Abstract
Early clinical features of lead toxicity are non-specific and an occupational history is particularly valuable.
Lead in the body comprises 2% in the blood (t1/2 35 days) and 95% in bone and dentine (t1/2 20–30 years). Blood lead may remain elevated for years after cessation from long exposure, due to redistribution from bone.
Blood lead concentration is the most widely used marker for inorganic lead exposure. Zinc protoporphyrin (ZPP) concentration in blood usefully reflects lead exposure over the prior 3 months.
Symptomatic patients with blood lead concentration >2.4 µmol l−1 (50 µg dl−1) or in any event >3.8 µmol l−1 (80 µg dl−1) should receive sodium calciumedetate i.v., followed by succimer by mouth for 19 days.
Asymptomatic patients with blood lead concentration >2.4 µmol l−1 (50 µg dl−1) may be treated with succimer alone.
Sodium calciumedetate should be given with dimercaprol to treat lead encephalopathy.
Keywords: chelating agents, lead paint, lead, occupational exposure, review, sodium calcium edetate, succimer, toxicity
Introduction
The danger to public health from lead in the environment continues to be a matter of concern, for subtle effects of the element on intelligence quotient and blood pressure have potentially widespread significance. Acute intoxication occurs sporadically, and when it does, the source of lead is commonly in the household. we report three cases of lead poisoning caused by old leaded paint, and review the sources and the management of this form of heavy metal poisoning.
Case reports
Case 1
A painter and decorator aged 40 years presented with a 6 week history of malaise, abdominal cramps, nausea, arthralgia and mild mental impairment. He had previously been working in a Georgian building in Bath using an industrial blowtorch and sander to remove paint. Two of the three floors had timber-panelled walls; all had 8 to 10 coats of paint and some were clearly very old. Although he had acquired a new respirator he did not wear it when other workmen were burning off or sanding in other rooms on the same floor, and during breaks he ate, drank and smoked cigarettes in the same building. No measurements of atmospheric lead concentrations were made.
Investigation by the patient's general practitioner showed a blood lead of 4.18 µmol l−1 (87.1 µg 100 ml−1) (Table 1) and he was referred for treatment.
Table 1.
Concentrations of blood lead (Pb)*(µmol l−1) and zinc protoporphyrin (ZPP) (µg g−1 Hb) before and after treatment with chelating agents.
| Case 1 | Case 2 | Case 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | R | Pb | ZPP | R | Pb | ZPP | R | Pb | ZPP |
| 1 | 4.18 | 7.2 | 4.04 | 10.6 | 4.09 | 28.2 | |||
| 7 | ↓ | 4.33 | 12.6 | ||||||
| 8 | ↓ | 4.13 | 8.6 | ↓ | ↓ | ||||
| 9 | ↓ | ↓ | 2.17 | 13.3 | ↓ | ||||
| 11 | ↓ | 2.86 | 9.2 | ↓ | 0.72 | 13.3 | ↓ | ||
| 22 | ↓ | ↓ | ↓ | ||||||
| 27 | ↓ | 2.13 | 11.4 | ↓ | |||||
| 29 | ↓ | ||||||||
| 36 | 2.47 | 32.2 | |||||||
| 64 | 2.51 | 23.6 | |||||||
| 79 | 1.93 | 13.9 | |||||||
| 143 | 2.45 | 11.6 | |||||||
| 245 | 2.06 | 12.8 | |||||||
| 366 | 1.53 | 3.6 | |||||||
| 647 | 1.85 | 7.4 | |||||||
| 758 | 1.35 | ||||||||
R = period of treatment with chelating agents.
ZPP values of 5–10 µg g−1 Hb represent borderline, and >10 µg g−1 Hb serious toxicity. See Discussion for interpretation of blood Pb values
It is the practice of United Kingdom Supra-regional Assay Service laboratories to use the SI molar system as the prime unit of reporting, but mass units are in general use, because the existing UK regulations for control of lead at work have not yet been converted. To convert Pb in µmol l−1 to µg 100 ml−1, multiply by 20.83 (1 µg 100 ml−1 = 0.048 µmol l−1).
Apart from mild abdominal tenderness, physical examination was unremarkable and in particular neurological examination was entirely normal.
Blood results were as follows: haemoglobin (Hb) 9.4 g l−1, mean corpuscular volume (MCV) 92.0 fl, white cell count (WCC) 13.2×109 l−1, platelet count 231×109 l−1. Plasma electrolytes and liver function tests were normal. basophilic stippling of erythrocytes was noted on the blood film.
He was treated initially with sodium calcium edetate (disodium calcium ethylenediamine-tetra-acetic acid) 40 mg kg−1 i.v. every 12 h for 48 h and then commenced succimer (2,3 dimercaptosuccinic acid, dmsa) 30 mg kg−1 by mouth for 5 days followed by 20 mg kg−1 for a further 14 days. At review 14 days later his Hb was 11.5 g l−1 and WCC 5.2×109 l−1; other relevant investigations appear in Table 1.
