Abstract
Aims
To investigate the pharmacokinetics and pharmacodynamics of epirubicin and paclitaxel in combination, as well as the effects of paclitaxel and its vehicle Cremophor EL on epirubicin metabolism.
Methods
Twenty-seven female patients with metastatic breast cancer received epirubicin 90 mg m−2 i.v. followed 15 min or 30 h later by a 3 h i.v. infusion of paclitaxel 175, 200 and 225 mg m−2. Plasma concentrations of paclitaxel, epirubicin and epirubicinol were measured and the relationship between neutropenia and drug pharmacokinetics was evaluated using a sigmoid maximum effect (Emax) model. Finally, the influence of paclitaxel and Cremophor EL on epirubicin metabolism by whole blood was examined.
Results
An increase in epirubicinol plasma concentrations occurred after the start of the paclitaxel infusion, resulting in a significant increase in the area under the plasma concentration-time curve (AUC) of epirubicinol (+0.5 µmol l−1 h [95% CI for the difference: 0.29, 0.71],+0.66 µmol l−1 h [95% CI for the difference: 0.47, 0.85] and +0.82 µmol l−1 h [95% CI for the difference: 0.53, 1.11] at paclitaxel doses of 175, 200 and 225 mg m−2, respectively), compared with epirubicin followed by paclitaxel 30 h later (0.61±0.1 µmol l−1 h). A significant increase in epirubicin AUC (+0.74 µmol l−1 h [95% CI for the difference: 0.14, 1.34] and +1.09 µmol l−1 h [95% CI for the difference: 0.44, 1.74]) and decrease in drug clearance (CLTB) (−25.35 l h−1 m−2[95% CI for the difference: −50.18, −0.52] and −35.9 l h−1 m−2[95% CI for the difference −63,4,−8,36]) occurred in combination with paclitaxel 200 and 225 mg m−2 with respect to the AUC (3.16±0.6 µmol l−1 h) and CLTB (74.4±28.4 l h−1 m−2) of epirubicin followed by paclitaxel 30 h later. An Emax relationship was observed between neutropaenia and the time over which paclitaxel plasma concentrations were equal to or greater than 0.1 µmol l−1 (tC0.1). The tC0.1 value predicted to yield a 50% decrease in neutrophil count was 7.7 h. Finally, Cremophor EL markedly inhibited the metabolism of epirubicin to epirubicinol in whole blood.
Conclusions
Paclitaxel/Cremophor EL affects the disposition of epirubicinol and epirubicin. Furthermore, the slope factor of the Emax relationship between neutropenia and tC0.1 of paclitaxel suggests that the drugs might also interact at the pharmacodynamic level.
Keywords: drug interaction, epirubicin, metabolism, paclitaxel, pharmacodynamics, pharmacokinetics
Introduction
The antimicrotubular drug paclitaxel shows therapeutic activity in breast cancer when used as a first- or second-line treatment [1]. In order to enhance the therapeutic potential of paclitaxel, combination regimens were designed with doxorubicin and epirubicin, two of the most active single agents in the treatment of patients with breast cancer [2]. Although response rates ranging from 83 to 94% were obtained with paclitaxel and doxorubicin, the incidence of congestive heart failure (CHF) was unexpectedly high (18–20%) [3, 4]. To explain the enhancement of anthracycline toxicity, the disposition and metabolism of doxorubicin in patients given paclitaxel were examined. Pharmacokinetic analysis showed higher peak plasma concentrations and reduced doxorubicin clearance when paclitaxel was given 24 h before doxorubicin compared with when it was given 24 h after the latter, with enhanced haematologic and nonhaematologic toxicities [5, 6]. Furthermore, doxorubicinol plasma concentrations increased during combined therapy, as compared with doxorubicin alone [5–7]. The use of epirubicin, a less cardiotoxic drug, was a introduced to improve treatment tolerability and to reduce heart damage. Epirubicin undergoes extensive hepatic metabolism and may be administered alone or in combination with alkylating agents or fluoropyrimidines to patients with early or advanced breast cancer [8]. The major adverse effects of epirubicin are acute dose-limiting haematologic toxicity and chronic cardiac damage. However, higher single and cumulative doses of epirubicin can be given compared with doxorubicin [8]. A phase II study of paclitaxel plus epirubicin administered as a first-line treatment to women with metastatic breast cancer showed high response rates and manageable adverse events [9]. However, the risk of cardiotoxicity was somewhat higher than expected with epirubicin alone [10]. The bone marrow and cardiac toxicity profile of epirubicin in combination with paclitaxel suggests the occurrence of a pharmacokinetic and pharmacodynamic interaction between the two drugs.
Therefore, the present study investigated (a) the pharmacokinetics of paclitaxel and its interaction with epirubicin and epirubicinol, (b) the effect of paclitaxel and its vehicle Cremophor EL on epirubicin metabolism in vitro, and (c) the pharmacokinetic–pharmacodynamic relationship for paclitaxel in combination with epirubicin in patients.
Methods
Patients and study design
The present study was performed in breast cancer patients enrolled in a dose-finding protocol on the combination of a fixed dose of epirubicin 90 mg m−2 with paclitaxel starting at 135 mg m−2 in three patients and increased by 20/25 mg m−2 steps in subsequent cohorts of six patients until dose limiting toxicity was experienced [9]. The maximal tolerated and administered doses of paclitaxel were 200 and 225 mg m−2, respectively [9]. For the purpose of the pharmacokinetic and pharmacodynamic investigation, a total of 21 patients at their first cycle of epirubicin 90 mg m−2 in combination with paclitaxel 175 (n = 6 patients), 200 (n = 9 patients) and 225 mg m−2 (n = 6 patients) underwent blood sampling for drug analysis, as well as monitoring for bone marrow toxicity. The pharmacokinetics of epirubicin and epirubicinol alone were examined in an additional group of six patients given epirubicin 90 mg m−2 followed by paclitaxel 200 mg m−2 30 h later, since plasma sampling was discontinued before paclitaxel administration. This group was named ‘epirubicin alone’ for pharmacokinetic purposes and provided drug disposition data for comparison with subjects given the epirubicin–paclitaxel combination with a 15 min time interval between drugs. The delayed administration of paclitaxel has been adopted to reduce the pharmacokinetic interaction that occurs when the drugs are given in close sequence, as demonstrated by this work and similar studies on doxorubicin [6].
