of 0.2 Hz. The spectral density curves for all artifact-free EEG epochs were averaged for a particular experimental situation. These mean spectral curves, containing data from 1.3 to 30 Hz, were quantified into 34 target variables: total power, absolute and relative power in 11 different frequency bands (delta [1.3–3.5 Hz], theta [3.5–7.5 Hz], alpha-1 [7.5–10.5 Hz], alpha-2 [10.5–13 Hz], beta-1 [13–16 Hz], beta-2 [16–20 Hz], beta-3 [20–25 Hz], beta-4 [25–30 Hz], combined delta-theta, alpha and beta), the dominant frequency in Hz, absolute and relative power of the dominant frequency, the centre-of-gravity frequency (centroids) and the frequency variability (centroid deviations) of the combined delta-theta, alpha and beta bands as well as of the total activity. Additionally, the vigilance alpha/delta-theta index was also calculated.
Topographic maps were computed by cubic interpolation of the values of the four nearest electrodes.
Subjective ratings
Volunteers were requested to answer the Hallucinogen Rating Scale (HRS), a self-report questionnaire specifically developed to quantify different aspects of psychedelic-induced subjective effects. The questionnaire includes six subscales: Somaesthesia, reflecting somatic effects; Affect, sensitive to emotional and affective responses; Volition, indicating the volunteer's capacity to willfully interact with his/her ‘self’ and/or the environment; Cognition, describing modifications in thought processes or content; Perception, measuring visual, auditory, gustatory and olfactory experiences; and finally Intensity, which reflects the strength of the overall experience [14]. In the present study a Spanish adaptation of the questionnaire was used [22].
Statistical analysis
EEG recordings
Statistical analysis of EEG recordings was performed following the IPEG (International Pharmaco-EEG Group) guideline on statistical design and analysis of pharmacodynamic trials [23]. Accordingly, the inferential strategy of descriptive data analysis (DDA) [24], as proposed for application to the mapping situation [25], was applied. In short, descriptive tests, preferably of simple null hypotheses such as equality of two treatment effects, are performed at all observation times, locations and measurements (variables). A nominal α-level for each test is chosen at 5%, and all P values lower than 0.05 are clearly distinguished in the graphical demonstration of the results. Therefore, the formal P value is calculated for each test, leading to certain pattern of P values in the whole data structure, of which the ‘small’ P values are indicative of areas of potentially true drug-effect-differences. Rather than considering these P values (should they be smaller than α) as a decision criterion for rejecting local null hypotheses (a procedure which would not be indicated in the absence of an α-correction measure), in DDA these patterns of small P values are analysed in a descriptive way in order to interpret results. This interpretation should be done not just by looking at the P values alone but by simultaneously taking into account the biomedical expectations based on the structure of the study. Therefore, the calculated P values and their pharmacologically sound patterns are used as ‘judgement criteria’. Statistics included multivariate methods such as Hotelling T2 to test overall differences between drugs, and paired t-tests to evaluate changes and interdrug differences in detail at different hours postadministration. According to the experimental design used, pharmacologically sound patterns of P values <0.05 would be those showing: (a) spatial clustering (b) time courses, and (c) dose dependencies. These results were displayed as significance probability maps. Additionally, dose/treatment-effect and time-effect relationships were explored by means of a multivariate, nonparametric approach [20]. Friedman tests and multiple Wilcoxon tests based on sign-adjusted changes in 28 V-EEG variables were applied. In all tests performed (parametric and nonparametric) PRE-2-values were considered as the predrug baseline, and comparisons were conducted with the randomized placebo.
Subjective ratings
HRS scores were analysed by means of a one-way analysis of variance (anova) with repeated measures, with treatment (randomized placebo, ayahuasca low dose, ayahuasca high dose) as factor. Greenhouse-Geisser epsilon was used to correct possible violations of the sphericity assumption and to reduce Type I errors. Differences were considered statistically significant for P<0.05. When anova showed significant differences between treatments, pairwise comparisons were carried out by means of t-tests, followed by Bonferroni correction.
Results
EEG recordings
(1) Pharmaco-EEG maps: multivariate analysis
In order to test the hypothesis that ayahuasca exerts significant central effects which induce modifications in brain electrical activity as compared with placebo, a multiple analysis of variance (manova) with repeated measures was performed for V-EEG for each of the 19 electrodes. Treatment (randomized placebo, ayahuasca), time (PRE-2, post) and the following set of variables: log-transformed absolute power values in the delta, theta, alpha-1, alpha-2, beta-1, beta-2, beta-3 and beta-4 frequency bands were considered in the manova. Hotelling T2 values were used in the significance probability maps to indicate differences between ayahuasca-induced and placebo-induced changes in brain electrical activity from baseline through 8 h after drug administration.
As shown in Figure 1, ayahuasca administration induced dose-dependent central effects as measured by the derived EEG variables, which were greater and longer lasting after the high dose. Thus, after the low 0.6 mg DMT kg−1 body weight dose, statistically significant differences with placebo were obtained only at isolated electrode locations between 45 min and 2.5 h postadministration. After the high 0.85 mg DMT kg−1 body weight dose, however, EEG changes were found over extensive scalp areas. These effects first attained statistical significance at 1 h, showed a peak between 1.5 and 2 h and gradually decreased thereafter, to disappear at 6–8 h. At the peak of the pharmacodynamic effects, variations in brain electrical activity were measured all over the scalp, with the greatest intensity in the central and right temporo-occipital electrodes.