Case 2
A self-employed decorator aged 51 years presented to his general practitioner with a 4 week history of nausea, constipation, headaches, intermittent dizziness, and paraesthesiae and weakness of the hands. He said that he had experienced prolonged exposure to lead paint whilst redecorating a late regency house (circa 1820) in Bath. His initial blood lead was 4.04 µmol l−1 (84.2 µg 100 ml−1). no abnormalities were detected on physical examination.
Investigation revealed Hb 13.2 g l−1, WCC 7.4×109 l−1, platelet count 233×109 l−1. Plasma electrolytes and liver function tests were normal. Basophilic stippling was not present on the blood film.
He received sodium calcium edetate i.v. for 48 h followed by succimer by mouth, as above. After 14 days he developed an urticarial reaction and the succimer was discontinued. Further data appear in Table 1.
Case 3
A man of 22 years presented with a 2 week history of lethargy, malaise, headaches, nausea and cramping abdominal pain. He had been working in the same site as case 1, though intermittently over 12 weeks. His blood lead was 4.09 µmol l−1 (85.2 µg 100 ml−1). On examination anaemia was noted but he was otherwise well and exhibited no neurological or intellectual impairment. Investigation showed: Hb 9.7 g 100 ml−1 with moderate polychromasia and basophilic stippling of the red cells. his blood count was otherwise within normal limits as were his electrolytes and liver function tests.
He received a 19 day course of succimer by mouth as above, but no sodium calciumedetate. At review 4 weeks later his symptoms had resolved and his Hb was 11.3 g 100 ml−1. Other relevant findings appear in Table 1.
Comment
All three patients presented with symptoms consistent with, and blood lead concentrations indicative of, acute poisoning. The setting of the exposure, removing leaded paint, represents an established source of danger. Cases 1 and 2 had more marked clinical features and received sodium calcium edetate followed by succimer. The urticarial reaction experienced by case 2 is a recognized adverse effect of succimer. Case 3 was thought to be well enough to justify treatment with succimer alone. Blood lead concentrations fell with chelation therapy in all cases. Despite removal from the source of exposure, cases 2 and 3 show sustained elevation of blood lead and zinc protoporphyrin (zpp) long after therapy; this is compatible with continued release of lead from the skeleton.
Discussion
Lead poisoning has long been recognized as an occupational hazard and indeed Hippocrates described the case of a metal extraction worker with abdominal colic [1]. The metal was used extensively for purposes that ranged from tool formation in ancient Egypt to the sweetening of wines in the Middle Ages. Famously, in 1767 Sir George Baker demonstrated that Devonshire colic was caused by lead in the production equipment of Devonshire cider [2]. In the 19th century lead poisoning was classified as a notifiable occupational disease in Britain, though the necessity of reducing workers' exposure was fully appreciated only in the latter half of the 20th century. The Control of Lead at Work Regulations [3, 4] prescribe safety limits. Blood lead values <1.45 µmol l−1 (30 µg 100 ml−1) represent reasonably well controlled occupational exposure provided there is 6 monthly monitoring, 1.45–2.4 µmol l−1 (30–50 µg 100 ml−1) require investigative action by the employer and 2.4–2.9 µmol l−1 (50–60 µg 100 ml−1) call for suspension of the worker from exposure. Special consideration is made for young workers and ‘genetically useful’ women. Most cases of acute lead poisoning now occur in employees of small companies, individuals renovating old buildings or in conjunction with hobbies that incur lead exposure, such as model soldier making or firearms use.
Chronic lead poisoning, by contrast, remained a problem and in 1988 some 3–4 million children in North America were considered at risk of toxicity judged by blood lead concentrations exceeding 0.72 µmol l−1 (15 µg 100 ml−1) [5]. Evidence that children chronically exposed to raised environmental lead concentrations suffer impaired intellectual and neurological development [6–8] has forced developed countries to reduce lead pollution, as witness the introduction of policies to promote lead free petrol [9] and to reduce the lead content of manufactured items such as ceramics and paint. In the years 1984–95, population blood lead concentrations fell by more than three-fold in the UK [10]. Recognized sources of exposure to lead appear in Table 2.
Table 2.
Sources of exposure to lead.
| Occupational | Environmental |
| Smelting or refining lead | Lead paint/pigments |
| Battery manufacture | Lead piping |
| Plastics manufacture | Leaded petrol |
| Housing renovation | Ceramic lead glaze |
| Lead crystal | |
| Recreational | Other |
| Model soldier making | Alternative medicines |
| Home jewellery making | (especially Indian) |
| Indoor range firearm use | Gunshot wounds |
| Ingestion of moonshine whisky | Mobilization of bone in |
| Petrol sniffing | hyperthyroidism |
| Eye shadow/cosmetics from less developed countries |
As adult lead poisoning becomes less common, it is important to be aware of the problem in persons who present with non-specific symptoms, and to take a full occupational history. The dangers of lead exposure in workers who strip paint in old buildings should be appreciated, for there are relatively few reports of this potentially important source of intoxication [11, 12].