The study was performed in compliance with the Helsinki Declaration and was approved by the Ethics Committee of Pisa University Hospital. Patients were advised of the investigational nature of this protocol and written, informed consent was obtained. Eligibility criteria for the dose-finding study were as follows [9]: (a) biopsy proven breast cancer, (b) no adjuvant chemotherapy within 6 months prior to enrolment, (c) total cumulative dose of doxorubicin ≤180 mg m−2 or epirubicin ≤360 mg m−2 and no prior radiotherapy on the mediastinal fields, (d) Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤1, (e) life expectancy ≥3 months, (f) age ≤70 years, (g) absolute neutrophil count ≥1.5×109 l−1, platelets ≥100×109 l−1, bilirubin ≤25 µmol l−1, creatinine ≤120 µmol l−1 and (h) left ventricular ejection fraction (LVEF) ≥50% at bidimensional ultrasonography. A differential blood count was performed twice weekly as well as 48 h or less before the next cycle, while blood chemistry and urinalysis were performed every 3 weeks. In case of grade 3/4 haematologic toxicity, the differential blood count was checked daily. The characteristics of patients included in the pharmacokinetic–pharmacodynamic study were as follows: (a) median age 54 years (range 41–66), (b) median PS 0 (range 0–1), (c) adjuvant chemotherapy in 14 patients (anthracyclines in 6 patients), (d) prior radiotherapy in 9 patients and (e) prior hormonal therapy in 13 subjects.
Drug administration and plasma sampling
Epirubicin (2 mg ml−1 in 0.9% NaCl) (Pharmacia & Upjohn, Milano, Italy) was infused i.v. over 5 min followed 15 min later by paclitaxel (0.6 mg ml−1 in 5% dextrose) (Bristol-Myers Squibb, Princeton, NJ) infused i.v. over 3 h with cycles repeated every 21 days. Dexamethasone 20 mg i.v., cimetidine 300 mg i.v. and orphenadrine 40 mg i.m. were given 0.5–1 h before paclitaxel. Blood samples (4 ml) were taken from an antecubital vein before epirubicin administration and 5, 10, 30 min, 1, 2, 3.25 (end of paclitaxel infusion), 4, 5, 6, 7, 9, 12, 18, 24 and 28 h after epirubicin administration and collected in heparinized tubes. Plasma was separated by centrifugation and stored at −20° C for a maximum of 30 days until drug assays were performed as described below.
Effect of paclitaxel and Cremophor EL on epirubicin metabolism in vitro
Epirubicin is extensively metabolized to epirubicinol at the C-13 carbonyl moiety by a NADPH-dependent cytosolic aldo-keto reductase. Biotransformation occurs in the blood as well as in the liver, due to the presence of these enzymes in erythrocytes [11, 12]. To study the effects of paclitaxel and its vehicle Cremophor EL (Fluka, Buchs, Switzerland) on epirubicin metabolism, heparinized blood was obtained from 10 healthy volunteers, pooled and then divided into 3 ml aliquots (n = 6 replicates for each dose of paclitaxel and Cremophor EL). Stock solutions of epirubicin (0.2 mmol l−1 in 0.9% NaCl) and paclitaxel (2.5 mmol l−1 in absolute ethanol) were prepared, while Cremophor EL was used as purchased without further dilution. Epirubicin (0.2 µmol l−1 final concentration) was added to blood after collection followed 15 min later by the addition of paclitaxel (2.5 and 5 µmol l−1) or Cremophor EL (2.5 and 5 ml l−1). Incubations were performed at 37° C. Aliquots of blood (0.5 ml) were taken at 0, 15, 30, 60 and 90 min of incubation, plasma was separated by centrifugation and stored at −20° C until assayed for epirubicin and epirubicinol. The concentrations of paclitaxel for these in vitro experiments were selected on the basis of the present in vivo study and correspond to drug concentrations achieved during the 3 h infusion of doses of 175–225 mg m−2. The administration of epirubicin 90 mg m−2 is associated with plasma concentrations exceeding 0.2 µmol l−1 for 60–90 min after i.v. injection. Finally, the concentrations of Cremophor EL were chosen on the basis of the findings of a previous report [13] and correspond to peak plasma concentrations achievable after the administration of a standard dose of paclitaxel (175 mg m−2 i.v. over 3 h).
Paclitaxel, epirubicin and epirubicinol assays
Paclitaxel was assayed by reverse-phase high performance liquid chromatography (h.p.l.c.) with u.v. monitoring [14] whereas the analysis of epirubicin and epirubicinol was performed by h.p.l.c. with fluorescence detection [15]. Limits of quantification of paclitaxel and epirubicin–epirubicinol were 10 nmol l−1 and 1 nmol l−1, respectively. Human blank plasma was used as calibrant matrix and the methods were linear (r2>0.995, linear regression analysis, weighting 1/X2) over the analytical range from 10 nmol l−1−1 mmol l−1 for paclitaxel and from 1 nmol l−1−10 mmol l−1 for epirubicin and epirubicinol. The accuracy (overall percent bias) of the assay was within the range of 1.5–7.9% for paclitaxel and −1.8–6.2% for epirubicin and epirubicinol. Both the intra-assay and interassay precision (coefficient of variation) were <6.5% for paclitaxel and <7.5% for epirubicin and epirubicinol.
Pharmacokinetic analysis
Paclitaxel, epirubicin and epirubicinol plasma concentration vs time data were modelled using MW/PHARM software (Mediware, the Netherlands [16]). Initial parameter estimates were determined by curve stripping with Kinstrip module and then fitted by Kinfit module. The nonlinear least-squares, iterative regression of Kinfit determines the slopes and intercepts of the logarithmically plotted curves of polyexponential functions and provides a correlation coefficient of the fitted curve. Modelling of the concentration-time data was done with the Nelder-Mead simplex procedure to determine the parameter values which minimize a weighted least squares criterion and the performance of the fitting procedure was controlled by an interactively defined accuracy factor [16]. While analysing the polyexponential pharmacokinetic data, the convergence was reached when the relative change in the sum of squares was less than 1×10−6 for nonlinear curve fitting/modelling. An open three-compartment model, with model input via constant infusion of drugs, best described the disposition kinetics of epirubicin [8] and paclitaxel [17] in the present study. The fitting of epirubicinol concentration vs time data was performed by a two-exponential decay equation [8], assuming that the conversion of epirubicin to epirubicinol is a first-order process. The following time-concentration polyexponential functions were obtained where Ct is the plasma concentration measured at time t, N is the number of compartments, Ci and Li are, respectively, the xi coefficient and exponent of polyexponential functions, tinf is the infusion time of paclitaxel and km is the rate of metabolite input into the central compartment:
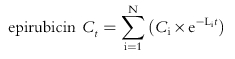 |
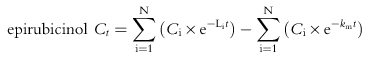 |
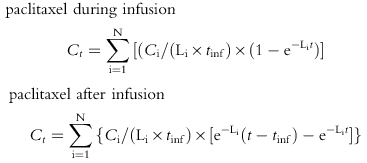 |
Curve fitting yielded the parameters Ci, Li, km and the intercompartmental rate constants kxy. Maximum plasma concentration (Cmax, µmol l−1 or nmol l−1) and time to reach Cmax (tmax, h) were determined graphically from the plasma concentration-time data. Half-lives (t1/2, h) were calculated as 0.693/λz, where λz (h−1) is the negative slope of the log-linear α, β, and γ phases of the plasma concentration-time profiles. The area under the plasma concentration-time curve (zero moment curve, AUC, µmol l−1 h) was calculated using the experimental values (trapezoidal rule) with extrapolation to infinity using the terminal elimination rate constant [18]. Mean residence time (MRT, h) for paclitaxel and epirubicin was determined by dividing the area under the first moment curve (AUMC; µmol l−1 h2) by AUC, with correction for infusion time, while the MRT of epirubicinol was calculated as AUMC AUC−1+[18]. Apparent total body clearance was normalized to the body surface (CLTB = dose AUC−1; l h−1 m−2) and the apparent volume of distribution at steady state (Vss, l m−2) was computed as Vss = V1×[1+k12/k21+k13/k31], where kxy are the intercompartmental rate constants and V1 is the volume of distribution of the central compartment [18]. Finally, the period in which paclitaxel concentrations were equal to or greater than 0.1 µmol l−1 (tC0.1, h) was estimated from individual fitted plasma concentration-time plots [19].
