Abstract
Aims
The antimalarial efficacy/pharmacodynamics and pharmacokinetics of intramuscular (i.m.) artemotil in Thai patients with acute uncomplicated falciparum malaria were studied to determine effective dose regimens and to compare these with the standard dose regimen of artemether.
Methods
In part I of the study three different artemotil dose regimens were explored in three groups of 6–9 patients for dose finding: 3.2 mg kg−1 on day 0 and 1.6 mg kg−1 on days 1–4 (treatment A), 1.6 mg kg−1 on day 0 and 0.8 mg kg−1 on days 1–4 (treatment B), 3.2 mg kg−1 on day 0 and 0.8 mg kg−1 on days 1–4 (treatment C). In part II of the study, artemotil treatments A and C were compared in three groups of 20–22 patients with standard i.m. artemether treatment: 3.2 mg kg−1 on day 0 and 0.8 mg kg−1 on days 1–4 (treatment R).
Results
Full parasite clearance was achieved in all patients in Part I, but parasite clearance time (PCT) and fever clearance time (FCT) tended to be longer in treatment B. Also the incidence of recrudescence before day 28 (RI) tended to be higher for treatment B. In part II, the mean PCT for each of the two artemotil treatments (52 and 55 h, respectively) was significantly longer than for artemether (43 h). The 95% CI for the difference A vs R was 0, 16 h (P = 0.0408) and for difference C vs R it was 2, 19 h (P = 0.0140). FCT was similar for the three treatments. The incidence of RI ranged from 5 out of 19 for treatment C to 3 out of 20 for treatment R. Plasma concentration-time profiles of artemotil indicated an irregular and variable rate of absorption after i.m. injection. A late onset of parasite clearance was associated with delayed absorption and/or very low initial artemotil plasma concentrations. Pharmacokinetic-pharmacodynamic evaluations supported a relationship between the rate of parasite clearance and exposure to artemotil during approximately the first 2 days of treatment, and suggested that artemotil has a slower rate of absorption than artemether. Safety assessment, including neurological and audiometric examinations showed no clinically relevant findings. Adverse events before and during treatment included headache, dizziness, nausea, vomiting and abdominal pain. These are characteristic of acute malaria infections and resolved during treatment.
Conclusions
The optimum dose regimen for artemotil in this study was identical to the standard dose regimen of artemether. The findings that artemotil is more slowly absorbed from the i.m. injection site than artemether, and that early systemic availability may be insufficient for an immediate onset of parasite clearance contributed to the decision to choose a higher loading dose of artemotil (divided over two injection sites) and to omit the fifth dose in later studies. With this optimized dosing schedule, the more pronounced depot characteristics of i.m. artemotil can be an advantage, since it may allow shorter hospitalization.
Keywords: arteether, artemether, artemotil, dose-finding, malaria, pharmacokinetics, Plasmodium falciparum
Introduction
Quinine is still widely used for treating severe chloroquine-resistant malaria. However, the disadvantages include the short half-life, possible toxic effects on the cardiovascular and central nervous systems, and painful local reactions after i.m. and i.v. administration. In addition, several studies show declining sensitivity and decreased clinical response of P. falciparum to quinine [1]. Artemisinin (qinghaosu) and its derivatives have been extensively studied during the past 20 years for the treatment of multidrug resistant Plasmodium falciparum[2–6]. They act early in asexual parasite development by destroying the small ring forms and have been reported to reduce parasitaemia by more than 90% after 24 h of treatment [3, 7–9].
Two artemisinin derivatives are currently used for the parenteral treatment of severe (cerebral) malaria. Intravenous artesunate, and i.m. artemether have been registered in China and Thailand. In addition, i.m. artemether is registered in some African countries. Although i.v. artesunate causes a faster onset of parasiticidal action, the artemisinin products for i.m. injection have the advantage of better chemical stability and the ease of once daily i.m. administration. This is not only a practical advantage, but it may also be life saving for patients with severe malaria in rural settings where treatment with intravenous medication is impossible, and where storage conditions are suboptimal.