Metabolism of lead
The discussion refers to the inorganic form of lead in which the different salts and oxides are regarded as acting identically once in the systemic circulation. The organic form, e.g. tetraethyl lead, possesses different absorption and disposition characteristics.
Marked age-related differences in the absorption and metabolism of lead contribute to the increased susceptibility to lead toxicity in children. The main route of absorption in adults is the respiratory tract where 30–70% of inhaled lead finds its way into the circulation. Particle size is the most important determinant of absorption. Gastrointestinal absorption in adults is generally less complete and averages approximately 10% of the ingested load. In children, alimentary absorption is about 50% and, together with their greater volume of air inhaled in relation to body size, total lead absorption from the environment is around three times higher than for adults [13]. Fasting, iron deficiency and low dietary calcium promote lead absorption [14]. Dermal absorption of inorganic lead is negligible. Organic lead enters the lipophilic body tissues and the body burden is less well reflected by the concentration of lead in the blood.
Lead deposition in the body comprises three major pools. The blood pool contains the rapidly exchangeable component but accounts for only 2% of the total body burden (95% is bound to the erythrocyte membrane and haemoglobin and 5% is in the plasma). Biochemically, the blood lead constitutes the most important component and the concentration reflects recent exposure; it has a biological t1/2 of about 35 days [15]. The remainder of the total body burden is distributed between an intermediate pool comprising skin and muscle, and a stable pool in dentine and the skeleton. This latter contains over 95% of the total body load and has a biological t1/2 of 20–30 years [16]. Lead is eliminated mainly in the urine.
Toxic effects of lead
General symptoms
The classical picture of abdominal colic and constipation is now not generally seen in developed countries, and patients more usually present with non-specific symptoms including fatigue, aches in muscles and joints and abdominal discomfort. Patients with poor dental hygiene (but not the edentulous) may exhibit a blue line at the dental margin of the gums due to deposition of lead sulphide. The clinical features are summarized in Table 3.
Table 3.
Symptoms and signs of lead poisoning.
| Mild | Lethargy |
| Anorexia | |
| Abdominal discomfort | |
| Arthralgia | |
| Moderate | Anaemia |
| Headache | |
| Abdominal cramps | |
| Gingival lead line | |
| Peripheral neuropathy (motor) | |
| Severe | Convulsions |
| Coma | |
| Encephalopathy | |
| Renal failure |
Biochemical and haematological
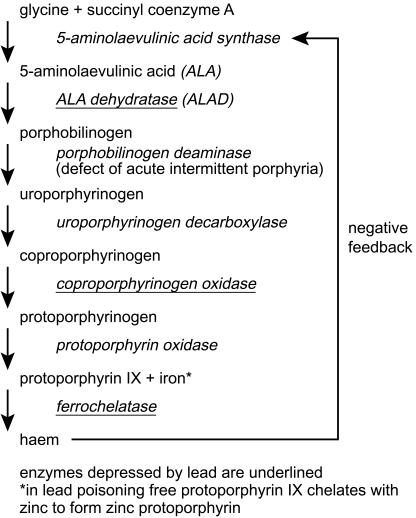
Lead has three important biochemical properties that contribute to its toxic effects on humans. Firstly, it is an electropositive metal with high affinity for sulfhydryl groups and thus inhibits sulphydryl dependent enzymes such as 5-aminolaevulinic acid dehydratase (ALAD, EC 4.2.1.24) and ferrochelatase (EC 4.99.1.2) which are essential for the synthesis of haem (Figure 1). Secondly, divalent lead acts in a manner similar to calcium and competitively inhibits its actions in important areas such as mitochondrial oxidative phosphorylation. In particular, lead impairs the intracellular messenger system normally regulated by calcium and thereby affects endocrine and neuronal function. Lead also changes the vasomotor action of smooth muscle by its effect on Ca ATPase. Thirdly, lead can affect the genetic transcription of DNA by interaction with nucleic acid binding proteins with potential consequences for gene regulation [17]. To date there has been no convincing evidence that lead is a human carcinogen although it has been shown to be so in animal models [18].
Figure 1.
Haem synthesis and the effects of lead.
Inhibition by lead of cytosolic ALAD prevents the formation of porphobilinogen and accumulation of the precursor 5-amino-laevulinic acid (ALA) in the plasma may play a significant role in the pathogenesis of lead poisoning by triggering an oxidative stress response, as in acute intermittent porphyria (Figure 1) [19]. The sensitivity of ALAD to lead is high, especially in children.