Pharmacodynamic analysis
The relationship between drug exposure and haematologic toxicity was evaluated. Scatterplots of percent decrease in absolute neutrophil count (ANC):
were constructed as a function of time over which plasma paclitaxel concentrations were above 0.1 µmol l−1, a threshold level associated with myelotoxicity [19] or parameters of epirubicin exposure (AUC and AUMC [21, 22]). Relationships were fitted according to sigmoidal maximum effect (Emax) model [18] using nonlinear least squares regression and a weighting factor of unity, defined as:
where κ is the shape factor that describes the sigmoidicity of the concentration-effect curve and PK50 is the value of the pharmacokinetic parameter (PK) that results in 50% of the Emax. The performance of the pharmacodynamic model was evaluated using the relative root mean square error (%RMSE) value and its standard error (%SE):
 |
where N is the number of P pairs (i.e. true with predicted values) and the prediction error is pe = [ln(Ppredicted value)−ln(Ptrue value)]; in the best model the percentage RMSE approaches zero.
Statistical analysis
Results are presented as mean values ± standard deviation (s.d.) as well as mean differences and 95% confidence intervals for the differences between the means of pharmacokinetic parameters and data from in vitro experiments on epirubicin metabolism [23]. If the confidence interval does not contain the value 0, then there is a statistically significant difference between the means of the two samples at the 95% confidence level. In addition to this, a two-tailed Student's t-test for unpaired observations was used to determine whether the difference between the two means was statistically significant. If the computed P value is less than 0.05, the null hypothesis can be rejected. Statistical comparisons were performed assuming that the variances of the samples were equal, which was confirmed by running an F-test to compare standard deviations. Statistical analyses were performed by the Statgraphics 5 Plus package (Manugistics, Rockville, MD).
Results
Pharmacokinetics of paclitaxel
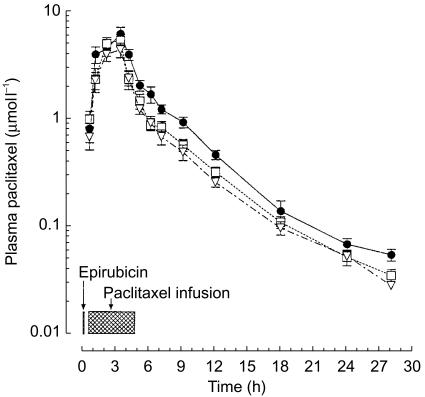
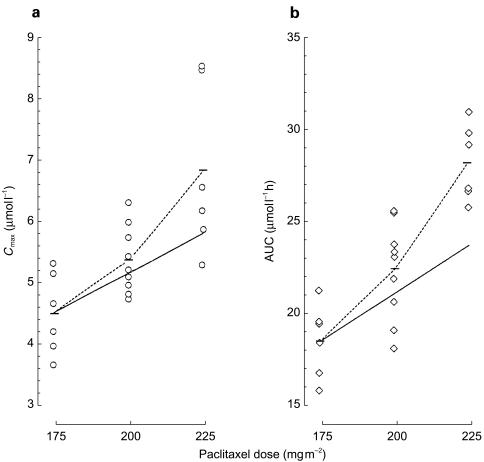
The plasma disposition of paclitaxel is shown in Figure 1 and the pharmacokinetic parameters are listed in Table 1. Cmax was reached at the end of the infusion and was significantly higher (P<0.05) by 0.86 µmol l−1 (95% CI for the difference: 0.19, 1.53) and 2.31 µmol l−1 (95% CI for the difference: 0.93, 3.68) at paclitaxel 200 and 225 mg m−2, respectively, with respect to a dose of 175 mg m−2 (4.53±0.66 µmol l−1). Likewise, dose escalation was associated with a significant increase (P<0.05) in the AUC at paclitaxel 200 mg m−2 (+3.97 µmol l−1 h; 95% CI for the difference: 0.93, 7.01) and 225 mg m−2 (+9.85 µmol l−1 h; 95% CI for the difference: 6.83, 12.87) compared with paclitaxel 175 mg m−2 (18.51±2.6 µmol l−1 h). A 28% increment in paclitaxel dose (from 175 to 225 mg m−2) resulted in a mean Cmax and AUC increase of 50% and 53%, respectively, indicating saturation kinetics (Figure 2). Accordingly, the CLTB of paclitaxel was significantly decreased (P<0.05) at a dose of 225 mg m−2 (−3.08 l h−1 m−2; 95% CI for the difference: −4.99, −1.16) as compared with 175 mg m−2 (12.29±1.84 l h−1 m−2; Table 1). Finally, additional pharmacokinetic parameters for paclitaxel, including t1/2, Vss, MRT changed to some extent with the dose increase, although the differences were not significant, with the exception of tC0.1 (+4.66 h; 95% CI for the difference: 0.17, 9.15) at 225 mg m−2, as compared with the 175 mg m−2 dose (15.20±2.78 h).
Figure 1.
Plasma concentration vs time curves for paclitaxel 175 (▿), 200 (□) and 225 (•) mg m−2 i.v. Symbols: mean values; vertical bars, s.d.
Table 1.
