Abstract
Aims
To determine whether metformin pretreatment has beneficial effects in clomiphene resistant infertile women with polycystic ovary syndrome (PCOS) in an infertility clinic.
Methods
This was a randomized placebo controlled double-blind crossover study of 3 months metformin (1500 mg day−1)/placebo, followed by 3 months metformin/placebo together with clomiphene (50–100 mg for 5 days) for three cycles in clomiphene resistant women with PCOS. The primary outcomes were restoration of spontaneous menses, ovulation induction (spontaneous or clomiphene induced) and pregnancy. Secondary endpoints were changes in biochemical parameters related to androgens and insulin.
Results
Twelve women completed the metformin arm and 14 the placebo arm. Spontaneous menstruation resumed in five metformin treated patients and in six placebo treated women, P = 0.63. No women given metformin spontaneously ovulated, although one patient given placebo did, P = 0.30. There was no difference in the efficacy of clomiphene between the two groups with ovulation being induced in five (out of 12) metformin treated women and four (out of 14) placebo treated women, P = 0.63. Pregnancy occurred in three (out of 12) women given metformin and two (out of 14) women given placebo, P = 0.59.
Conclusions
Metformin is not always beneficial when given to clomiphene resistant infertile women with PCOS in clinical practice.
Keywords: androgens, clomiphene, insulin, menstruation, metformin, ovulation, PCOS
Introduction
Polycystic ovary syndrome (PCOS) is a common condition characterized by hirsutism, anovulation and menstrual irregularity. There is a broad clinical spectrum with compromised fertility an important issue for patients. Given that insulin resistance is thought to play a key part in the syndrome [1, 2], it is theoretically appealing to treat PCOS by ameliorating insulin resistance. Both biguanide and thiazolidinedione drugs are insulin sensitizing agents used in the treatment of Type 2 diabetes. A number of reports have now been published suggesting beneficial effects from both metformin [3] and troglitazone [4] in women with PCOS. Metformin acts by decreasing gluconeogenesis as well as increasing peripheral utilization of glucose in the presence of endogenous insulin [5].
Metformin has not been associated with any congenital abnormalities when given to diabetic women who have subsequently become pregnant [6, 7]. The UK National Teratology Service records endorse this view.
Current fertility therapy includes the use clomiphene citrate to stimulate ovulation. Concerns have been raised about the number of clomiphene courses that a woman should undergo with reports linking clomiphene to ovarian cancer [8]. It is prudent therefore to limit the number of clomiphene cycles a woman is exposed to. Given that a proportion of women with PCOS do not respond to clomiphene [9], pretreatment with metformin offers a theoretically sound therapeutic manoeuvre that could enhance the efficacy of clomiphene and further reduce the number of clomiphene cycles required to induce fertility.
We have studied the effects of metformin in a cohort of clomiphene resistant infertile women attending an infertility clinic to see whether in clinical practice pretreatment enhances ovulation and pregnancy rates. Women selected for this study had therefore already tried a minimum two courses of clomiphene at 50 and 100 mg, and had not ovulated in response to this treatment.
Methods
This study was approved by the Nottingham City Hospital Ethics Committee. Written informed consent was obtained in all women recruited. Women (aged 18–40 years) who wished to conceive were recruited from the Fertility and Endocrinology clinics at City Hospital, Nottingham. For inclusion, they had to have exhibited preceding chronic oligomenorrhoea (menstrual cycle greater than 40 days for 6 months) or amenorrhoea and a day 20–22 serum progesterone ≤ 10 nmol l−1. They had to have demonstrated persisting anovulation, defined on ultrasound scanning as an endometrial thickness of ≤ 5 mm and absence of an ovarian follicle ≥ 14 mm, despite the administration of clomiphene citrate 100 mg for 5 days in the follicular phase.
At baseline physical observations including body mass index, lying blood pressure (after 5 min supine) and Ferriman Gallwey (a semiquantitative, clinical) score for hirsutism were measured and an ovarian ultrasound scan performed. The following biochemical investigations were undertaken, performed on day 5 in women with periods and randomly in amenorrhoeic women: Serum oestradiol, testosterone, LH, FSH, prolactin (automated immunoassay on a Bayer ACS:Centaur); SHBG, insulin (Automated immunoassay on a DPC Immulite analyser); 17OH progesterone (in house r.i.a.); and fasting glucose (Olympus AU 600 Assay). Previously validated HOMA-IR equations were then used to assess insulin resistance (fasting insulin×fasting glucose)/22.5 and β cell function (20×fasting insulin)/(fasting glucose −3.5) [10]. Free androgen index was calculated from the formula (Testosterone×1000)/SHBG.