In 1985, the World Health Organization (WHO) Steering Committee for the Scientific Working Group on the Chemotherapy of Malaria decided to evaluate another derivative of artemisinin, artemotil (previously named beta-arteether). Artemotil was chosen for development for three major reasons: artemotil would not have the instability problems of sodium artesunate; its biochemical breakdown would not give methanol, as does artemether; and lastly it is easily formulated in oil for parenteral administration. Chinese investigators had already synthesized artemotil and documented its antimalarial activity [10–12]. The objective of WHO was to seek drug registration of artemotil in a European country and in malaria endemic countries world-wide, in order to be available at the lowest possible cost. Registration in an EU-member state would serve as a means to supply nongovernmental organizations (NGOs) for medical/humanitarian aid working in malaria endemic areas. With WHO support, the preclinical development was undertaken and co-ordinated by the Walter Reed Army Institute of Research (WRAIR, Washington, USA) [13–16].
For the clinical development, a collaborative project was initiated in 1991 between WHO and the Dutch company ARTECEF BV (Maarssen, the Netherlands). In May 2000, artemotil was registered and received market approval by the Dutch authorities under the name of Artecef® 50 or Artecef® 150 (containing 50 and 150 mg artemotil ml−1, respectively, in sesame oil). Like artemether, artemotil has been developed as an intramuscular formulation for the treatment of severe (cerebral) malaria in children and adolescents. The severity may range from patients who are no longer able to swallow or retain oral medication up to comatose patients with severe cerebral malaria.
Artecef® is the only artemisinin-derived product for the indication of severe malaria with a European format registration. Because of WHO's involvement in the development, Artecef® is available at a cost-price plus limited margin for the nonprofit sector (NGOs) of the market.
The tolerability, safety and pharmacokinetics of artemotil in healthy adult volunteers has been demonstrated in phase I clinical studies [17]. Phase II/III clinical studies in Thailand and Africa have demonstrated the efficacy of artemotil in adults and children against chloroquine- and quinine-resistant P. falciparum in severe malaria [18–21].
In the present paper, we report the findings of the first phase II study designed to determine the best dose regimen for i.m. artemotil (part I) and to compare its safety and efficacy with artemether (part II) in patients with acute uncomplicated P. falciparum malaria. The rate of parasite clearance was considered the primary efficacy parameter in this study, since it was considered the most relevant endpoint to predict the efficacy for the treatment of severe malaria.
Methods
Patients
In part I of the study, 25 young Thai male and female adults (16–40 years), were selected who presented with acute uncomplicated P. falciparum malaria and parasite count 1.000–50 000/mm3. In the second part, 63 Thai male or female subjects (16–50 years) with acute uncomplicated P. falciparum malaria and higher parasite counts (10 000–100 000 per mm3), were included. All patients were recruited from the population of malaria patients referred to the Hospital for Tropical Diseases in Bangkok, Thailand between March 1994 and July 1995. Pre-study assessments included clinical laboratory evaluation, full physical and neurological examination, ECG, urine screen for antimalarial drugs and a pregnancy test for female patients.
Patients were included who had a medical history without major pathology and without deviations in clinical laboratory parameters in blood and urine that could place the patients at risk during the study (e.g. indications for renal failure, hypoglycaemia or severe anaemia). A predose blood smear was taken for parasite count and identification. Reasons for exclusion were complicated or severe malaria [22], pretreatment with antimalarial drugs within 7 days (14 days for mefloquine and pyrimethamine-sulfadoxine), pregnancy or participation in a drug trial or donation of blood within 90 days prior to the start of the study.
Ethics
Both studies were conducted after obtaining the approval of the Medical Ethics Committee of the Mahidol University, Bangkok. Informed consent was obtained from all patients and the studies were performed in accordance with the Declaration of Helsinki and its subsequent amendments.
Design, study drugs, treatments and assessments
Part I was an open-label dose-finding design in which three sequential groups of patients received gluteal i.m. injections of artemotil once daily for 5 days with an interim safety evaluation between successive dose groups. In the protocol, seven dose regimens were considered for dose-finding and the selection of each dose was based on the efficacy of the preceding dose regimen. Ultimately, three dose regimens were actually given in part I.