Pharmacokinetic parameters for paclitaxel administered as a 3 h i.v. infusion.
| Paclitaxel 175 mg m−2 Mean±s.d. | Paclitaxel 200 mg m−2 Difference between means 200 vs 175 mg m−2 (95% CI) | Paclitaxel 225 mg m−2 Difference between means 225 vs 175 mg m−2 (95% CI) | |
|---|---|---|---|
| Patients | 6 | 9 | 6 |
| Cmax (µmol l−1) | 4.53±0.66 | 0.86 (0.19, 1.53)* | 2.31 (0.93, 3.68)* |
| t½,α (h) | 0.72±0.42 | −0.13 (−0.51, 0.25) | −0.19 (−0.64, 0.26) |
| t½,β (h) | 1.66±0.43 | 0.07 (−0.29, 0.43) | 0.52 (−0.37, 1.41) |
| t½,γ (h) | 13.93±3.29 | −1.85 (−5.57, 1.87) | 0.69 (−2.96, 4.34) |
| AUC (µmol l−1 h) | 18.51±2.6 | 3.97 (0.93, 7.01)* | 9.85 (6.83, 12.87)* |
| CLTB (l h−1 m−2) | 12.29±1.84 | −2.02 (4.49, −0.46) | −3.08 (−4.99, −1.16)* |
| Vss (l m−2) | 52.4±23.5 | 18.1 (−8.51, 44.71) | 34.4 (−13.23, 82.03) |
| MRT (h) | 6.64±1.27 | −1.2 (−2.62, 0.22) | −1.44 (−3.15, 0.27) |
| tC0.1 (h) | 15.20±2.78 | 2.64 (−1.04, 6.32) | 4.66 (0.17, 9.15)* |
P<0.05 unpaired t-test vs paclitaxel 175 mg m−2.
Figure 2.
Scatter plots of individual values of Cmax (a) and AUC (b) after increasing doses of paclitaxel in 21 subjects. The dotted line links the observed mean values (horizontal bars) of Cmax and AUC, while the solid line intersects the theoretical mean values if Cmax and AUC increased proportionally with dose.
Pharmacokinetics of epirubicin
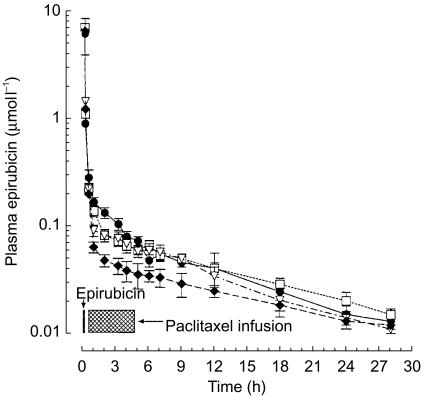
The plasma concentration-time profiles of epirubicin are presented in Figure 3 and the pharmacokinetic parameters are listed in Table 2. Plasma concentrations of epirubicin decreased sharply after injection, due to rapid tissue distribution, and then slowly declined as a result of drug elimination (Figure 3). Most pharmacokinetic parameters for epirubicin administered in combination with paclitaxel, including Cmax, t1/2, Vss and MRT displayed minor changes across all groups of patients, and no significant differences were observed with respect to epirubicin alone (Table 2). The AUC of epirubicin in combination with paclitaxel at a dose of 200 and 225 mg m−2 increased significantly (P<0.05) by 0.74 µmol l−1 h (95% CI for the difference: 0.14, 1.34) and 1.09 µmol l−1 h (95% CI for the difference: 0.44, 1.74), respectively, as compared with treatment with epirubicin plus paclitaxel (175 mg m−2) (3.16±0.6 µmol l−1 h; Table 2). Finally, combined treatment with paclitaxel at doses of 175, 200 and 225 mg m−2 was associated with a progressive decrease in the CLTB of epirubicin that reached the accepted level of significance (P<0.05) at paclitaxel dose of 200 mg m−2 (−25.35 l h−1 m−2; 95% CI for the difference: −50.18, −0.52) and 225 mg m−2 (−35.9 l h−1 m−2; 95% CI for the difference: −63.4, −8.36) compared with epirubicin alone (74.4±28.4 l h−1 m−2; Table 2).
Figure 3.
Plasma concentration vs time curves of epirubicin 90 mg m−2 i.v. administered alone (♦) or followed 15 min later by paclitaxel 175 (▿), 200 (□) and 225 (•) mg m−2 i.v. Symbols: mean values; vertical bars, s.d.
Table 2.
Pharmacokinetic parameters for epirubicin administered at 90 mg m−2 i.v. alone or in combination with paclitaxel.
| Epirubicin alone Mean±s.d. | Epirubicin+paclitaxel 175 mg m−2 Difference in means 175 vs epirubicin alone (95% CI) | Epirubicin+paclitaxel 200 mg m−2 Difference in means 200 vs epirubicin alone (95% CI) | Epirubicin+paclitaxel 225 mg m−2 Difference in means 225 vs epirubicin alone (95% CI) | |
|---|---|---|---|---|
| Patients | 6 | 6 | 9 | 6 |
| Cmax (µmol l−1) | 6.44±1.2 | −0.28 (−1.76, 1.2) | 0.5 (−0.53, 1.53) | −0.25 (−1.38, 0.88) |
| t½,α (h) | 0.06±0.03 | 0 (−0.04, 0.04) | 0.02 (−0.05, 0.01) | 0 (−0.03, 0.03) |
| t½,β (h) | 1.37±0.4 | 0.21 (−0.35, 0.77) | 0.25 (−0.07, 0.58) | 0.31 (−0.12, 0.74) |
| t½,γ (h) | 16.0±4.8 | 0.44 (−6.31, −7.19) | 0.01 (−7.02, 7.04) | 1.42 (−3.96, 6.8) |
| AUC (µmol l−1 h) | 3.16±0.6 | 0.41 (−0.46, 1.28) | 0.74 (0.14, 1.34)* | 1.09 (0.44, 1.74)* |
| CLTB (l h−1 m−2) | 74.4±28.4 | −20.2 (−49.16, 8.76) | −25.35 (−50.18, −0.52)* | −35.9 (−63.4, −8.36)* |
| Vss (l m−2) | 480.1±153.5 | −111.3 (−276.4, 53.76) | −31.6 (−270.4, 207.2) | −124 (−308, 60.4) |
| MRT (h) | 8.97±4.99 | −0.39 (−5.68, 4.90) | 1.63 (−4.38, 7.64) | 3.76 (−5.17, 12.7) |
P<0.05 unpaired t-test vs epirubicin alone.