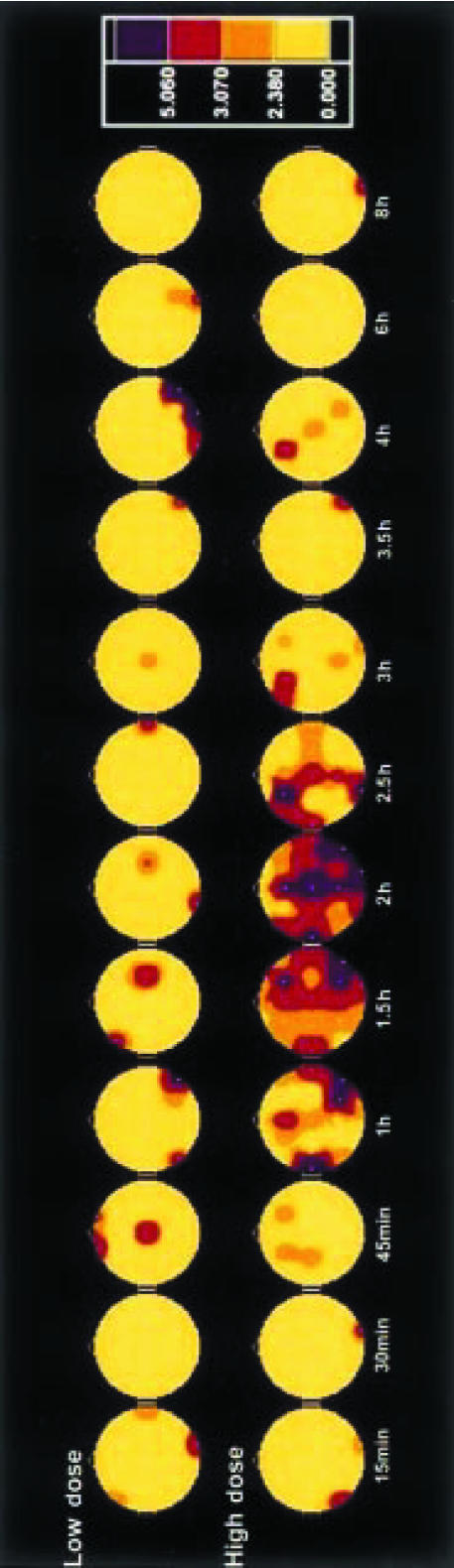
Figure 1.
Significance probability maps showing differences between ayahuasca-induced and placebo-induced central effects at 12 time points vs baseline values (PRE-2) after low (upper row) and high (lower row) doses of ayahuasca (n = 18). The vertex view shows the nose at the top, the left ear to the left, the right ear to the right. Electrode positions are indicated by white dots. Maps are based on Hotelling T2 obtained from multivariate tests in repeated measures anovas on eight logarithmically transformed absolute power values in delta, theta, alpha-1, alpha-2, beta-1, beta-2, beta-3 and beta-4 frequency bands. The colour key shows T2 values with hot/red colours indicating significant differences: T2>2.38 = P<0.10, >3.07 = P<0.05 and >5.06 = P<0.01.
(2) Pharmaco-EEG maps: univariate analysis
Topographic brain maps based on t-tests are described to show detailed drug-induced changes in the individual EEG variables.
Total power
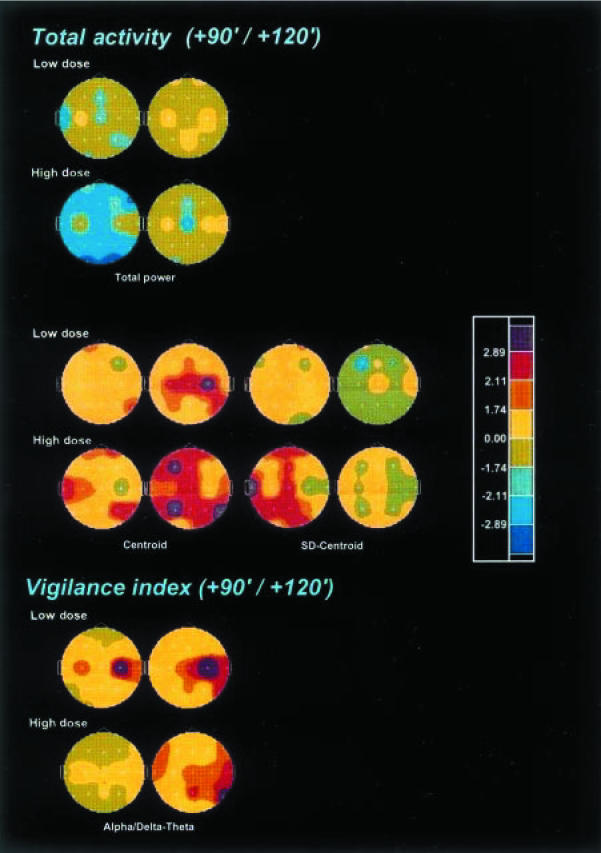
As shown in Figure 2, ayahuasca produced a significant and dose-dependent reduction in total power in electrodes located all over the scalp, with a temporal peak at 90 min after administration of the high dose. Both the centroid of the total activity and its deviation showed significant and dose-dependent increases peaking at 120 and 90 min, respectively.
Figure 2.
Significance probability maps showing differences between ayahuasca-induced and placebo-induced changes in total power and frequency variables of the EEG total activity (1.3–30 Hz), and in the alpha/delta-theta vigilance index, after low (upper rows) and high (lower rows) doses of ayahuasca (n = 18) at 90 min (left) and 120 min (right) after administration vs baseline values (PRE-2). The vertex view shows the nose at the top, the left ear to the left, the right ear to the right. Electrode positions are indicated by white dots. Eight-colour scale represents drug-induced changes as compared with placebo based on t-values: lilac, increase at P<0.01; red, increase at P<0.05; ochre, increase at P<0.10; pale yellow, trend towards increase; pale green, trend towards decrease; bright green, decrease at P<0.10; light blue, decrease at P<0.05; dark blue, decrease at P<0.01.
Slow activity
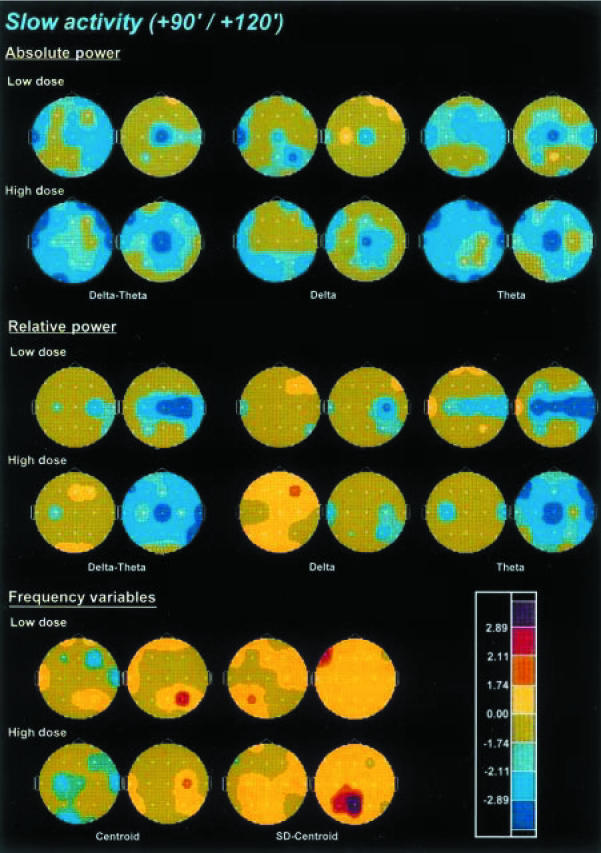
The effects of ayahuasca on slow activity are shown in Figure 3. Absolute power of the combined delta-theta activity was decreased in a dose-dependent manner after dosing with ayahuasca, with the peak decreases at 90 min for the low dose and between 90 and 120 min for the high dose. When examined separately, both the delta and theta frequency bands showed decreases in absolute power. However, the most dramatic decreases were found in the theta band, an effect which showed a dose-dependent pattern and peaked between 90 and 120 min.