Part II was an open-label, randomized efficacy study in three parallel groups of patients; Treatments A and C from the first part were compared with the standard regimen of artemether as reference treatment (R). Patient numbers were assigned according to the order of inclusion, and treatments had been assigned to patient numbers by a computer-generated randomization schedule.
Artemotil was formulated as ampoules containing 1 ml of a solution of 150 mg ml−1 in sesame oil for intramuscular injection (batch no. ACE93 P070/332009).
Artemether was available as ampoules containing 1 ml of a solution of 80 mg ml−1 in peanut oil for intramuscular injection (Injectio Artemtheri; Kunming Pharmaceutical Factory P.R. China).
The dosing regimens for part I and part II, are listed below:
Treatment Part I
A-13.2 mg kg−1 artemotil on day 0 and 1.6 mg g−1 on days 1–4
B-11.6 mg kg−1 artemotil on day 0 and 0.8 mg kg−1 on days 1–4
C-13.2 mg kg−1 artemotil on day 0 and 0.8 mg kg−1 on days 1–4
Treatment Part II
A-II3.2 mg kg−1 artemotil on day 0 and 1.6 mg kg−1 on days 1–4
C-II3.2 mg kg−1 artemotil on day 0 and 0.8 mg kg−1 of on days 1–4
R (reference treatment)3.2 mg kg−1artemether on day 0 and 1.6 mg kg−1 of on days 1–4
Most patients stayed at the Hospital for Tropical Diseases for the full 28 days following drug administration. Patients who left the hospital returned for the day 14, 21 and 28 assessments and were required to stay in Bangkok during the study period to preclude re-infection.
Artemotil was injected for 5 days, each day at the same time. Injections were administered in the upper 1/3 of the lateral gluteal muscle and the injection site was changed alternately. Injection sites were inspected daily. Body temperature, pulse and respiration rate were evaluated every 4 or 6 h until body temperature was below 37° C. Blood films for parasite counts were obtained every 4–6 h after the first injection until films were negative. Thereafter, blood films and temperature readings were taken once daily in order to determine recrudescence.
In the dose-finding part of the study (part I) a series of blood samples (5 ml) were taken for analysis of artemotil. These were collected just before and 3 h after injection on days 0–3 and just before and 3, 6, 12, 24, 48, 72, 96 h after dosing on day 4. For safety assessments during the study, blood pressure was recorded daily and ECGs were recorded on days 2, 4, 7, 14 and 28. All ECG recordings were specifically analysed and evaluated with respect to potential QTc-interval prolongation. Adverse events were monitored regularly throughout the study. Blood and urine samples were collected for clinical laboratory tests. The safety assessments also included neurological examinations before and 3 h after injection on days 0, 2 and 4 and on days 7, 14 and 28. These neurological examinations also included audiometric assessments (Rinne test at 512 cp, Weber test at 512 cp and high-tone audiogram).
Parameters for efficacy and pharmacodynamics
For parasite counting, Giemsa-stained thick and thin blood films were obtained. Parasite density was determined by counting parasitized and unparasitized red blood cells (500) on a thin film. The number of parasitized cells per µl was calculated on the basis of the patient's most recent red cell count. If parasite numbers were too low to be counted on thin films, these were determined by counting parasites against 200 white blood cells on thick films. The number of parasites per µl were calculated from the patient's most recent white blood cell count. A film was considered negative if no malaria parasites were detected after examining a minimum of 200 oil-fields on the thick blood film. Parasite clearance time (PCT) was defined as the time (in hours) from the start of the treatment until the time of the first of three successive negative slides. In addition, a 99% parasite clearance time (PCT99%) was determined. This was the time from start of treatment to reach a 99% reduction relative to maximum parasitemia as determined by interpolation of individual parasite count vs time plots. The PCT99% was to be used for correlation with pharmacokinetic parameters.
Failure of parasite response to treatment was rated as RI and RII according to standard WHO definitions: RI = recrudescent parasitaemia within 28 days after complete initial clearance; RII = persistent parasitaemia until day 7.
The fever clearance time (FCT) was defined as the time of the first of the three observations of body temperature ≤37.0° C.