Pharmacokinetics of epirubicinol
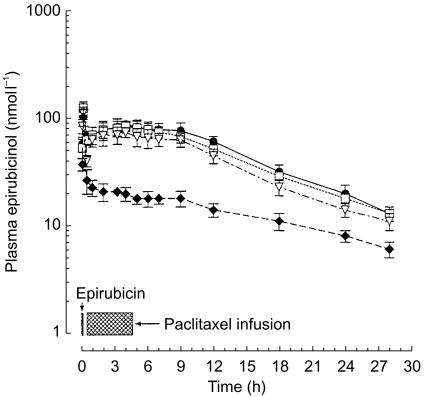
The infusion of epirubicin was followed 0.17 h later (tmax 1) by peak plasma concentrations of epirubicinol (Cmax 1: 103.5±18.1 nmol l−1; Figure 4, Table 3). After the start of paclitaxel infusion, the metabolite showed a rebound phenomenon, and a second peak (Cmax 2) was observed approximately 4 h (tmax 2) after the bolus of epirubicin. The mean concentration of epirubicinol reached at Cmax 2 was 75 nmol l−1 with paclitaxel 175 mg m−2; the administration of higher doses of the taxane was associated with a significant increase in the Cmax 2 of epirubicinol (+10.7 nmol l−1; 95% CI for the difference: 0.65, 20.75, at paclitaxel 200 mg m−2 and +13.7 nmol l−1; 95% CI for the difference: 3.35, 24.06, at paclitaxel 225 mg m−2; Table 3). Epirubicinol showed a biexponential decline and plasma concentrations of the metabolite persisted for some time after the end of the paclitaxel infusion (Figure 4). The t½,α of epirubicinol was significantly increased (P<0.05) by 1.03, 1.26 and 01.38 h in patients given epirubicin followed by paclitaxel at doses 175, 200 and 225 mg m−2, compared with epirubicin alone (0.30±0.08 h). Finally, a marked increase (P<0.05) was also observed in the plasma AUC values of epirubicinol (+0.5,+0.66 and +0.82 µmol l−1 h after administration of epirubicin in combination with paclitaxel 175, 200 and 225 mg m−2, respectively) compared with epirubicin alone (AUC: 0.61±0.1 µmol l−1 h; Table 3). Other pharmacokinetic parameters, i.e. t1/2,β and MRT, displayed minor changes below the level of statistical significance (Table 3).
Figure 4.
Plasma concentration vs time curves for epirubicinol in patients given epirubicin 90 mg m−2 i.v. administered alone (♦) or followed 15 min later by paclitaxel 175 (▿), 200 (□) and 225 (•) mg m−2 i.v. Symbols: mean values; vertical bars, s.d.
Table 3.
Pharmacokinetic parameters for epirubicinol after administration of epirubicin 90 mg m−2 i.v. alone or in combination with paclitaxel.
| Treatment data | Epirubicin alone Mean±s.d. | Epirubicin+paclitaxel 175 mg m−2 Difference in means 175 vs epirubicin alone (95% CI) | Epirubicin+paclitaxel 200 mg m−2 Difference in means 200 vs epirubicin alone (95% CI) | Epirubicin+paclitaxel 225 mg m−2 Difference in means 225 vs epirubicin alone (95% CI) |
|---|---|---|---|---|
| Patients | 6 | 6 | 9 | 6 |
| Cmax 1 (nmol l−1) | 103.5±18.1 | 10.7 (−10.86, 32.26) | 23.2 (−2.73, 43.67) | 17.9 (−3.6, 39.4) |
| Cmax 2 (nmol l−1) | / | 75.0±8.1¶ | 10.7 (0.65, 20.75)** | 13.7 (3.35, 24.06)** |
| tmax 1 (h) | 0.17±0.0 | 0 | 0 | 0 |
| tmax 2 (h) | / | 3.88±0.42¶ | −0.13 (−0.81, 0.55)# | −0.25 (−0.79, 0.29)# |
| t½,λ1 (h) | 0.30±0.08 | 1.03 (0.79, 1.27)* | 1.26 (0.86, 1.66)* | 1.38 (1.11, 1.65)* |
| t½,λ2 (h) | 20.85±7.71 | −2.75 (−11.15, 5.65) | −3.14 (−10.03, 3.75) | −2.02 (−9.9, 5.86) |
| AUC (µmol l−1 h) | 0.61±0.1 | 0.5 (0.29, 0.71)* | 0.66 (0.47, 0.85)* | 0.82 (0.53, 1.11)* |
| MRT (h) | 22.2±8.4 | −3.72 (−14.23, 6.79) | −2.53 (−12.61, 7.55) | −6 (−17.2, 5.17) |
P<0.05 unpaired t-test vs epirubicin alone.
Mean difference and 95% CI for the difference vs epirubicin+paclitaxel 175 mg m −2; P<0.05 unpaired t-test
Mean difference and 95% CI for the difference vs epirubicin+paclitaxel 175 mg m−2.
Mean±s.d.
Effects of paclitaxel and Cremophor EL on epirubicin metabolism in vitro
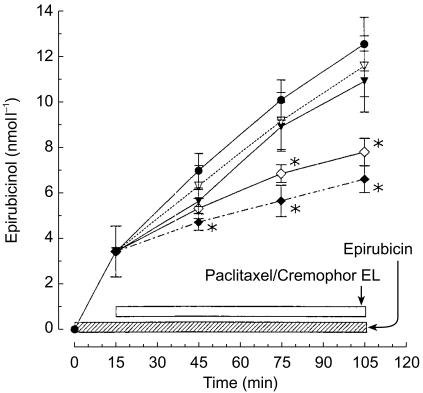
Epirubicin was metabolized by whole blood, as shown by the time-dependent production of epirubicinol up to a mean value 12.5±1.1 nmol l−1 after 105 min of incubation (Figure 5). The addition of paclitaxel to the samples to give a final concentration of 2.5 and 5 µmol l−1 resulted in a slightly decreased production of epirubicinol (−0.93 and −1.6 nmol l−1, respectively), after 90 min of incubation, which was not significant. However, a marked inhibition of epirubicinol production was observed at various time points in the presence of Cremophor EL (2.5 and 5 ml l−1) (Figure 5), its concentration being decreased by −4.71 nmol l−1 (95% CI for the difference: −5.85, −3.57) and −5.91 nmol l−1 (95% CI for the difference: −7.04, −4.78), respectively, after 90 min of incubation (P<0.05 vs epirubicin alone).
Figure 5.
Effect of paclitaxel (2.5 (▿) and 5 (▾) µmol l−1) or Cremophor EL (2.5 (⋄) and 5 (♦) ml l−1) on the production of epirubicinol by whole blood containing epirubicin 0.2 µmol l−1. • epirubicin. Symbols: mean values; vertical bars, standard deviation of the mean. *P<0.05.