Figure 3.
Significance probability maps showing differences between ayahuasca-induced and placebo-induced changes in absolute power, relative power and frequency variables of the combined slow activity (1.3–7.5 Hz), delta (1.3–3.5 Hz) and theta (3.5–7.5 Hz) frequency bands after low (upper rows) and high (lower rows) doses of ayahuasca (n = 18), at 90 min (left) and 120 min (right) after administration vs baseline values (PRE-2). For technical description of the maps and explanation of the colour key see Figure 2.
Relative power of the combined delta-theta bands was also dose-dependently decreased, with the peak reductions at 120 min. Decreases in relative power were marginal for the delta band, while they were prominent and dose-dependent for the theta band. These reductions in relative power were maximal at 120 min, showing a widespread distribution all over the scalp.
The centroid of the combined delta-theta activity showed a significant though modest deceleration, with a significant increase of its deviation. Nevertheless, although dose-dependent, the deceleration of the centroid was not uniformly distributed over the scalp, showing the greatest decreases at C3, T4 and O1 at the high ayahuasca dose at 90 min after administration. At the high dose, the significant increase seen for the deviation of the centroid was obtained at 120 min and was restricted to the Pz and P3 leads.
Alpha activity
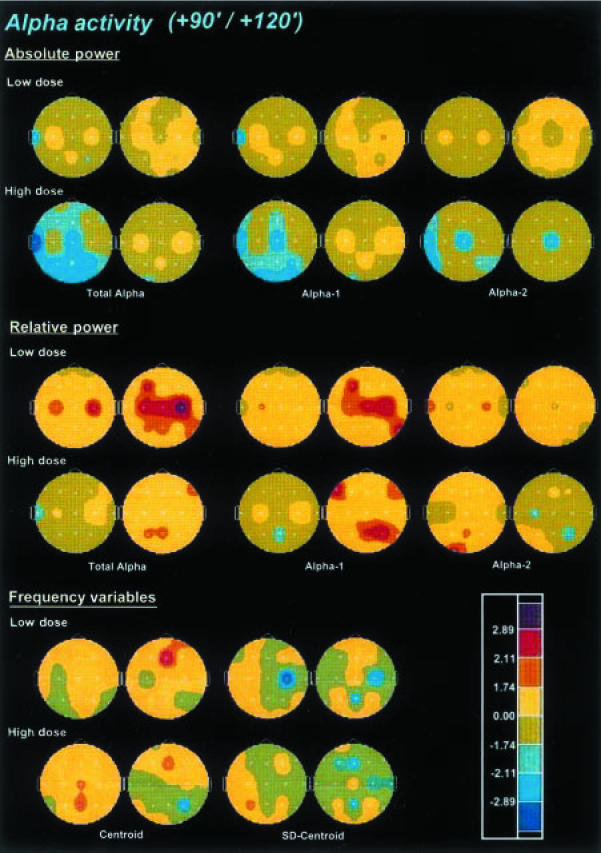
The effects of ayahuasca on alpha activity are shown in Figure 4. Absolute alpha activity was significantly and dose-dependently decreased after ayahuasca. The decreases were more prominent at the high dose in the left-temporal and centro-parieto-occipital electrodes. The maximal decrease was observed at 90 min after administration. When separately examined, the alpha-2 band showed more significant and more widely distributed decreases than the alpha-1 band. Differently from the maximal total alpha and alpha-1 power decreases, the reductions in absolute power for the alpha-2 band peaked at 60 min after administration (not shown).
Figure 4.
Significance probability maps showing differences between ayahuasca-induced and placebo-induced changes in absolute power, relative power and frequency variables of total alpha activity (7.5–13 Hz), alpha-1 (7.5–10.5 Hz), and alpha-2 (10.5–13 Hz) frequency bands after low (upper rows) and high (lower rows) doses of ayahuasca (n = 18), at 90 min (left) and 120 min (right) after administration vs baseline values (PRE-2). For technical description of the maps and explanation of the colour key see Figure 2.
Relative alpha activity was significantly increased at 120 min after administration, showing an inverse dose-reponse pattern, with maximal increase after the low dose. While this increase was consistently observed in the alpha-1 sub-band, in the alpha-2 sub-band a decrease which reached the highest significance at 60 min after the intake was seen (not shown).
No consistent pattern of changes was observed after ayahuasca in the dominant frequency within the alpha band (not shown). A tendency towards statistical significance was seen in the absolute power of the dominant frequency (predominantly decreases) which reached significance marginally in some electrode sites between 45 and 120 min after administration of the high dose. Conversely, relative power of the dominant frequency did show statistically significant increases after the low and the high ayahuasca doses at 120 min after administration. Finally, no consistent drug-induced effects were found either for the centroid of the alpha activity or its deviation.
Fast activity
The effects of ayahuasca on fast activity are shown in Figure 5. The absolute power of global beta activity was dose-dependently decreased by ayahuasca, with a maximal decrement at 90 min after administration. When split between the four frequency subbands, absolute power decreases were found to be more intense in the beta-1 range, with power decreases becoming less prominent as one moved to beta-2, beta-3 and beta-4. Peak decreases were observed at 90 min after administration, except for beta-3 which were more prominent at 45 min (not shown).
Figure 5.