Pharmacokinetics
Plasma concentrations of artemotil were measured in plasma samples using a reversed-phase h.p.l.c. method with reductive electrochemical detection [23]. The calibration range was 5- to 1000 ng ml−1. Calibration curves showed a linear response vs concentration, with r2 values of at least 0.997. The within and between day precision were better than 15% at different concentration levels of the quality control samples (25, 100 and 250 ng ml−1). All reported concentrations above 5 ng ml−1 have been included in the pharmacokinetic evaluations.
The following pharmacokinetic parameters were derived from the artemotil plasma concentrations measured by h.p.l.c.: Cmax (peak plasma concentration on day 4), t½ (elimination half-life after the last dose) and AUC(96,120 h) (area under plasma concentration-time curve over 24 h after the last dose). t½ was derived by log-linear regression on the terminal part of the plasma concentration profile after the last dose. AUC(96,120 h) was calculated using the linear trapezoidal rule. In addition, for the determination of pharmacokinetic/pharmacodynamic relationships the area under the plasma concentration-time curve from 0 to 51 h after the first dose was calculated (AUC(0,51 h)) following the linear trapezoidal rule.
Evaluation and statistics
Patients were included in the assessment of efficacy/pharmacodynamics if they could be followed up until full parasite clearance or if they had at least 7 days follow-up of parasitaemia. For the parameters for efficacy/pharmacodynamics, descriptive statistics were presented per treatment. For the comparative part of the study (Part II), Wilcoxon scores (rank sums) were compared by treatment, and treatments were compared on a pair wise basis (A-II vs C-II and B-II vs C-II) using the Wilcoxon 2-sample test with continuity correction of 0.5). All patients receiving study medication were included in the safety evaluation.
Pharmacokinetic parameters were also presented descriptively per treatment. The relationship between pharmacodynamics (PCT99%) and pharmacokinetics was explored by correlation of PCT99%vs AUC(0,51 h).
Results
Patients
Of the 25 patients with acute uncomplicated malaria enrolled in the dose-finding study (part I), 23 were evaluated for efficacy/pharmacodynamics and for pharmacokinetics, and 25 for safety and tolerability. Two patients (who received treatment C-I) were excluded from efficacy and pharmacokinetic evaluation since they had participated in another antimalaria study within 3 months of enrolment in the present study. Evidence of use of other antimalarials was found in the pre-dose plasma samples of these subjects.
Apart from the excluded patients, evidence of previous use of other antimalarials was found in the pre-dose plasma samples of two subjects in treatment A-I (2×quinine), two in treatment B-I (quinine and chloroquine) and three in treatment C-I (mefloquine and 2×chloroquine).
The demographic data in Table 1 show that the three successive treatment groups in the dose-finding study (A-I, B-I and C-I) were similar at baseline except for the predose geometric mean level of parasitaemia in group C-I which was somewhat lower compared with the other two treatments. However, the duration and extent of fever before dosing was similar for all groups.
Table 1.
Mean demographic and baseline characteristics per treatment group.
| Part I: Dose-finding | Part II: Comparative study | |||||
|---|---|---|---|---|---|---|
| A-I | B-I | C-I | A-II | C-II | R | |
| Total (male/female) | 8(7/1) | 6(3/3) | 11(7/4) | 21(15/6) | 20(11/9) | 22(11/11) |
| Mean age (years) | 21.0 | 23.7 | 21.9 | 24 | 25 | 25 |
| (range) | (16–30) | (17–30) | (16–40) | (16–34) | (16–48) | (16–50) |
| Mean height (cm) | 159.6 | 157.7 | 159.9 | 161 | 160 | 158 |
| Mean weight (kg) | 48.6 | 47.5 | 47.5 | 50.1 | 48.7 | 49.5 |
| Duration of predose fever (days) | 5.6 | 2.8 | 3.8 | 5.8 | 6.2 | 5.8 |
| Highest temperature predose (°C) | 38.0 | 37.9 | 37.8 | 38.4 | 38.4 | 38.5 |
| Parasitaemia (/µl)* | 13 955 | 12 971 | 8614 | 29 504 | 26 787 | 21 470 |
| Range: high | 85 200 | 64 430 | 19 000 | 159 680 | 134 090 | 103 600 |
| low | 3220 | 5080 | 3430 | 4820 | 5760 | 7360 |
Geometric mean (range).