Pharmacokinetic–pharmacodynamic relationships
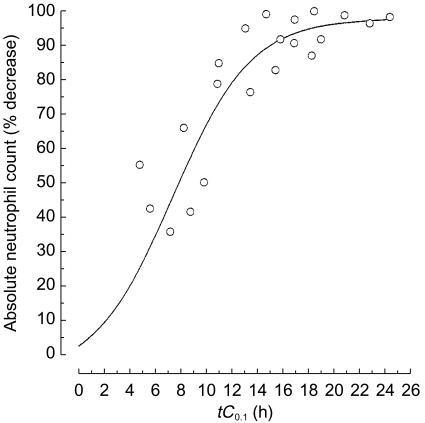
The nadir of the absolute neutrophil count was reached more frequently on day 12 (range, 8–17) and the percentage of courses with neutrophil count of less than 0.5×109 l−1 were 88, 49 and 84% at paclitaxel doses 175, 200 and 225 mg m−2, respectively. The incidence of febrile neutropaenia was low (4% of courses), because of the lack of mucositis and the limited duration of severe (grade IV) neutropaenia (median, 4 days) [9]. Analysis demonstrated that the percentage decrease in absolute neutrophil count was related to the time of exposure to paclitaxel concentrations greater than or equal to 0.1 µmol l−1 (tC0.1), as described by the sigmoid maximum effect (Emax) pharmacodynamic model. The plot in Figure 6 depicts the Emax relationship using the data obtained from all patients treated with epirubicin at a dose of 90 mg m−2 followed by paclitaxel at a dose of 175, 200 and 225 mg m−2. On the basis of Emax model fitting, the tC0.1 predicted to yield a 50% decrease in absolute neutrophil count was 7.7 h. Indeed, the shape of the curve was steep (slope = 3.1) and this model provided a strong correlation between pharmacokinetics and dose-limiting neutropaenia (r2 = 0.95, P<0.05). Data analysis by either linear or other nonlinear functions was unsatisfactory and no correlation could be found (r2<0.25). Finally, no correlation was found between the AUC or AUMC of epirubicin or epirubicinol and neutropaenia (data not shown).
Figure 6.
Pharmacokinetic–pharmacodynamic correlation between the percent decrease in absolute neutrophil count and tC0.1 of paclitaxel in patients treated with epirubicin and paclitaxel. The solid line indicate the best fit of a sigmoid Emax pharmacodynamic model to the data.
Discussion
The present study investigated the pharmacokinetic and pharmacodynamic profile of paclitaxel and epirubicin in breast cancer patients. The experimental findings suggest that the changes in epirubicin and epirubicinol disposition are likely to be the consequence of the influence of paclitaxel/Cremophor EL on drug elimination rather than on epirubicin metabolism. In addition to this, the work presents original evidence of the unidirectional interaction of paclitaxel/Cremophor EL on epirubicin disposition, since the pharmacokinetics of paclitaxel were similar when the latter was given alone and in combination with epirubicin [17]. The increased plasma AUC of epirubicin and epirubicinol might contribute, at least in part, to the higher risk of cardiotoxicity in patients given the combined treatment compared with epirubicin alone [10]. Finally, the enhanced neutropenic effect of paclitaxel given in combination with epirubicin, as indicated by the steeper slope factor of the Emax relationship between neutrophil count decrease and tC0.1 compared with paclitaxel alone [19], may represent the consequence of the combined effect of the taxane and the anthracycline at the bone marrow level.
The data from this work are in agreement with previous studies, revealing the occurrence of significant differences in pharmacokinetics and toxicity depending on the sequence and schedule of administration of paclitaxel in combination with doxorubicin or epirubicin [5–7, 24, 25]. Thus, the 24 h administration of paclitaxel 125 mg m−2 immediately before a 48 h infusion of doxorubicin 48 mg m−2 resulted in 70% increase in doxorubicin Cmax and 32% reduction in drug clearance compared with the reverse sequence of drug administration [5]. The simultaneous 72 h infusion of paclitaxel 180 mg m−2 and doxorubicin 60 mg m−2 was associated with increased steady-state plasma concentrations of doxorubicinol (mean 25 nmol l−1) compared with doxorubicin alone (16 nmol l−1), with no changes in paclitaxel or doxorubicin distribution [7, 26]. Furthermore, a 3 h infusion of paclitaxel was associated with nonlinear doxorubicin disposition and increased concentrations of doxorubicin and doxorubicinol [6]. A pharmacokinetic study in patients given epirubicin 90 mg m−2 in combination with paclitaxel 175 mg m−2 showed significant changes in epirubicinol disposition compared with patients administered epirubicin 90 mg m−2[27].
Previous studies have not investigated the pharmacokinetic–pharmacodynamic relationship for paclitaxel and epirubicin and have not determined whether paclitaxel affects the pharmacokinetic profile of epirubicin in a dose-dependent manner.
The rebound phenomenon observed for epirubicinol after beginning the infusion of paclitaxel 175–225 mg m−2 and the changes in epirubicin AUC and CLTB after the administration of paclitaxel 200–225 mg m−2 are most likely the result of impairment of epirubicin elimination. Biliary clearance of epirubicin and metabolites occurs by active excretion mediated by P-glycoprotein (P-gp [22]), the product of the mdr1a multidrug resistance gene. Paclitaxel and Cremophor EL are good substrates for P-gp [28, 29] and competition for this carrier protein may result in decreased hepatic clearance and increased AUC of anthracyclines, as previously shown for doxorubicin and doxorubicinol [6]. In the present work, an in vitro study was performed in order to test the hypothesis that epirubicin metabolism by aldo-keto reductase was induced, causing the marked increase in epirubicinol concentrations following paclitaxel administration. However, the in vitro findings seem to rule out the involvement of aldo-keto reductase in the changes in the pharmacokinetics of epirubicinol, since paclitaxel did not significantly affect the production of epirubicinol by whole blood. The marked inhibition of epirubicinol formation displayed by Cremophor EL might be dependent either on direct inhibition of enzyme activity or on entrapment of epirubicin in micelles, as previously suggested for paclitaxel [30]. Whatever the mechanism might be, its clinical relevance is likely to be negligible since the extremely low volume of distribution of Cremophor EL in humans suggests that it is limited to the central plasma compartment, and tissue penetration is insignificant [31]. Therefore, the inhibition of epirubicinol excretion through competition for P-gp by paclitaxel and Cremophor EL appears the most likely explanation of the pharmacokinetic interaction, although studies that directly address this hypothesis are lacking. The absence of significant changes in epirubicin pharmacokinetics in combination with paclitaxel at doses below 200 mg m−2 may be influenced by the extensive metabolism of epirubicin by the liver, which might offset any impairment of elimination. Indeed, in addition to C4-aglycone formation by glycosidic cleavage and C-13 alcohol formation by carbonyl reduction, epirubicin and epirubicinol are conjugated with glucuronic acid, whereas doxorubicin and doxorubicinol are not [8].