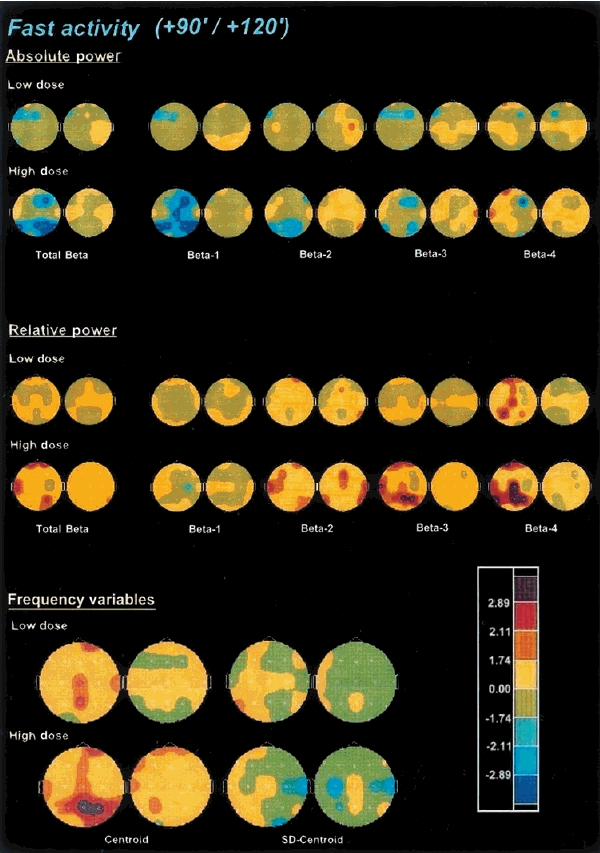
Significance probability maps showing differences between ayahuasca-induced and placebo-induced changes in absolute power, relative power and frequency variables of the combined fast activity (13–30 Hz), beta-1 (13–16 Hz), beta-2 (16–20 Hz), beta-3 (20–25) and beta-4 (25–30) frequency bands after low (upper rows) and high (lower rows) doses of ayahuasca (n = 18), at 90 min (left) and 120 min (right) after administration vs baseline values (PRE-2). For technical description of the maps and explanation of the colour key see Figure 2.
As far as relative power in the beta frequency range is concerned, statistically significant increases were found, these being more intense and longer-lasting at the high relative to the low ayahuasca dose. The maximal increments were obtained between 45 and 90 min after administration. Compared with absolute power values, the examination of relative power in the individual beta subbands rendered an inverse pattern of variation. Thus, relative power increases were marginally significant for the beta-1 band, became more widespread over the scalp for beta-2, more significant for beta-3 and were maximal for beta-4. Increases in the relative power of the beta-4 frequencies showed a predominant central and parieto-temporal distribution. Statistical significance for relative power increases for beta-2, beta-3 and beta-4 was obtained between 45 and 120 min after administration, with the maximal increase at 90 min.
The centroid of the beta frequency range showed a statistically significant and dose-dependent shift toward the higher values after ayahuasca, which also peaked at 90 min after administration. The deviation of the centroid was not significantly modified by the drug.
Table 1 lists 95% confidence intervals for changes in absolute (µV2) and relative (%) power in all frequency bands at 90 and 120 min following the administration of the low and high ayahuasca doses in a single representative electrode (P3).
Table 1.
95% confidence intervals for changes in absolute (µV2) and relative (%) power in all frequency bands at 90 and 120 min, following the administration of the low 0.6 mg DMT kg−1 body weight, and high 0.85 mg DMT kg−1 body weight ayahuasca doses, in a single representative electrode (P3). All changes vs baseline (PRE-2) and randomized placebo. Data from 18 volunteers, showing mean change ±1.96 s.e.mean.
| Low dose | High dose | |||
|---|---|---|---|---|
| 90 min | 120 min | 90 min | 120 min | |
| Absolute power (µV2) | ||||
| Total power (1.3–30 Hz) | −5.70±18.62 | −5.60±13.72 | −20.20±15.23* | −8.30±18.07 |
| Delta (1.3–3.5 Hz) | −1.20±1.57 | −1.30±1.82 | −1.40±1.10* | −1.70±1.84 |
| Theta (3.5–7.5 Hz) | −1.10±2.70 | −1.70±1.45* | −2.70±2.21* | −2.00±2.45 |
| Alpha-1 (7.5–10.5 Hz) | −0.40±7.84 | −3.00±8.41 | −11.30±11.07* | −1.70±11.11 |
| Alpha-2 (10.5–13 Hz) | −2.00±3.58 | 0.70±2.74 | −2.60±3.65 | −2.00±4.90 |
| Beta-1 (13–16 Hz) | −0.30±0.53 | 0.01±1.96 | −0.80±0.49* | −0.40±0.71 |
| Beta-2 (16–20 Hz) | −0.50±0.82 | −0.20±0.57 | −1.00±0.98* | −0.30±0.84 |
| Beta-3 (20–25 Hz) | −0.20±0.35 | 0.10±0.65 | −0.40±0.53 | −0.10±0.49 |
| Beta-4 (25–30 Hz) | 0.01±1.96 | −0.10±0.12 | −0.01±0.10 | −0.01±0.06 |
| Relative power (%) | ||||
| Delta (1.3–3.5 Hz) | −1.20±3.35 | −1.80±2.70 | 0.50±1.63 | −1.20±1.31 |
| Theta (3.5–7.5 Hz) | −1.30±3.65 | −3.20±2.98* | −1.40±2.12 | −3.30±2.59* |
| Alpha-1 (7.5–10.5 Hz) | 1.70±6.66 | 3.10±5.06 | −2.70±5.88 | 4.40±5.39 |
| Alpha-2 (10.5–13 Hz) | 0.20±3.92 | 1.90±2.86 | 2.00±3.57 | 0.10±1.96 |
| Beta-1 (13–16 Hz) | −0.20±0.65 | 0.01±1.96 | −0.20±0.78 | −0.40±0.61 |
| Beta-2 (16–20 Hz) | 0.30±0.59 | 0.10±0.39 | 0.40±0.57 | 0.30±0.53 |
| Beta-3 (20–25 Hz) | 0.20±0.49 | −0.10±0.65 | 1.00±0.88* | 0.20±0.65 |
| Beta-4 (25–30 Hz) | 0.20±0.14* | −0.10±0.16 | 0.30±0.24* | −0.10±0.27 |
Statistically significant differences vs placebo (P < 0.05) obtained after Student's t-test are indicated.