Inhibition of mitochondrial ferrochelatase prevents the incorporation of ferrous iron into protoporphyrin IX. Consequently free protoporphyrin IX accumulates and forms a metal chelate with zinc, which remains in the erythrocyte for its life-time. Zinc protoporphyrin (ZPP) is thus an indicator of exposure to lead over the prior 3 months.
Elevation of ZPP also occurs in iron deficiency anaemia, thalassaemia trait, haemoglobin E and protoporphyria [20]. Ferrochelatase is less sensitive than ALAD to the effects of lead [21], and recent work has suggested that inhibition of the related enzyme ferrireductase is more important [22]. Anaemia in lead poisoning is typically hypochromic and microcytic with basophilic stippling of red cells (due to inhibition of pyrimidine 5′-nucleotidase, EC 3.2.2.10); it is a late complication and generally appears only when blood lead concentrations exceed 2.40 µmol l−1 (50 µg 100 ml) [23].
Neurological
Symptoms in adults range from mild lethargy and fatigue to a severe motor neuropathy, characteristically with weakness of forearm extensor muscles. The presentation in children is commonly with a severe encephalopathy. Chronic, low-level exposure of young children is associated with deficits in central nervous system function including impaired intelligence [4, 24]. Environmental contamination may result from home renovation that disturbs lead-based paints, creating a dust that is inhaled or ingested by children [25].
The mechanisms of lead neurotoxicity have not been fully elucidated. Acute encephalopathy appears to follow disruption of the blood–brain barrier. Impairment of the intracellular action of calcium in second messenger signalling leads to loss of integrity of the tight junctions between brain endothelial cells and the subsequent passage of plasma into the brain causes cerebral oedema [14]. Lead also appears to be capable of acting either as a developmental toxin in the central nervous system in children or as a direct toxicant on neurotransmission [26]. Cognitive impairment in children chronically exposed to low concentrations of lead in early life may be due to an effect on cell:cell connections in the immature brain. Interference with neuronal cell adhesion molecules, demonstrated in rodents, has been proposed as a mechanism of developmental toxicity [27]. In contrast, at the neurosynaptic junction, it appears that lead interferes with transmitter release and signal transduction. In adults the classical picture of severe lead toxicity includes bilateral wrist drop. The histology shows segmental axonal demyelination and degeneration, and decreased nerve conduction velocities have been demonstrated in lead workers with blood lead concentrations of 1.9 µmol l−1 (40 µg 100 ml−1) [28]. The underlying mechanism may be substitution of calcium and zinc by lead at the synapse, affecting intracellular messengers such as cAMP and protein kinase [29].
Renal
Lead nephropathy used to be relatively common in industrial workers and the distillers of illegal ‘moonshine’ whisky in America. Chronic lead exposure causes interstitial nephritis and chronic renal failure. Acute severe lead exposure may give rise to proximal tubular dysfunction with glycosuria, hyperphosphaturia, and aminoaciduria. The diagnosis of chronic lead nephropathy is based on the typical clinical picture, chronic tubulointerstitial nephritis on biopsy and the exclusion of other causes. Evaluating the lead stores by X-ray fluorescence or sodium calcium edetate mobilization test may be helpful in adding weight to the diagnosis [30] but there is no single diagnostic test.
Diagnosis
The diagnosis is usually based on the blood lead concentration in association with compatible clinical symptoms. The issues have recently been reviewed [31]. Blood lead concentrations are the most widely used marker for inorganic lead exposure. The concentrations gradually decline over 2–4 weeks after the patient has been removed from the source. Thus a subject may experience symptomatic lead poisoning with a blood lead concentration within the acceptable range, and the ZPP should additionally be measured (see below). By contrast, after long exposure, the blood lead may remain elevated for years after cessation, due to redistribution from bone. Urinary lead excretion following a dose of sodium calcium edetate has been used to estimate the body burden [32]. Exposure to organic lead is best reflected by its urinary excretion.
Inhibition of ALAD leads to an accumulation of ALA, which is excreted in the urine. Urinary ALA excretion and ALAD activity are both sensitive indicators of lead exposure, but are not used routinely.
Inhibition of mitochondrial ferrochelatase results in accumulation of erythrocyte precursors including protoporphyrin, which binds to available zinc. ZPP remains in the blood for the life-time of the erythrocyte and reflects lead exposure over the prior three months.