Preclinical studies have shown that paclitaxel/Cremophor EL significantly increase cardiac tissue concentrations of epirubicin in mice [32] and rats [33]. Although there is no evidence that this also occurs in man and that the increased AUC of epirubicin is associated with an enhanced risk of cardiac toxicity, the animal findings and the perturbances of epirubicin pharmacokinetics in patients suggest caution and strict cardiac minitoring in the management of subjects given paclitaxel–epirubicin in combination. Indeed, the risk of CHF was somewhat higher in patients given epirubicin in combination with paclitaxel (7.7, 19 and 48.7% at epirubicin 720, 1000 and 1080 mg m−2 cumulative dose respectively) [10], compared with epirubicin alone (3.1, 15 and 33% at 850, 1000 and 1080 mg m−2 cumulative dose, respectively) [34], although clinical cardiac toxicity did not develop more rapidly. In contrast, the combination of doxorubicin and paclitaxel markedly increased the occurrence of CHF [3], which might be explained by a greater pharmacokinetic interaction between doxorubicin and paclitaxel, as shown by the present study and that of Gianni et al.[6], or by the differing cardiotoxicity of parent drugs and their metabolites [35]. The cardiac damage caused by anti-tumour anthracyclines has been attributed to the formation of drug semiquinones which generate superoxide anion, which reduces ferritin- and non-ferritin-bound Fe(III), favouring the release of Fe(II), which is then involved in free radical reactions in a NADPH-dependent process [35]. There is evidence for an active role of the alcohol metabolites of anthracyclines in their cardiotoxic effects [35]. In particular, human myocardial tissue metabolizes anthracyclines by enzymatic two-electron reduction of the C-13 carbonyl group in the side chain of the drug. The formation of secondary alcohol metabolites from doxorubicin is much greater than from epirubicin [35].
The disposition of paclitaxel was found to be non-linear, since Cmax and AUC displayed a non-proportional increase with respect to dose. Moreover, the Emax sigmoid curve representing the relationship between tC0.1 and the severity of neutropaenia, displayed a steeper profile for the combination of paclitaxel and epirubicin compared with paclitaxel given alone [19], and the tC0.1 time interval associated with a 50% reduction in absolute neutrophil count was 7.7 h in the present study compared with a value of 11.16 h in a previous report using paclitaxel alone [19]. The difference in the slope factor in the nonlinear relationship between neutropaenia and paclitaxel plasma concentrations equal to or greater than 0.1 µmol l−1 might be partly the result of the combined myelotoxic effect of epirubicin and paclitaxel.
In conclusion, the findings of the present study provide evidence of a significant influence of paclitaxel/Cremophor EL on epirubicinol and epirubicin disposition, as well as of enhancement of neutropaenia. Therefore, modified regimens for epirubicin–paclitaxel treatment should be considered, with drug administration to be separated by a suitable time interval in order to minimize an interaction and to avoid antagonistic effects on the cell cycle [36] which may impair therapeutic efficacy.
Acknowledgments
This work was supported in part by research grants from the Italian Association for Cancer Research (AIRC, Milano, Italy) and from Bristol Myers-Squibb (Roma, Italy).
References
- 1.Miller KD, Sledge GW., Jr Taxanes in the treatment of breast cancer: a prodigy comes of age. Cancer Invest. 1999;17:121–136. [PubMed] [Google Scholar]
- 2.Kaufman PA. Paclitaxel and anthracycline combination chemotherapy for metastatic breast cancer. Semin Oncol. 1999;26:39–46. [PubMed] [Google Scholar]
- 3.Gianni L, Munzone E, Capri G, et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer. High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol. 1995;13:2688–2699. doi: 10.1200/JCO.1995.13.11.2688. [DOI] [PubMed] [Google Scholar]
- 4.Gehl J, Boesgaard M, Paaske T, Vittrup Jensen B, Dombernowsky P. Combined doxorubicin and paclitaxel in advanced breast cancer: effective and cardiotoxic. Ann Oncol. 1996;7:687–693. doi: 10.1093/oxfordjournals.annonc.a010717. [DOI] [PubMed] [Google Scholar]
- 5.Holmes FA, Madden T, Newman RA, et al. Sequence-dependent alteration of doxorubicin pharmacokinetics by paclitaxel in a phase I study of paclitaxel and doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1996;14:2713–2721. doi: 10.1200/JCO.1996.14.10.2713. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Viganò L, Locatelli A, et al. Human pharmacokinetic characterization and in vitro study of the interaction between doxorubicin and paclitaxel in patients with breast cancer. J Clin Oncol. 1997;15:1906–1915. doi: 10.1200/JCO.1997.15.5.1906. [DOI] [PubMed] [Google Scholar]
- 7.Berg SL, Cowan KH, Balis FM, et al. Pharmacokinetics of taxol and doxorubicin administered alone and in combination by continuous 72-hour infusion. J Natl Cancer Inst. 1994;86:143–145. doi: 10.1093/jnci/86.2.143. [DOI] [PubMed] [Google Scholar]
- 8.Coukell AJ, Faulds D. Epirubicin. An updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of breast cancer. Drugs. 1997;53:453–482. doi: 10.2165/00003495-199753030-00008. [DOI] [PubMed] [Google Scholar]
- 9.Conte PF, Baldini E, Gennari A, et al. Dose-finding study and pharmacokinetics of epirubicin and paclitaxel over 3 hours: a regimen with high activity and low cardiotoxicity in advanced breast cancer. J Clin Oncol. 1997;15:2510–2517. doi: 10.1200/JCO.1997.15.7.2510. [DOI] [PubMed] [Google Scholar]
- 10.Gennari A, Salvadori B, Donati S, et al. Cardiotoxicity of epirubicin/paclitaxel-containing regimens: role of cardiac risk factors. J Clin Oncol. 1999;17:3596–3602. doi: 10.1200/JCO.1999.17.11.3596. [DOI] [PubMed] [Google Scholar]
- 11.Qin KN, Cheng KC. Structure and tissue-specific expression of the aldo-keto reductase superfamily. Biochemistry. 1994;33:3223–3228. doi: 10.1021/bi00177a012. [DOI] [PubMed] [Google Scholar]