In part II, comparison with artemether, 63 subjects were enrolled and all were evaluated for efficacy, safety and tolerability. Three subjects were excluded from the analysis of 28 day cure. Also one patient was excluded from the FCT analysis due to a urinary tract infection.
In part II, baseline characteristics were also similar for the three treatment groups except for a lower initial mean parasite count in group R which was not statistically significant (Table 1). The prescreen for antimalarial drugs was not performed due to logistical reasons.
Efficacy/pharmacodynamics
The results on efficacy/pharmacodynamics for the dose-finding part are summarized in Table 2. In all patients full parasite clearance was achieved before day 7. Consequently, there were no type II treatment failures. In almost all patients, a clear decline in parasite count could be observed from 8 h after the first dose.
Table 2.
Efficacy/pharmacodynamic results. Part I: dose-finding.
| Ttreatment A-I artemotil 3.2/1.6×4 | Treatment B-I artemotil 1.6/0.8×4 | Treatment C-I artemotil 3.2/0.8×4 | |
|---|---|---|---|
| PCT (h) | n = 8 | n = 6 | n = 9 |
| Median (range) | 32 (24–52) | 50 (28–132) | 32 (24–52) |
| PCT99% (h) | n = 8 | n = 6 | n = 9 |
| Mean (range) | 25.5 (16–41) | 39.6 (9–92) | 24.5 (20–30) |
| FCT (h) | n = 8 | n = 6 | n = 9 |
| Median (range) | 37 (4–102) | 57 (8–238) | 32 (6–36) |
| Patients with 28 days follow-up | n = 6 | n = 5 | n = 8 |
| RI recrudescence | n = 1 | n = 3 | n = 1 |
| Recrudescence on day | 23 | 21, 26, 27 | 20 |
PCTs were similar (about 35 h) for treatments A-I and C-I but PCT tended to be longer for treatment B-I (about 60 h). This longer PCT was partly caused by one individual whose PCT was 132 h. The mean PCT for treatment B-I without the value of this patient was 45.4±16.4 h (n = 5), which is still longer than the mean PCTs for treatments A-I and C-I. The patient with an exceptionally long PCT in treatment B-I also had a very long FCT (238 h). The mean FCT for treatment B-I without the value of this patient was 44.8 h (n = 5), which was comparable with the mean FCT for treatment A-I. For treatment C-I a mean FCT of about 22 h was found (Table 2).
Treatments A-I and C-I resulted in one RI out of six or eight patients, respectively. However, decreasing the loading dose to 1.6 mg kg−1 followed by a maintenance dose of 0.8 mg kg−1 (treatment B-I) resulted in three RI cases out of 5 patients.
Based on these results from part I of the study, the artemotil dose regimens of treatment A-I and treatment C-I were selected for comparison with the standard dose regimen of artemether in part II of the study.
The results on efficacy/pharmacodynamics of this comparison are summarized in Table 3. For all three treatments, individual parasite counts vs time profiles were similar to those as described in Part I (data not shown). There was an immediate decline after the first dose in several patients, while in general a clear decline was observed from around 12 h after the first dose. The mean PCT for each of the two artemotil treatments (52 and 55 h, respectively) was significantly longer than for artemether (43 h). The median difference between treatments A-II and R was 7.5 h, and the 95% CI of this difference was 0, 16 h (P = 0.0408). The median difference between treatments C-II and R was 10 h, and the 95% CI of this difference was 2, 19 h (P = 0.0140).
Table 3.
Efficacy/pharmacodynamic results. Part II: comparison artemotil with artemether.
| Treatment A-II artemether 3.2/1.6×4 | Treatment C-II artemotil 3.2/0.8×4 | Treatment R artemether 3.2/1.6×4 | |
|---|---|---|---|
| PCT (h) | n = 21 | n = 20 | n = 22 |
| Mean (range) | 51.7 (27–92) | 54.9 (33–97) | 43.1 (21–74) |
| FCT (h) | n = 21 | n = 19** | n = 22 |
| Mean (range) | 50.4 (6–152) | 48.5 (6–124) | 52.4 (8–136) |
| Patients with 28 days follow-up | n = 21 | n = 19 | n = 20 |
| RI recrudescence | n = 4 | n = 5 | n = 3 |
| Recrudescence on day | 21, 23, 23, 24 | 16, 18, 19, 20, 27 | 19, 20, 21 |
One patient was excluded due to a urinary tract infection.