Vigilance index: alpha/delta-theta
The alpha/delta-theta ratio (Figure 2) was also calculated for each of the recorded time points. This index showed a significant increase, relative to placebo, both after the low and the high ayahuasca doses between 90 and 150 min, with the maximal increase at 120 min.
(3) Non-parametric multilead EEG analysis
Dose/treatment-effect relationships were calculated using Friedman and multiple Wilcoxon tests of sign-adjusted changes from PRE-2-values in 28 V-EEG variables obtained in the 19 leads. As shown in Table 2, based on the rank-sums, administered at the low dose ayahuasca could only be differentiated from randomised placebo at 45 min and 60 min after dosing. At the high dose, however, statistically significant differences were found from 45 min through 120 min after administration. Pairwise comparisons considering the total rank-sum showed statistically significant differences between randomised placebo and each of the ayahuasca doses, and between the low and high ayahuasca doses.
Table 2.
Dose/treatment-effect relationships after single oral doses of randomized placebo (A), low dose 0.6 mg DMT kg−1 body weight ayahuasca (B), high dose 0.85 mg DMT kg−1 body weight ayahuasca (C), and non-randomized placebo administered on the first (adaptation) experimental session (D). Data from 18 volunteers, based on sign-adjusted changes in 28 V-EEG variables (rank-sums, means of 19 electrodes, differences from PRE-2 baseline values).
| Time (min) | Randomized placebo (A) | Low dose (B) | High dose (C) | Adaptation placebo (D) | χ2 | Multiple Wilcoxon |
|---|---|---|---|---|---|---|
| 15 | 71.8 | 69.3 | 69.9 | 69.0 | 0.1 | |
| 30 | 63.5 | 76.8 | 79.9 | 59.8 | 6.3 | |
| 45 | 51.4 | 76.7 | 92.6 | 59.4 | 22.3** | A : B*, A : C**, D : C** |
| 60 | 53.1 | 85.6 | 85.9 | 55.4 | 21.5** | A : B**, A : C**D : B**, D : C** |
| 90 | 55.7 | 77.8 | 94.8 | 51.7 | 26.3** | A : C**D : B*, D : C** |
| 120 | 62.0 | 72.5 | 90.3 | 55.2 | 15.2** | A : C*D : C* |
| 150 | 62.1 | 74.8 | 86.3 | 56.8 | 11.3** | A : C(*) D : C* |
| 180 | 65.9 | 74.7 | 74.4 | 64.9 | 1.5 | |
| 210 | 75.1 | 60.8 | 73.4 | 70.7 | 2.7 | |
| 240 | 76.3 | 62.1 | 71.4 | 70.3 | 2.6 | |
| 360 | 80.6 | 62.0 | 75.1 | 62.4 | 5.9 | |
| 480 | 70.2 | 62.7 | 83.7 | 63.3 | 5.9 | |
| Total | 787.7 | 855.8 | 977.7 | 738.9 | 57.7** | A : B*, C**D : B**, C**B : C** |
= P<0.1
P<0.05
P < 0.01.
Time-effect relationships were calculated using Friedman and multiple Wilcoxon tests for randomised placebo-corrected sign-adjusted changes from PRE-2-values in 28 V-EEG variables obtained in the 19 leads, as shown in Figure 6. After ayahuasca administration, changes on EEG variables were seen as early as 15–30 min, followed by a steep increase at 45 min in rank-sum values. At the high dose, ayahuasca showed the pharmacodynamic peak between 45 and 90 min, with rank-sum values gradually decreasing thereafter and approaching baseline at 4–6 h after administration. At the low dose, an analogous curve was found, with the pharmacodynamic peak between 45 and 90 min having an analogous subsequent decrease to that of the high dose. Compared to baseline values, at the low dose increases in rank-sum values did not reach statistical significance at any of the time points evaluated. At the high dose, statistically significant differences were found at 45, 60 and 90 min after administration.
Figure 6.
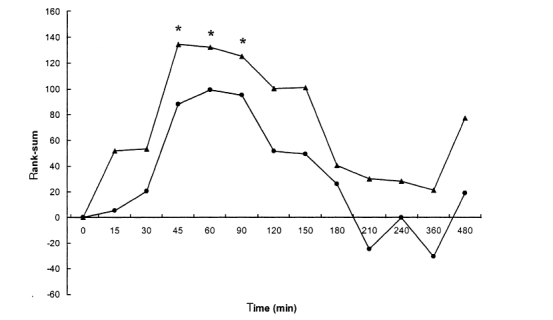
Time-effect relationships after single oral doses of 0.6 mg DMT kg−1 body weight ayahuasca (low dose) [•], and 0.85 mg DMT kg−1 body weight ayahuasca (high dose) [▴]. Plots show differences from baseline values (PRE-2) of sign-adjusted changes in 28 V-EEG variables (rank-sums, means of 19 electrodes, randomized placebo-corrected) from 18 volunteers. An asterisk indicates significant differences form baseline values obtained by means of multiple Wilcoxon.
Subjective ratings
As shown in Table 3, ayahuasca induced significant dose-dependent increases in all subscales of the HRS, an instrument specifically designed to quantify the effects of psychedelic drugs. Ayahuasca was thus capable of inducing a modified state of awareness in which a psychedelic profile was prominent. At the low dose, all HRS subscales showed statistically significant increases relative to placebo, except for Volition, a measure of impairment in the capacity of the volunteer to interact with his/herself and his/her surroundings. This subscale however, reached statistical significance at the high dose, indicating that of the six aspects measured by the HRS, this was the least modified by ayahuasca. Qualitatively, the profile of effects induced by ayahuasca included paresthesias and perceptual modifications of predominantly visual, and to a lower extent, auditive nature. This coexisted with more elaborated modifications in thought, associations and emotion, in a global experience described as similar to dreaming activity.
Table 3.