- 12.Dockham PA, Sreerama L, Sladek NE. Relative contribution of human erythrocyte aldehyde dehydrogenase to the systemic detoxification of the oxazaphosphorines. Drug Metab Dispos. 1997;25:1436–1441. [PubMed] [Google Scholar]
- 13.Sparreboom A, van Zuylen L, Brouwer E, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59:1454–1457. [PubMed] [Google Scholar]
- 14.Jamis-Dow CA, Klecker RW, Sarosy G, Reed E, Collins JM. Steady-state plasma concentrations and effects of taxol at a 250 mg/m2 dose in combination with granulocyte-colony stimulating factor in patients with ovarian cancer. Cancer Chemother Pharmacol. 1993;33:48–52. doi: 10.1007/BF00686022. [DOI] [PubMed] [Google Scholar]
- 15.Fogli S, Danesi R, Innocenti F, et al. An improved HPLC method for therapeutic drug monitoring of daunorubicin, idarubicin, doxorubicin, epirubicin, and their 13-dihydro metabolites in human plasma. Ther Drug Monit. 1999;21:367–375. doi: 10.1097/00007691-199906000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Proost JH, Meijer DKF. MW/PHARM, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med. 1992;22:155–163. doi: 10.1016/0010-4825(92)90011-b. [DOI] [PubMed] [Google Scholar]
- 17.Gianni L, Kearns CM, Giani A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic–pharmacodynamic relationships in humans. J Clin Oncol. 1995;13:180–190. doi: 10.1200/JCO.1995.13.1.180. [DOI] [PubMed] [Google Scholar]
- 18.Rowland M, Tozer TN. In: Clinical Pharmacokinetics: Concepts and Applications. 3. Rowland M, Tozer TN, editors. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 19.Huizing MT, Keung AC, Rosing H, et al. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. J Clin Oncol. 1993;11:2127–2135. doi: 10.1200/JCO.1993.11.11.2127. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Vermorken JB. The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs. 1998;55:5–30. doi: 10.2165/00003495-199855010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Bastholt L, Dalmark M, Gjedde SB, et al. Dose–response relationship of epirubicin in the treatment of postmenopausal patients with metastatic breast cancer: a randomized study of epirubicin at four different dose levels performed by the Danish Breast Cancer Cooperative Group. J Clin Oncol. 1996;14:1146–1155. doi: 10.1200/JCO.1996.14.4.1146. [DOI] [PubMed] [Google Scholar]
- 22.Gurney HP, Ackland S, Gebski V, Farrell G. Factors affecting epirubicin pharmacokinetics and toxicity: evidence against using body-surface area for dose calculation. J Clin Oncol. 1998;16:2299–2304. doi: 10.1200/JCO.1998.16.7.2299. [DOI] [PubMed] [Google Scholar]
- 23.Zar JH. In: Biostatistical Analysis. Zar JH, editor. Englewood Cliffs: Prentice Hall; 1984. [Google Scholar]
- 24.Venturini M, Lunardi G, Del Mastro L, et al. Sequence effect of epirubicin and paclitaxel treatment on pharmacokinetics and toxicity. J Clin Oncol. 2000;18:2116–2125. doi: 10.1200/JCO.2000.18.10.2116. [DOI] [PubMed] [Google Scholar]
- 25.Grasselli G, Viganò L, Capri G, et al. Clinical and pharmacologic study of the epirubicin and paclitaxel combination in women with metastatic breast cancer. J Clin Oncol. 2001;19:2222–2231. doi: 10.1200/JCO.2001.19.8.2222. [DOI] [PubMed] [Google Scholar]
- 26.Fisherman JS, Cowan KH, Noone M, et al. Phase I/II study of 72-hour infusional paclitaxel and doxorubicin with granulocyte colony-stimulating factor in patients with metastatic breast cancer. J Clin Oncol. 1996;14:774–782. doi: 10.1200/JCO.1996.14.3.774. [DOI] [PubMed] [Google Scholar]
- 27.Esposito M, Venturini M, Vannozzi MO, et al. Comparative effects of paclitaxel and docetaxel on the metabolism and pharmacokinetics of epirubicin in breast cancer patients. J Clin Oncol. 1999;17:1132–1140. doi: 10.1200/JCO.1999.17.4.1132. [DOI] [PubMed] [Google Scholar]
- 28.Parekh H, Wiesen K, Simpkins H. Acquisition of taxol resistance via P-glycoprotein- and non-P-glycoprotein-mediated mechanisms in human ovarian carcinoma cells. Biochem Pharmacol. 1997;53:461–470. doi: 10.1016/s0006-2952(97)83383-7. [DOI] [PubMed] [Google Scholar]
- 29.Sikic BI, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40:S13–S19. doi: 10.1007/s002800051055. [DOI] [PubMed] [Google Scholar]
- 30.van Tellingen O, Huizing MT, Panday VR, Schellens JH, Nooijen WJ, Beijnen JH. Cremophor EL causes (pseudo-) non-linear pharmacokinetics of paclitaxel in patients. Br J Cancer. 1999;81:330–335. doi: 10.1038/sj.bjc.6690696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparreboom A, Verweij J, van der Burg ME, et al. Disposition of Cremophor EL in humans limits the potential for modulation of the multidrug resistance phenotype in vivo. Clin Cancer Res. 1998;4:1937–1942. [PubMed] [Google Scholar]
- 32.Colombo T, Gonzalez Paz O, Zucchetti M, et al. Paclitaxel induces significant changes in epidoxorubicin distribution in mice. Ann Oncol. 1996;7:801–805. doi: 10.1093/oxfordjournals.annonc.a010758. [DOI] [PubMed] [Google Scholar]
- 33.Platel D, Pouna P, Bonoron-Adèle S, Robert J. Preclinical evaluation of the cardiotoxicity of taxane-anthracycline combinations using the model of isolated perfused rat heart. Toxicol Appl Pharmacol. 2000;163:135–140. doi: 10.1006/taap.1999.8847. [DOI] [PubMed] [Google Scholar]
- 34.Ryberg M, Nielsen D, Skovsgaard T, Hansen J, Jensen BV, Dombernowsky P. Epirubicin cardiotoxicity: an analysis of 469 patients with metastatic breast cancer. J Clin Oncol. 1998;16:3502–3508. doi: 10.1200/JCO.1998.16.11.3502. [DOI] [PubMed] [Google Scholar]
- 35.Minotti G, Cavaliere AF, Mordente A, et al. Secondary alcohol metabolites mediate iron delocalization in cytosolic fractions of myocardial biopsies exposed to anticancer anthracyclines. Novel linkage between anthracycline metabolism and iron-induced cardiotoxicity. J Clin Invest. 1995;95:1595–1605. doi: 10.1172/JCI117833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoli W, Ricotti L, Barzanti F, et al. Schedule–dependent interaction of doxorubicin, paclitaxel and gemcitabine in human breast cancer cell lines. Int J Cancer. 1999;80:413–416. doi: 10.1002/(sici)1097-0215(19990129)80:3<413::aid-ijc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]