The FCT for all three treatments was similar (around 50 h).
The incidence of RI recrudescence was highest for treatment C-II (5 out of 19 patients), and lowest for treatment R (3 out of 20 patients), but the small numbers do not allow firm conclusions to be drawn.
Pharmacokinetics and PK-PD relationships
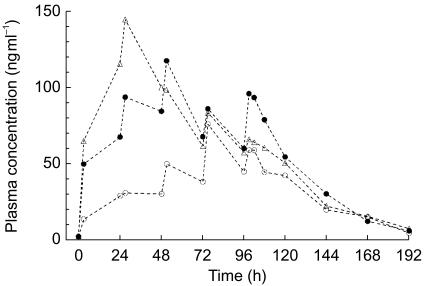
Artemotil plasma concentration-time curves as assessed in part I of the study were irregular, and indicated considerable variability among patients, also within the same treatment group. There were several occurrences of a pronounced absorption lag-time after injection. Mean artemotil plasma concentration-time curves are presented in Figure 1. The lower loading dose in treatment B-I was associated with on average lower plasma concentrations up to 72 h postdose, i.e. after the first three injections. Comparable steady-state concentrations were obtained from the fourth injection onwards for treatments B-I and C-I (Figure 1). Summary statistics of the main pharmacokinetic parameters derived from the plasma concentrations after the last dose are summarized in Table 4. The geometric mean Cmax and AUC(96,120 h) show higher values for treatment A compared with the other two treatments, while the plasma half-life was somewhat longer for Treatment C-I.
Figure 1.
Mean plasma concentration-time curves of artemotil (arteether) as observed during multiple dose gluteal i.m. injections of artemotil as three regimens in adult patients with uncomplicated P. falciparum malaria. Treatments: A: •, n = 7(excluding subject 04); B:○, n = 6; C: ▵, n = 9.
Table 4.
Summary statistics of artemotil pharmacokinetic parameters after the last dose.
| Parameter | Treatment | Geometric mean | Range | n |
|---|---|---|---|---|
| Cmax (ng ml−1) | A-I | 105.8 | 44.0–162 | 7 |
| B-I | 66.2 | 33.0–132 | 6 | |
| C-I | 77.7 | 61.0–131 | 9 | |
| AUC(96,120 h) (ng ml−1 h) | A-I | 1956 | 682–3339 | 7 |
| B-I | 1181 | 700–1923 | 6 | |
| C-I | 1443 | 1236–2146 | 9 | |
| t1/2 (h) | A-I | 22.4 | 12.5–38.5 | 4 |
| B-I | 26.1 | 15.5–51.6 | 4 | |
| C-I | 34.6 | 16.8–91.2 | 9 |
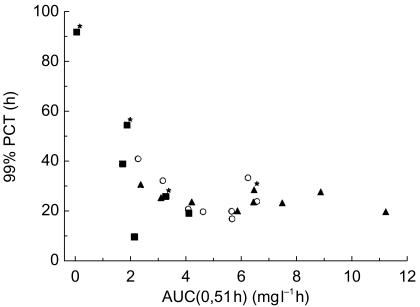
To study the relationship between artemotil pharmacokinetics and pharmacodynamics, the relationship between parasite count-time profile and artemotil plasma concentration-time profile was considered for each patient. It appears that patients with a late onset of parasite clearance also showed a delayed absorption of artemotil and/or very low initial artemotil plasma concentrations. For example, the patient with an outlying long PCT and FCT in treatment B-I showed no measurable artemotil plasma concentrations until after the third injection (i.e. at 51 h after the first injection). A scatter plot of PCT99%vs AUC(0,51 h) (Figure 2) suggests a relationship between the rate of parasite clearance and the exposure during the first 2 days of treatment.