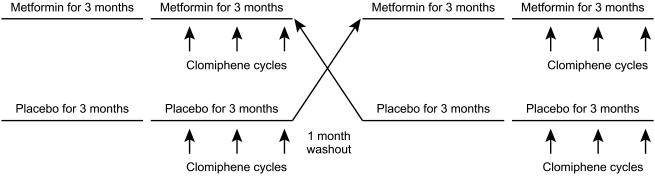
This was a randomized double-blind placebo controlled crossover design trial of metformin/placebo (Figure 1). Patients were exposed to metformin/placebo for 6 months with a minimum 1 month washout period observed. The dose of Metformin/Placebo was built up gradually over a 3 week period to minimize side-effects (500 mg three times daily final dose; beginning at 500 mg daily with main meal for 1 week, followed by 500 mg twice daily for 1 week and increased to 500 mg three times daily, provided there were no side-effects). Metformin/placebo was given for 12 weeks, at which time an assessment of menstruation frequency and ovulation, as indicated by serial ovarian scans and luteal phase progesterone estimations (> 10 nmol l−1), were made. If metformin/placebo alone induced regular menses and ovulation, metformin/placebo was continued and cycles were observed for a further 3 months. If menstruation had not occurred by week 12, it was induced by a progesterone challenge test (medroxyprogesterone 5 mg daily for 5 days). If ovulation had not occurred by week 12, metformin/placebo was continued and ovulation induction attempted with clomiphene. Follicular phase clomiphene citrate (50 mg daily for 5 days on days 2–6) was administered and evidence of ovulation was monitored as before. If ovulation did not occur with clomiphene 50 mg, the dose of clomiphene citrate was increased to 100 mg. A total of three clomiphene courses were given. Repeat biochemical estimations were carried out after the 3 month treatment period.
Figure 1.
The study design.
Primary outcomes were ovulation (spontaneous or clomiphene induced), pregnancy and restoration of spontaneous menses. Secondary endpoints were changes in biochemical parameters.
If pregnancy occurred during the first treatment phase, then the subject was not then eligible for crossover into the second phase. Regular requests for prescriptions of study drugs were used as a means of assessing compliance.
We based our power calculation on the results of Nestler et al. [11] that showed that metformin/clomiphene resulted in 90% ovulation induction compared with 8% ovulation induction with placebo/clomiphene. Setting α as 0.05 and β as 0.2 we calculated that a crossover sample size of 12 patients would give an 80% chance of detecting a treatment difference on ovulation induction at a two-sided 0.05 significance level. We allowed for a 10% drop out and therefore aimed to recruit at least 14 women into the study. Data are given as mean and 95% confidence intervals and were analysed parametrically using students unpaired t-test. Categorical variable data were analysed using contingency tables. The statistical software package used was Stat 100 (Biosoft Inc.).
Results
Nineteen women were recruited into the study. Only two women did not have ultrasound appearances consistent with PCOS. At recruitment, the mean age was 30.1 (28.3–31.8) years, weight 86.3 (78.1–94.6) kg, BMI 33.3 (30.2–36.3), blood pressure 118 (111–126)/77 (73–82), mmHg and the Ferriman Gallwey score 9 (7–12). Baseline clinical chemistry is given in Table 1.
Table 1.
Baseline clinical chemistry data for all 19 women recruited into the study.
| Parameter | Mean (95% confidence interval) |
|---|---|
| Fasting glucose (mmol l−1) | 5.2 (5.0, 5.4) |
| Fasting insulin (mIU l−1) | 15.9 (11.9, 19.8) |
| Insulin resistance | 3.7 (2.7, 4.8) |
| β cell function | 189 (150, 229) |
| Testosterone (nmol l−1) | 2.3 (2.0, 2.5) |
| SHBG (nmol l−1) | 29 (24, 35) |
| Free androgen index | 89 (68, 110) |
| Oestradiol (pmol l−1) | 267 (164, 371) |
| 17 Hydroxyprogesterone (nmol l−1) | 8.9 (6.8, 10.9) |
| LH (U l−1) | 12.6 (9.2, 15.9) |
| FSH (U l−1) | 5.4 (4.2, 6.6) |
| Prolactin (mU l−1) | 314 (222, 407) |
Twelve women completed the metformin arm and 14 the placebo arm. Spontaneous menstruation resumed in five metformin treated patients and in six placebo treated women, P = 0.63. No women given metformin spontaneously ovulated, although one patient given placebo did, P = 0.30. There was no difference in the efficacy of clomiphene between the two groups with ovulation being induced in five (out of 12) metformin treated women and four (out of 14) placebo treated women, P = 0.63. Pregnancy occurred in 3 (out of 12) women given metformin and 2 (out of 14) women given placebo, P = 0.59.