Treatment
The most important initial aspect of management of lead poisoning is the removal of the patient from the source of exposure. With adults this usually means a change in work or at least in working practice, or the cessation of hobbies that involve lead exposure. If there is no occupational hazard then sources of lead at home must be eliminated, particularly in the case of children. Isotopic analysis of lead can usefully detect the domestic source of the toxin [33]. Lead occurs as a mixture of four isotopes (atomic weights 204, 206, 207 and 208), which behave identically with respect to their metabolism. The relative abundance of these varies according to the source of the lead; where there are several sources of lead in the environment, comparing their relative abundance with that in the patient may identify the cause of the poisoning.
The mainstay of treatment for lead poisoning is the use of chelating agents which form complexes with lead, prevent its binding to cell constituents and, being hydrophilic, are eliminated in the urine. The concept of accelerating the elimination of a toxic metal by giving a complexing substance was proposed in 1942 [34] and was given practical expression by the development of dimercaprol (2,3-dimercapto-1-propanol) for arsenic poisoning.
The most widely examined agents are the polyaminocarboxylic acids, of which sodium calcium edetate (EDTA) is particularly effective for lead poisoning because of its capacity to exchange calcium for lead [35]. The resulting lead chelate is rapidly excreted in the urine. Sodium calcium edetate principally mobilises lead from bone and the extracellular compartment [36]. It is given intravenously as less than 5% is absorbed from the gut following oral administration, and intramuscular injection is extremely painful. The t1/2 is 20–60 min following i.v. injection. It is usually given in a dosage of 50–80 mg kg−1 in 1–2 doses day−1 for 2–5 days. Sodium calcium edetate is generally well tolerated, the principal side-effect being nephrotoxicity, especially in subjects with underlying renal impairment, in whom the maintenance of adequate hydration during administration is essential. Other reported adverse effects include headache, fatigue, myalgia, thirst, fever, nausea and vomiting, sneezing, lacrimation, nasal congestion, rashes, anaemia and hypotension.
More recently succimer (2,3-dimercaptosuccinic acid, DMSA), a water-soluble analogue of dimercaprol, has been increasingly used. Originally employed as the antimony chelate to treat schistosomiasis [37], succimer was later developed for heavy metal poisoning [38] including lead, arsenic and mercury. The advantages of succimer include its high affinity for lead and suitability for administration by mouth. It is better tolerated and has a wider therapeutic index than dimercaprol [39]. Succimer is 95% protein bound in plasma and has a t1/2 of 3 h. The usual dosage is 10–30 mg kg−1 day−1 for the first 5–7 days, and then at a reduced dose for a further 10–14 days [40]. Adverse effects are uncommon and mainly comprise of nausea, diarrhoea, skin rashes, and transient elevation of liver enzymes [41]. Haemolytic anaemia was reported in a patient with glucose-6-phosphate dehydrogenase deficiency following exposure to succimer [42].
Previously, the treatment of severe lead poisoning or lead encephalopathy was with a combination of sodium calcium edetate and dimercaprol [43]. Dimercaprol is more effective than sodium calcium edetate in chelating lead from the soft tissues such as the brain and combination therapy appeared superior to monotherapy. Recently succimer has been employed in preference to dimercaprol due to its ease of use and lesser toxicity. There is less published experience with succimer (which is not formally licenced for treating lead poisoning in the UK, although it is in the USA). Animal studies have shown it to reduce tissue lead concentrations [44, 45]. Succimer appears to enhance excretion of lead from both the intracellular and extracellular compartments. It does not, however, promote urinary lead excretion to the same degree as EDTA [46] and following cessation of treatment blood lead levels may rebound. Administration over 19 days may prevent the rebound phenomenon [47, 48] making oral monotherapy with this agent attractive for the treatment of mild to moderate lead poisoning. In severe lead poisoning sodium calcium edetate is commonly used to initiate lead excretion and occurs through chelation of lead from bone and the extracellular space, with the urinary excretion of lead diminishing over approximately 5 days as extracellular lead is exhausted. Worsening colic and encephalopathy following initiation of treatment have been attributed to redistribution of lead from bone to brain [49]. Redistribution of lead from the skeleton to the soft tissues by sodium calcium edetate has been demonstrated in animals. It appears that combination treatment with succimer can prevent this occurrence [50, 51] although another study produced conflicting results [52]. It is not known if combination therapy prevents lead redistribution in humans.
Depletion of essential trace elements may accompany chelation therapy. Sodium calcium edetate significantly increases urinary excretion of calcium, copper, zinc and iron. Quantification of such loss showed that the urinary excretion of zinc increased by a factor of 22 and iron by 3.8 [53]. Succimer, by contrast, does not appear significantly to influence the excretion of trace elements other than urinary copper and zinc, effects which do not appear to be clinically important [54, 55]. Combination therapy, however, does increase urinary zinc excretion, an outcome which may be more relevant to children, as adults are less sensitive to trace element deficiencies. It seems reasonable, nevertheless, to ensure an adequate dietary intake of calcium, iron, and zinc in all subjects undergoing treatment.