Means (s.d.) of the scores obtained for the HRS questionnaire subscales (n = 18) after single oral doses of randomized placebo, low dose 0.6 mg DMT kg−1 body weight ayahuasca and high dose 0.85 mg DMT kg−1 body weight ayahuasca, and results of the statistical analyses performed. Student's t-tests were followed by Bonferroni correction.
| Student's t-test | |||||
|---|---|---|---|---|---|
| anova | vs Placebo | vs Low dose | |||
| Variable | P value | Placebo | Low dose | High dose | High dose |
| HRS | |||||
| Somaesthesia | *** | 0.07 (0.10) | 0.50 (0.41)** | 0.97 (0.40)** | ** |
| Perception | *** | 0.09 (0.19) | 0.55 (0.49)** | 1.10 (0.67)** | ** |
| Cognition | *** | 0.06 (0.16) | 0.4 (0.45)** | 0.96 (0.59)** | ** |
| Volition | * | 0.81 (0.79) | 1.11(0.69) | 1.35 (0.61)* | NS |
| Affect | *** | 0.32 (0.21) | 0.65 (0.36)** | 1.02 (0.38)** | ** |
| Intensity | *** | 0.24 (0.45) | 1.32 (0.73)** | 1.85 (0.51)** | ** |
P<0.05
P<0.01
P<0.001; NS = not significant.
Discussion
The administration of ayahuasca to a group of healthy volunteers induced a dose-dependent pattern of subjective effects typical of the psychedelics, replicating the profile obtained in a previous study [12]. In addition to results obtained by means of self-assessment instruments, the implementation of q-EEG demonstrated a significant effect of ayahuasca, as compared with placebo, on the human CNS. These effects consisted of an overall decrease in absolute power for all the frequency bands evaluated, and an acceleration of the centre-of-gravity frequency. Absolute power decreases were most prominent in theta, delta and slow beta bands, while the alpha and fast beta rhythms were less intensely affected. Relative power was found to be significantly decreased in the theta, and to a lower extent, delta band. In the alpha band, relative power showed an increase, predominantly in the alpha-1 subband, and significant increases were also obtained in relative power in the beta frequency band. These increases in relative fast activity were most prominent in the beta-3 and beta-4 subbands. Additionally, the alpha/delta-theta ratio, an index of activation, was found to be increased after ayahuasca.
The evaluation of the plots of the rank-sums of changes measured at the 19 leads over time showed the first increases between 15 and 30 min, which were followed by a steep rise at 45 min, reaching the maximum effects between 45 and 90 min EEG measures gradually declined thereafter to reach baseline values around 4–6 h after administration. Most remarkably, these objectively measured effects of the drug on the spontaneous brain electrical activity closely paralleled the time course of subjectively experienced effects, measured by means of self-report visual analogue scales, as previously reported [12].
To our knowledge, only one previous study has addressed the evaluation of EEG activity in humans after the ingestion of ayahuasca. A recent article reported the evaluation of the EEG effects of ayahuasca in a group of nine subjects in field conditions [26]. In the cited study, EEG recordings were obtained in the course of a ritual Daime session in Brazil. The study was conducted in the absence of a placebo control, and only with an approximate knowledge of the ingested ayahuasca dose, this being on average 0.67 mg DMT kg−1 body weight. These investigators reported significant changes after ayahuasca in relation to baseline values only in the 36–44 Hz band. Given that this frequency range was not evaluated in the present study, it is impossible to establish a comparison with the results obtained in the aforementioned study. Nevertheless, Don et al. also reported a pattern of changes in the classical frequency bands which did not reach statistical significance but which bore similarities to that observed in the present study. These nonsignificant variations included a ‘slight increase in beta’, and a ‘slight decrease in theta and alpha’.
The changes in brain electrical activity observed in the present study are difficult to relate entirely to any pharmaco-EEG profile characteristic of the main psychotropic drug groups. Even a direct comparison with other psychedelics is far from easy. Virtually no studies have been conducted in the last 30 years regarding the effects of these drugs on the human EEG. The quantitative approach to the effects of psychedelics on the human EEG was absent at the time they attracted the greatest interest from psychiatry and psychopharmacology researchers in the 1950s and 1960s. Most of the information available from the early research conducted with these compounds is essentially qualitative. In these studies only marginal changes were described after the administration of psilocybin, mescaline or LSD on the visually inspected EEG trace, reporting at most an increase in fast rhythms and an overall decrease in signal amplitude [27]. Itil and coworkers, however, conducted a number of studies combining visual inspection and power spectrum analysis of the recordings obtained after administering anticholinergic compounds with true hallucinogenic properties, such as atropine, and serotonergic psychedelics like LSD. These researchers found almost opposite EEG patterns for these two groups of compounds. While atropine caused the alpha rhythm to disappear and the predominance of low-voltage slow waves, they concluded that the most characteristic effects of LSD were a reduction of theta activity and slow waves in general, as well as an increase in fast activity [27, 28]. In line with these observations, in the present study both absolute and relative power of slow activity decreased after ayahuasca, specially in the theta band. With regard to fast activity, while absolute power was decreased following ayahuasca administration, a marked enhancing effect was obtained for relative power. The milder increases were found for the slower beta-1 and beta-2 sub-bands and the most intense in the faster beta-3 and beta-4 sub-bands.
Ayahuasca shares the decremental effects seen on delta and theta power with both psychostimulants, such as amphetamine and methylphenidate, and serotonin releasers such as fenfluramine [29, 30]. Interestingly, psychostimulants act predominantly enhancing dopaminergic neurotransmission, in contrast with the serotonergic properties of psychedelics. However, a recent neuroimaging study in humans has shown that dopamine release takes place in the basal ganglia and the ventral striatum after the administration of psilocybin to humans [31], pointing to a role of dopaminergic neurotransmission in the effects of the classical psychedelics. Additional similarities are also to be found between the relative beta-3 and beta-4 band enhancing properties found for ayahuasca, and the analogous effect obtained after tricyclic antidepressants, which characterizes the group [29]. Drugs belonging to this pharmacological class inhibit the re-uptake of monoamines, which leads to increased levels of these endogenous compounds in the synapse [32]. (+)-Fenfluramine and the selective serotonin reuptake inhibitor fluoxetine also lead to increases in relative beta power [30, 33], an effect which is also shared by antidepressants showing MAOI properties [34]. It is consequently reasonable to assume that the blocking effects of the β-carbolines on MAO may have led to increased levels of monoamines, due to the blockade of their metabolism, which in turn may have contributed to the relative beta-promoting effect of ayahuasca. Regarding slow activity, the theta-dampening activity of psychostimulants and psychedelics is diametrically opposed to the theta-enhancing action of the classical neuroleptics such as haloperidol and chlorpromazine [30, 35]. This theta-enhancing action has also been observed in drugs with a mixed anti-D2 and anti-5-HT2 profile, such as risperidone [36], or the more selective 5-HT2 blocker ketanserin [37], suggesting a pro-dopaminergic and pro-serotonergic activity for ayahuasca.