Excluding data from those patients who became pregnant during the study, by the end of 6 months therapy, there were no significant differences in the following parameters comparing metformin with placebo: change in weight −1.0 kg (−8.4 to +8.7) vs +0.2 (−4.8 to +6.2) P = 0.73, change in BMI −1.1(−3.3 to +3.5) vs +0.1(−1.8 to +2.45) P = 0.41, change in blood pressure 0(−26 to + 18)/0 (−4 to + 12) mmHg vs 0(−12 to + 15)/2 (−8 to +6) P = 0.91 systolic; P = 0.37 diastolic or change in Ferriman-Gallwey score −2(−3 to +1) vs −2(−6–0) P = 0.57. The changes in clinical chemistry parameters after 6 months metformin/placebo are given in Table 2. There was no significant difference in any of these parameters comparing metformin with placebo.
Table 2.
Change in clinical chemistry after 6 months therapy (mean (95% confidence interval)).
| Parameter | Change in placebo group (n = 12) | Change in metformin group (n = 9) | P value |
|---|---|---|---|
| Fasting glucose (mmol l−1) | −0.1 (−0.45, 0.001) | −0.2 (−0.4, 0.03) | 0.85 |
| Fasting insulin (mIU l−1) | −2.1 (−4.6,+4.0) | −1.5 (−4.8,+4.8) | 0.83 |
| Insulin resistance | −0.37 (−1.35,+1.50) | −0.60 (−1.35,+0.85) | 0.73 |
| β cell function | −29.17 (−44.1,+73.1) | +9.64 (−13.4,+67.7) | 0.73 |
| Testosterone (nmol l−1) | −0.15 (−0.33,+0.96) | +0.4 (−0.8,+0.3) | 0.17 |
| SHBG (nmol l−1) | +3 (−25.98,+14.6) | +4.5 (+0.33,+9.66) | 0.29 |
| Free androgen index | −2.3 (−29.5,+35.7) | −24.7 (−28.6,−4.3) | 0.36 |
Based on the request and dispensing of prescriptions we have no reason to doubt our patients' adherence to treatment.
Discussion
Our randomized placebo controlled trial in clomiphene resistant PCOS women has suggested that in clinical practice not every woman with polycystic ovary syndrome will benefit from metformin treatment to enhance ovulation. This was a practical clinic based study in patients wishing to become pregnant. As such, we opted for a cross-over design to allow every patient the opportunity to try metformin therapy. It is one of the longest metformin exposure studies published and inevitably some women dropped out, in general opting to try in vitro fertilization therapy instead. No women stated side-effects as a main reason for withdrawing. It is a small single centre study, although patient numbers are comparable with many published studies.
We have found that metformin used in practice in a fertility clinic was no more likely than placebo to normalize menstrual cycles, or ovulation rates, whether spontaneous or stimulated. We tried to replicate the results of the study reported by Nestler et al. [11] who carried out a 1 month study of metformin (1500 mg day−1) vs placebo. In that study, one third of metformin treated women spontaneously ovulated compared with only 4% placebo controlled women. That study also gave clomiphene therapy after metformin/placebo and found that 90% of the metformin pretreated women ovulated in response to clomiphene compared with only 8% of the placebo pretreated women [11]. In a follow-up study by the same group, metformin was given for 7 weeks to women who had been shown to be clomiphene resistant. The rate of ovulation induction with clomiphene fell to 75%, with 27% placebo women also ovulating with clomiphene, suggesting that the cohort were not all truly clomiphene resistant [12]. In that study, the pregnancy rate per ovulatory cycle in the metformin group was 21% and in the placebo group was 16%. Such data are in keeping with our findings. Our study has given the longest course of metformin to clomiphene resistant patients (6 months) and we have found inconsistent effects on ovulation induction. It appears that some women are more likely to respond to metformin than others are. Characteristics of patients that have previously been shown to favour response to metformin include a higher body mass index, higher fasting insulin and lipid concentrations, higher blood pressure, lower androstenedione concentrations, and less severe menstrual irregularities [13]. The characteristics of our patients were similar to the nonresponder group reported in this study [13]. It is also accepted that weight reduction per se is beneficial in women with PCOS [14, 15]. One report found that metformin and diet was no more effective than placebo/diet in women with PCOS [16]. The beneficial effect of metformin may therefore lie in its abilities to enhance weight reduction. Our study subjects did not lose any significant amount of weight during treatment, and this may be another reason why no positive effects were seen.
Metformin has not been shown to influence androgen concentrations when compared with placebo in PCOS women. Our study found no change in total testosterone, SHBG or free androgen index following 6 months therapy with metformin. Studies of 2 months therapy with metformin have all concluded that there is no change in androgen concentrations [11, 12, 17]. A comparable duration of metformin therapy to our study (7 months) also found that the serum testosterone concentration in the metformin group was 0.49 ng ml−1 whilst in the placebo group was 0.47 ng ml−1[18].