As there is individual variation in response to lead exposure [56], the decision to treat is based on clinical symptoms and length of exposure as well as the blood lead concentration. A reasonable approach is to treat symptomatic patients with blood lead concentration >2.4 µmol l−1 (50 µg 100 ml−1) or in any event >3.8 µmol l−1 (80 µg 100 ml−1). This may be achieved with sodium calcium edetate by i.v. infusion followed by succimer by mouth for at least 5 days. Administration of succimer over 19 days may prevent rebound elevation of blood lead after treatment has stopped. Asymptomatic patients with blood lead concentration >2.4 µmol l−1 (50 µg 100 ml−1) may be treated with succimer alone, with regular monitoring of blood lead concentration [47].
Acknowledgments
The authors wish to thank Dr P. Astley and the Clinical Chemistry Laboratory, Southmead Hospital, Bristol for measuring blood lead and ZPP concentrations.
References
- 1.Browder AA, Joselow MM, Louria DB. The problem of lead poisoning. Medicine. 1973;52:121–139. doi: 10.1097/00005792-197303000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Waldron HA, Scott A. In: Hunter's Diseases of Occupations. Raffle PAB, Adams PH, Baxter PJ, Lee WR, editors. London: Edward Arnold; 1994. pp. 90–138. [Google Scholar]
- 3.Approved Code of Practice. London: HMSO; 1985. Control of lead at work. [Google Scholar]
- 4.London: The Stationery Office Ltd; 1998. Control of lead at work regulations. [Google Scholar]
- 5.Department of Health and Human Services Agency for Toxic Substances and Disease Registry. Washington DC: Government Printing Office; 1988. The nature and extent of lead poisoning in children in the United States; pp. 1–13. [Google Scholar]
- 6.Fulton M, Raab G, Thomson G, Laxen D, Hunter R, Hepburn W. Influence of blood lead on the ability and attainment of children in Edinburgh. Lancet. 1987;i:1221–1226. doi: 10.1016/s0140-6736(87)92683-3. [DOI] [PubMed] [Google Scholar]
- 7.Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood. N Engl J Med. 1990;322:83–88. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Geneva: World Health Organization; 1995. Inorganic lead. Environmental Health Criteria 165. [Google Scholar]
- 9.Annest JL, Pirkle JL, Makuc D, Neese JW, Bayse DD, Kovar MG. Chronological trend in blood lead levels between 1976 and 1980. N Engl J Med. 1983;308:1373–1377. doi: 10.1056/NEJM198306093082301. [DOI] [PubMed] [Google Scholar]
- 10.Delves HT, Daiper SJ, Oppert S, et al. Blood lead concentrations in United Kingdom have fallen substantially since 1984. Br Med J. 1996;313:883–884. doi: 10.1136/bmj.313.7061.883d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart SP, McIver B, Frier BM, Agius RM. Abdominal pain and vomiting in a paint stripper. Postgrad Med J. 1996;72:253–255. doi: 10.1136/pgmj.72.846.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marino PE, Landrigan PJ, Graef J, et al. A case report of lead paint poisoning during renovation of a Victorian farmhouse. Am J Public Health. 1990;80:1183–1185. doi: 10.2105/ajph.80.10.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler EE, Edwards BB, Jensen RL, Mahaffey KR, Fomon SJ. Absorption and retention of lead by infants. Pediatr Res. 1978;12:29–34. doi: 10.1203/00006450-197801000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Mahaffey KR. Environmental lead toxicity: nutrition as a component of intervention. Environ Health Perspect. 1990;89:75–78. doi: 10.1289/ehp.908975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barltrop D, Smith AM. Kinetics of lead interactions with human erythrocytes. Postgrad Med J. 1985;51:770–773. doi: 10.1136/pgmj.51.601.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans. J Clin Invest. 1976;58:260–270. doi: 10.1172/JCI108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goering PL. Lead–protein interactions as a basis for lead toxicity. Neurotoxicology. 1993;14:45–60. [PubMed] [Google Scholar]
- 18.Zelikoff JT, Li JH, Hartwig A, Wang ZW, Costa M, Rossman TG. Genetic toxicology of lead compounds. Carcinogenesis. 1988;9:1727–1732. doi: 10.1093/carcin/9.10.1727. [DOI] [PubMed] [Google Scholar]