DMT, the main psychotropic agent in ayahuasca, not only binds to the 5-HT2A/2C receptors, located mainly at a postsynaptic level, but also shows affinity for the 5-HT1A sites, which in certain brain regions correspond predominantly to somatodendritic autoreceptors [38]. Thus, DMT probably displays agonist activity also at the 5-HT1A sites, a pattern shared by other indole psychedelics, in contrast with the phenethylamines like mescaline, which interact only with the 5-HT2A/2C receptors [39]. The pharmaco-EEG profile of drugs displaying selective agonist or partial agonist activity at the 5-HT1A site has been described, allowing a more detailed discussion on the probable biochemical mechanisms involved in the EEG effects of ayahuasca. Indeed buspirone, a partial 5-HT1A agonist, has been shown to produce marked increases in theta power, in the absence of other relevant EEG modifications [40]. As an opposed pattern was seen for the theta band after ayahuasca, one could postulate that 5-HT1A agonism does not seem to be the predominant contribution at a molecular level to the EEG effects of ayahuasca. This is consistent with data from a previous study, in which increases in the intensity of the psychological effects elicited by intravenous DMT following blockade of the 5-HT1A sites by pindolol were reported [41]. The observed increases suggest both that agonism at the 5-HT1A site is not essential to obtain a psychedelic effect profile, and that a decreased binding of DMT at the 5-HT1A sites leads to an increase in the amount of DMT available to interact with the 5-HT2 receptors, and consequently to the enhanced subjective effects experienced by the volunteers. Thus, the present q-EEG findings would rather support a preponderant involvement of the 5-HT2 receptor in the genesis of the central effects of the beverage.
To sum up, the cerebral bioavailability and psychoactivity of ayahuasca could be objectively measured by means of q-EEG, which evidenced a clear dose-dependent effect at the doses administered. Remarkably, the time pattern obtained for EEG effects closely paralleled that of previously reported subjective effects. The global reduction in total power and the shift toward higher frequencies after ayahuasca are in line with older reports on the classical serotonergic psychedelics, which described an amplitude reduction and a suppresion of slow activity in the human EEG. Finally, the detailed assessment of ayahuasca effects on the different EEG variables indicated common features with the profile shown by pro-dopaminergic and pro-serotonergic drugs, and supports the involvement of serotonergic 5-HT2 and dopaminergic D2-receptor agonism in the central effects of ayahuasca.
Acknowledgments
We would like to thank Esther Martínez, Félix González and José María Fábregas for their continued support to our research project, and also CEFLURIS in Brazil for providing the ayahuasca (Daime) used in the present study. We are also greatful to James C. Callaway of the Department of Pharmaceutical Chemistry of the University of Kuopio, Finland, for quantifying the DMT in ayahuasca, and Maria Montero, Hospital de Sant Pau, Barcelona, for conducting the psychiatric interviews. Finally, our thanks to Rosa Antonijoan, Sylvie Cotxet, Llúcia Benito, Susanna Clos and David Martínez for their assistance during data-collection, and to Angeles Funes for editing the figures.
References
- 1.Dobkin de Rios M. Visionary Vine: Hallucinogenic Healing in the Peruvian Amazon. Prospect Heights, Illinois: Waveland Press; 1984. [Google Scholar]
- 2.Schultes RE, Hofmann A. Plantas de los dioses: orígenes del uso de los alucinógenos. México D.F. Fondo de Cultura Económica; 1982. [Google Scholar]
- 3.Anonymous L' Ayahuasca: de l' Amazonie à la Jungle Urbaine. La Géopolitique Mondiale Des Drogues 1998/1999, Paris: Observatoire Géopolitique Des Drogues. 2000. pp. 102–106.
- 4.Callaway JC, Grob CS. Ayahuasca preparations and serotonin reuptake inhibitors: a potential combination for severe adverse interactions. J Psychoactive Drugs. 1998;30:367–369. doi: 10.1080/02791072.1998.10399712. [DOI] [PubMed] [Google Scholar]
- 5.Rivier L, Lindgren JE. ‘Ayahuasca’, the South American hallucinogenic drink. An ethnobotanical and chemical investigation. Econ Bot. 1972;26:101–129. [Google Scholar]
- 6.McKenna DJ, Towers GHN, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants. Tryptamine and β-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- 7.Schultes RE, Hofmann A. The Botany and Chemistry of Hallucinogens. Springfield, Illinois: Charles C. Thomas; 1980. [Google Scholar]
- 8.Marek GJ, Aghajanian GK. Indoleamine and phenethylamine hallucinogens: mechanisms of psychotomimetic action. Drug Alcohol Depend. 1998;51:189–198. doi: 10.1016/s0376-8716(98)00076-3. [DOI] [PubMed] [Google Scholar]
- 9.Smith RL, Canton H, Barrett RJ, Sanders-Bush E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav. 1998;61:323–330. doi: 10.1016/s0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki O, Katsumata Y, Oya M. Characterization of eight biogenic indoleamines as substrates for type A and type B monoamine oxidase. Biochem Pharmacol. 1981;30:1353–1358. [PubMed] [Google Scholar]
- 11.Callaway JC, McKenna DJ, Grob CS, et al. Pharmacokinetics of Hoasca alkaloids in healthy humans. J Ethnopharmacol. 1999;65:243–256. doi: 10.1016/s0378-8741(98)00168-8. [DOI] [PubMed] [Google Scholar]
- 12.Riba J, Rodríguez-Fornells A, Urbano G, et al. Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology. 2001;154:85–95. doi: 10.1007/s002130000606. [DOI] [PubMed] [Google Scholar]
- 13.Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, et al. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Psychopharmacology. 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- 14.Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose–response study of N,N-dimethyltryptamine in humans, II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- 15.Strassman RJ. Human psychopharmacology of LSD, dimethyltryptamine and related compounds. In: Pletscher A, Ladewig D, editors. 50 Years of LSD. Current Status and Perspectives of Hallucinogens. New York: Parthenon; 1994. pp. 145–174. [Google Scholar]