The HOMA-IR index is a validated estimate of insulin resistance [10]. Published studies have shown HOMA-IR indices in normal subjects to be 1.7. Subjects with impaired fasting glucose (IFG) have a value of 2.6, and impaired glucose tolerance (IGT) have values of 2.4 [19]. Our values of above 3.0 are well above normal, indicating insulin resistance. We have been unable to demonstrate any improvement in insulin resistance or β cell function when metformin is given to PCOS women. Other placebo-controlled studies on the effect of metformin on insulin have concurred with our findings that fasting insulin concentrations do not alter [11, 18]. Insulin sensitivity assessed by i.v. insulin tolerance test and insulin concentrations in response to an oral glucose tolerance test have also not improved after 2 months metformin therapy [17], although one study did find a reduction in the stimulated insulin concentrations in response to a glucose load [11].
In conclusion, our clinical study suggests that caution needs to be applied when considering the value of metformin in women with PCOS. Current evidence suggests metformin may be beneficial in a subset, but by no means all, of patients with PCOS.
References
- 1.Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62:904–910. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- 2.Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF, Sheikh Z. Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med. 1992;327:157–162. doi: 10.1056/NEJM199207163270304. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinaemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism: Clin Exp. 1994;43:647–654. doi: 10.1016/0026-0495(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R. The insulin sensitising agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 1996;81:3299–3306. doi: 10.1210/jcem.81.9.8784087. [DOI] [PubMed] [Google Scholar]
- 5.Davidson MB, Peter AL. An overview of metformin in the treatment of type 2 diabetes mellitus. Am J Med. 1997;102:99–110. doi: 10.1016/s0002-9343(96)00353-1. [DOI] [PubMed] [Google Scholar]
- 6.Piacquadio K, Hollingsworth DR, Murphy H. Effects of in-utero exposure to oral hypoglycaemic drugs. Lancet. 1991;338:866–869. doi: 10.1016/0140-6736(91)91512-s. [DOI] [PubMed] [Google Scholar]
- 7.Helmuth E, Damm P, Molsted-Pedersen L. Congenital malformations in offspring of diabetic women treated with oral hypoglycaemic agents during embryogenesis. Diabetic Med. 1994;11:471–474. doi: 10.1111/j.1464-5491.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 8.Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumours in a cohort of infertile women. N Engl J Med. 1994;331:771–776. doi: 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- 9.Lobo RA, Gysler M, March CM, Goebelsmann U, Mishell DR. Clinical and laboratory predictors of clomiphene response. Fertil Steril. 1982;37:168–174. [PubMed] [Google Scholar]
- 10.Matthews DR, Hasker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostatic model assessment. Insulin resistance and [beta]-cell function from plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med. 1998;338:1876–1880. doi: 10.1056/NEJM199806253382603. [DOI] [PubMed] [Google Scholar]
- 12.Vandermolen DT, Ratts VS, Evans WS, Stovall DW, Kauma SW, Nestler JE. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril. 2001;75:310–315. doi: 10.1016/s0015-0282(00)01675-7. [DOI] [PubMed] [Google Scholar]
- 13.Moghetti P, Castello R, Negri C, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in Polycystic Ovary Syndrome: a randomized, double blind, placebo controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–146. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 14.O'Dea JPK, Wieland RG, Hallberg MC, Llerena LA, Zorm EM, Genuth SM. Effect of dietary weight loss on sex steroid binding, sex steroids, and gonadotrophins in obese premenopausal women. J Lab Clin Med. 1979;93:1004–1008. [PubMed] [Google Scholar]
- 15.Kiddy DS, Hamilton-Fairley D, Seppala M, et al. Diet-induced changes in sex hormone binding globulin and free testosterone in women with normal or polycystic ovaries: correlation with serum insulin and insulin-like growth factor-1. Clin Endocrinol. 1989;31:757–763. doi: 10.1111/j.1365-2265.1989.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 16.Crave J-C, Fimbel S, Lejeune H, Cugnardey N, Dechaud H, Pugeat M. Effects of diet and metformin administration on sex hormone-binding globulin, androgens, and insulin in hirsute and obese women. J Clin Endocrinol Metab. 1995;80:2057–2062. doi: 10.1210/jcem.80.7.7608255. [DOI] [PubMed] [Google Scholar]
- 17.Acbay O, Gundogdu S. Can metformin reduce insulin resistance in polycystic ovary syndrome? Fertil Steril. 1996;65:946–949. [PubMed] [Google Scholar]
- 18.Pasquali R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the Polycystic Ovary Syndrome. J Clin Endocrinol Metabolism. 2000;85:2767–2773. doi: 10.1210/jcem.85.8.6738. [DOI] [PubMed] [Google Scholar]
- 19.Tripathy D, Carlsson M, Almgren P, et al. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49:975–980. doi: 10.2337/diabetes.49.6.975. [DOI] [PubMed] [Google Scholar]