- 19.Costa CA, Trivelato GC, Pinto AM, Bechara EJ. Correlation between plasma 5-aminolaevulinic acid concentrations and indicators of oxidative stress in lead-exposed workers. Clin Chem. 1997;43:1196–1202. [PubMed] [Google Scholar]
- 20.Graham EA, Felgenhauer J, Detter JC, Labbe RF. Elevated zinc protoporphyrin associated with thalassaemia trait and haemoglobin E. J Pediatr. 1996;129:105–110. doi: 10.1016/s0022-3476(96)70196-8. [DOI] [PubMed] [Google Scholar]
- 21.Lockitch G. Perspectives on lead toxicity. Clin Biochem. 1993;26:371–381. doi: 10.1016/0009-9120(93)90113-k. [DOI] [PubMed] [Google Scholar]
- 22.Labbe RF. Lead poisoning enzymes. (editorial) Clin Chem. 1990;36:1870–1871. [PubMed] [Google Scholar]
- 23.Rempel D. The lead exposed worker. JAMA. 262:532–534. [PubMed] [Google Scholar]
- 24.Needleman HL, Gatsonis CA. Low-level lead exposure and IQ of children. JAMA. 1990;263:673–678. [PubMed] [Google Scholar]
- 25.Children with elevated blood lead levels attributed to home renovation and remodeling – New York, 1993–1994. JAMA. 1997;277:1030–1031. Report. [PubMed] [Google Scholar]
- 26.Silbergeld EK. Mechanisms of lead neurotoxicity, or looking beyond the lamppost. Faseb J. 1992;6:3201–3206. doi: 10.1096/fasebj.6.13.1397842. [DOI] [PubMed] [Google Scholar]
- 27.Regan CM. Lead-impaired neurodevelopment: mechanisms and threshold values in the rodent. Neurotoxicol Teratol. 1989;11:533–537. doi: 10.1016/0892-0362(89)90033-0. [DOI] [PubMed] [Google Scholar]
- 28.Seppalainen AM, Hernberg S, Vesanto R, Kock B. Early neurotoxic effects of occupational lead exposure: a prospective study. Neurotoxicology. 1983;4:181–192. [PubMed] [Google Scholar]
- 29.Goldstein GW. Evidence that lead acts as a calcium substitute in second messenger metabolism. Neurotoxicology. 1993;14:97–102. [PubMed] [Google Scholar]
- 30.Perazella MA. Lead and the kidney: nephropathy, hypertension and gout. Conn Med. 1996;60:521–526. [PubMed] [Google Scholar]
- 31.Baldwin DR, Marshall WJ. Heavy metal poisoning and its laboratory investigation. Ann Clin Biochem. 1999;36:267–300. doi: 10.1177/000456329903600301. [DOI] [PubMed] [Google Scholar]
- 32.Piomelli S, Rosen JF, Chisholm JJ, Graef JW. Management of childhood lead poisoning. Paediatrics. 1984;105:523–532. doi: 10.1016/s0022-3476(84)80414-x. [DOI] [PubMed] [Google Scholar]
- 33.Campbell MJ, Delves HT. Accurate and precise determination of lead isotope ratios in clinical and environmental samples using inductively coupled plasma source mass spectrometry. J Anal Atom Spectrum. 1989;4:235–236. [Google Scholar]
- 34.Kety SS. The lead citrate complex ion and its role in the physiology and therapy of lead poisoning. J Biol Chem. 1942;142:181–192. [Google Scholar]
- 35.Leckie WJH, Tompsett SL. The diagnostic and therapeutic use of edathamil calcium disodium in excessive inorganic lead absorption. Q J Med. 1958;27:65–82. [PubMed] [Google Scholar]
- 36.Hammond PB. The effect of chelating agents on the tissue distribution and excretion of lead. Toxicol Appl Pharmacol. 1971;18:296–310. doi: 10.1016/0041-008x(71)90121-9. [DOI] [PubMed] [Google Scholar]
- 37.Friedheim EAH, Da Silva JR, Martins AV. Treatment of Schistosomiasis mansoni with antimon-a. a′-dimercapto-potassium succinate (TWSb) Am J Trop Med Hyg. 1954;3:714–727. doi: 10.4269/ajtmh.1954.3.714. [DOI] [PubMed] [Google Scholar]
- 38.Aposhian HV. DMSA and DMPS – water-soluble antidotes for heavy metal intoxication. Ann Rev Pharmacol Toxicol. 1983;23:193–215. doi: 10.1146/annurev.pa.23.040183.001205. [DOI] [PubMed] [Google Scholar]
- 39.Graziano JH, Siris ES, Lolacono N, Silverberg SJ, Turgeon L. 2,3-dimercaptosuccinic acid as an antidote for lead intoxication. Clin Pharmacol Ther. 1985;37:431–438. doi: 10.1038/clpt.1985.67. [DOI] [PubMed] [Google Scholar]
- 40.Porru S, Alessio L. The use of chelating agents in occupational lead poisoning. Occup Med. 1996;46:41–48. doi: 10.1093/occmed/46.1.41. [DOI] [PubMed] [Google Scholar]