- 16.Saletu B. The use of pharmaco-EEG in drug profiling. In: Hindmarch I, Stonier PD, editors. Human Psychopharmacology. Measures and Methods. Vol. 1. Chichester: John Wiley Sons; 1987. pp. 173–200. [Google Scholar]
- 17.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 18.Callaway JC, Raymon LP, Hearn WL, et al. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J Anal Toxicol. 1996;20:492–497. doi: 10.1093/jat/20.6.492. [DOI] [PubMed] [Google Scholar]
- 19.Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 20.Anderer P, Saletu B, Kinsperger K, Semlitsch H. Topographic brain mapping of EEG in neuropsychopharmacology – Part I. Methodological aspects. Meth Find Exp Clin Pharmacol. 1987;9:371–384. [PubMed] [Google Scholar]
- 21.Anderer P, Semlitsch HV, Saletu B, Barbanoj MJ. Artifact processing in topographic mapping of electroencephalografic activity in neuropsychopharmacology. Psychiatry Res Neuroimaging. 1992;45:79–93. doi: 10.1016/0925-4927(92)90002-l. [DOI] [PubMed] [Google Scholar]
- 22.Riba J, Rodríguez-Fornells A, Strassman RJ, Barbanoj MJ. Psychometric assessment of the Hallucinogen Rating Scale. Drug Alcohol Depend. 2001;62:215–223. doi: 10.1016/s0376-8716(00)00175-7. [DOI] [PubMed] [Google Scholar]
- 23.Ferber G, Abt K, Fichte K, Luthringer R. IPEG guideline on statistical design and analysis for pharmacodynamic trials. International Pharmaco-EEG group. Neuropsychobiology. 1999;39:92–100. doi: 10.1159/000026567. [DOI] [PubMed] [Google Scholar]
- 24.Abt K. Descriptive data analysis. A concept between confirmatory and exploratory data analysis. Meth Inf Med. 1987;26:77–78. [PubMed] [Google Scholar]
- 25.Abt K. Statistical aspects of neurophysiologic topography. J Clin Neurophysiol. 1990;7:519–534. doi: 10.1097/00004691-199010000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Don NS, McDonough BE, Moura G, et al. Effects of Ayahuasca on the human EEG. Phytomedicine. 1998;5:87–96. doi: 10.1016/S0944-7113(98)80003-2. [DOI] [PubMed] [Google Scholar]
- 27.Oughourlian JM, Rougeul A, Verdeaux J. Action des hallucinogènes sur l'électroencéphalogramme. Thérapie. 1971;26:953–968. [PubMed] [Google Scholar]
- 28.Itil T, Fink M. Klinische Untersuchungen und quantitative EEG-Daten bei experimentellen Psychosen. Arzneimittelforschung. 1966;16:237–239. [PubMed] [Google Scholar]
- 29.Herrmann WM, Schaerer E. Pharmaco EEG: computer EEG analysis to describe the projection of drug effects on a functional cerebral level in humans. In: Lopes da Silva FH, Storm van Leeuwen W, Rémond A, editors. Handbook of Electroencephalography and Clinical Neurophysiology, Clinical application of computer analysis of EEG & other neurophysiological signals. Vol. 2. Amsterdam: Elsevier; 1986. pp. 385–445. [Google Scholar]
- 30.Saletu B, Barbanoj MJ, Anderer P, Sieghart W, Grünberger J. Clinical-pharmacological study with two isomers (d-, l-) of fenfluramine and its comparison with chlorpromazine and d-amphetamine: blood levels, EEG mapping and safety evaluation. Meth Find Exp Clin Pharmacol. 1993;15:291–312. [PubMed] [Google Scholar]
- 31.Vollenweider FX, Vontobel P, Hell D, Leenders KL. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man. A PET study with [11C]raclopride. Neuropsychopharmacology. 1999;20:424–433. doi: 10.1016/S0893-133X(98)00108-0. [DOI] [PubMed] [Google Scholar]
- 32.Baldessarini RJ. Drugs and the treatment of psychiatric disorders: depression and mania. In: Hardman JG, Limbird LE, editors. The Pharmacological Basis of Therapeutics. Ninth. New York: McGraw-Hill; 1996. pp. 431–459. [Google Scholar]
- 33.Barbanoj MJ, Antonijoan RM, Morte A, Riba J, Jané F. Study of human psychotropic drug interactions by means of q-EEG. In: Saletu B, Krijzer F, Ferber G, Anderer P, editors. Electrophysiological Brain Research in Preclinical, Clinical Pharmacology, Related Fields – An Update. Vienna: International Pharmaco-EEG Group; 2000. pp. 164–172. [Google Scholar]
- 34.Saletu B, Grünberger J. On acute and chronic CNS effects of antidepressants in normals: neurophysiological, behavioral and pharmacokinetic studies with pirlindol. Meth Find Exp Clin Pharmacol. 1985;7:137–151. [PubMed] [Google Scholar]
- 35.McClelland GR, Cooper SM, Pilgrim AJ. A comparison of the central nervous system effects of haloperidol, chlorpromazine and sulpiride in normal volunteers. Br J Clin Pharmacol. 1990;30:795–803. doi: 10.1111/j.1365-2125.1990.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DY, Lee KU, Kwon JS, et al. Pharmacokinetic-pharmacodynamic modeling of risperidone effects on electroencephalography in healthy volunteers. Psychopharmacology. 1999;144:272–278. doi: 10.1007/s002130051003. [DOI] [PubMed] [Google Scholar]
- 37.Reimann IW, Ziegler G, Ludwig L, Frölich JC. Central and autonomic nervous system side effects of ketanserin. Arzneimittelforschung. 1986;36:1681–1684. [PubMed] [Google Scholar]
- 38.Glennon RA, Dukat M. Serotonin receptor subtypes. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology, the Fourth Generation of Progress. New York: Raven Press; 1995. pp. 415–429. [Google Scholar]
- 39.Aghajanian GK. 50 Years of LSD. Current Status and Perspectives of Hallucinogens. New York: Parthenon; 1994. LSD and phenethylamine hallucinogens: common sites of neuronal action; pp. 27–41. [Google Scholar]
- 40.Barbanoj MJ, Anderer P, Antonijoan RM, Torrent J, Saletu B, Jané F. Topographic pharmaco-EEG mapping of increasing doses of buspirone and its comparison with diazepam. Hum Psychopharmacol Clin Exp. 1994;9:101–109. [Google Scholar]
- 41.Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73:121–124. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]