- 41.Mann KV, Travers JD. Succimer, an oral lead chelator. Clin Pharm. 1991;10:914–922. [PubMed] [Google Scholar]
- 42.Gerr F, Frumkin H, Hodgins P. Haemolytic anaemis following succimer administration in a glucose-6-phosphate dehydrogenase deficient patient. J Toxicol Clin Toxicol. 1994;32:569–575. doi: 10.3109/15563659409011061. [DOI] [PubMed] [Google Scholar]
- 43.Chisholm JJ., Jr Treatment of acute lead intoxication – choice of chelating agents and supportive therapeutic measures. Cin Toxicol. 1970;3:527–540. doi: 10.3109/15563657008990125. [DOI] [PubMed] [Google Scholar]
- 44.Smith D, Bayer L, Strupp BJ. Efficacy of succimer in reducing brain Pb levels in a rodent model. Environ Res. 1998;78:168–176. doi: 10.1006/enrs.1998.3854. [DOI] [PubMed] [Google Scholar]
- 45.Kostial K, Blanusa M, Piasek M, Restek-Samarzija N, Jones MM, Singh PK. Combined chelation therapy in reducing tissue lead concentrations in suckling rats. J Appl Toxicol. 1999;19:143–147. doi: 10.1002/(sici)1099-1263(199905/06)19:3<143::aid-jat562>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 46.Glotzer DE. The current role of 2,3-dimercaptosuccinic acid (DMSA) in the management of childhood lead poisoning. Drug Safety. 1993;9:85–92. doi: 10.2165/00002018-199309020-00002. [DOI] [PubMed] [Google Scholar]
- 47.Graziano JH, Lolacono NJ, Moulton T, Mitchell ME, Slavkovich V, Zarate C. Controlled study of mes-2,3-dimercaptosuccinic acid for the management of childhood lead intoxication. J Pediatr. 1992;120:133–139. doi: 10.1016/s0022-3476(05)80618-3. [DOI] [PubMed] [Google Scholar]
- 48.Lifshitz M, Hashkanazi R, Phillip M. The effect of 2,3 dimercaptosuccinic acid in the treatment of lead poisoning in adults. Ann Med. 1997;29:83–85. doi: 10.3109/07853899708998747. [DOI] [PubMed] [Google Scholar]
- 49.Besunder JB, Super DM, Anderson RL. Comparison of dimercaptosuccinic acid and calcium disodium ethylenediaminetetraacetic acid versus dimercaptopropanol and ethylenediaminetetraacetic acid in children with lead poisoning. J Pediatr. 1997;130:966–971. doi: 10.1016/s0022-3476(97)70285-3. [DOI] [PubMed] [Google Scholar]
- 50.Flora SJ, Bhattacharya R, Vijayaraghavan R. Combined therapeutic potential of meso-2,3-dimercaptosuccinic acid and calcium disodium edetate in the mobilization and distribution of lead in experimentally intoxicated rats. Fundam Appl Toxicol. 1995;25:233–240. doi: 10.1006/faat.1995.1059. [DOI] [PubMed] [Google Scholar]
- 51.Tandon SK, Singh S, Prasad S, Mathur N. Mobilization of lead by calcium versenate and dimercaptosuccinate in the rat. Clin Exp Pharmacol Physiol. 1998;25:686–692. doi: 10.1111/j.1440-1681.1998.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 52.Kostial K, Blanusa M, Piasek M, Restek-Samarzija N, Jones MM, Singh PK. Combined chelation therapy in reducing tissue lead concentration in suckling rats. J Appl Toxicol. 1999;19:143–147. doi: 10.1002/(sici)1099-1263(199905/06)19:3<143::aid-jat562>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 53.Powell JJ, Burden TJ, Greenfield SM, Taylor PD, Thompson RP. Urinary excretion of essential metals following intravenous injection of calcium disodium edetate: an estimate of free zinc and zinc status in man. J Inorg Biochem. 1999;75:159–165. doi: 10.1016/s0162-0134(99)00054-9. [DOI] [PubMed] [Google Scholar]
- 54.Friedheim E, Graziano JH, Popovac D, Dragovic D, Kaul B. Treatment of lead poisoning by 2,3-dimercaptosuccinic acid. Lancet. 1978;ii:1234–1236. doi: 10.1016/s0140-6736(78)92103-7. [DOI] [PubMed] [Google Scholar]
- 55.Aposhian HV, Maiorino RM, Dart RC, Perry DF. Urinary excretion of meso-2,3-dimercaptosuccinic acid in human subjects. Clin Pharmacol Ther. 1989;45:520–526. doi: 10.1038/clpt.1989.67. [DOI] [PubMed] [Google Scholar]
- 56.Milkovic-Kraus S, Restek-Samarzija N, Samarzija M, Kraus O. Individual variation in response to lead exposure. A dilemma for the occupational health physician. Am J Ind Med. 1997;31:631–635. doi: 10.1002/(sici)1097-0274(199705)31:5<631::aid-ajim19>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]