Figure 2.
Time to 99% clearance of parasites (PCT99%) vs artemotil AUC up to 51 h after the first dose (AUC(0,51 h) for acute malaria patients in the dose finding (part I). ○ Treatment A: n = 8 (including subject 04); ▪ = Treatment B; n = 6; ▴ = Treatment C, n = 9; * = Recrudescence.
Safety and tolerability
The safety evaluation as well as the neurologic and audiometric examinations showed no clinically relevant findings, which is in line with observations in healthy volunteers [17]. The ECG recordings provided no indication for QTc-interval prolongation. Two patients complained of mild pain at the site of injection. Symptoms occurring before and after treatment included headache, dizziness, nausea, vomiting, and abdominal pain. These symptoms are characteristic of acute malaria infections and are in general resolved during the first 2 days of treatment.
Discussion
This study was initiated to determine the optimal 5 day dose regimen of i.m. artemotil (part I), and to compare the efficacy with the standard regimen of i.m. artemether in patients with acute uncomplicated malaria (part II). Starting with treatment A, decisions for further dose selection in part I were based on the response to treatment in at least five patients. In view of the small patient numbers, the results needed cautious interpretation. However, the response to treatment B-I suggested that a loading dose of 1.6 mg kg−1 was insufficient for a rapid onset of parasite clearance. Moreover, the recovery from fever and the 28 day cure rate were less adequate than for the treatments with 3.2 mg kg−1 as loading dose (treatments A-I and C-I). The latter two treatments showed similar results with respect to efficacy/pharmacodynamic parameters, and were selected for comparison with artemether. The presence of other antimalarials in the baseline samples of some of the patients could have influenced their PCT and FCT values, but it is unlikely that treatment comparison was affected in view of the even distribution of these cases over the treatment groups.
Each of the treatments in part II of the study produced an adequate therapeutic response; although the incidence of RI failures was relatively high in treatment C-II (0.8 mg kg−1 artemotil as maintenance dose). The most important difference between the treatments in part II of the study was that PCT for the artemether treatment was significantly shorter than for the two artemotil treatments. This difference may partly reflect the lower baseline parasite count for the artemether group (treatment R). Nonetheless, the difference in PCT also correlates with the different pharmacokinetic properties of artemotil compared to artemether.
An important feature of the pharmacokinetics of artemotil after intramuscular administration is the long plasma half-life (mean half life ranged from 22.4 h to 34.6 h) and the resulting accumulation during a 5 day treatment course. Based on pharmacokinetic studies on artemotil in animals, it seems likely that this long plasma half-life results from sustained absorption from the injection site [14]. A similar pattern has been observed for artemether in healthy volunteers and patients. The plasma half-life of artemether is 1–3 h after oral administration [24–26], and after intramuscular administration the plasma half-life is substantially longer, indicating that (also for artemether) the half-life after intramuscular administration is determined by sustained absorption from the injection site [27, 28]. However, it should be noted that studies on the disposition of i.m. artemether have produced remarkably disparate results, even though assay methodology similar to that in the present study was used [20]. Reports from a Thai group [25, 26] suggest that individual artemether plasma concentration profiles are relatively uniform with peak concentrations being attained around 4 h after administration, and a mean terminal half-life of 6–7 h. These reports also suggest a prompt and substantial biotransformation of artemether to artenimol (dihydroartemisinin). Results for i.m. artemether more in keeping with the present study (slower and more variable absorption and much lower biotransformation to artenimol) have been reported by others [24, 29]. While this discrepancy needs further clarification, it is conceivable that the absorption rate from the intramuscular depot is variable, depending on muscle tone and activity, and could explain the highly variable plasma concentration profiles within and between subjects [27]. In addition, since there is no first-pass metabolism after i.m. administration, one would expect substantially lower concentrations of the metabolite artenimol than after oral administration. For i.m. artemotil, biotransformation to artenimol seems lower than for artemether. In an earlier study in healthy volunteers receiving i.m. artemotil, we found very low concentrations of artenimol, that were below the limit of quantification limit in several subjects [17]. In a more recent study, we used an LC-MS/MS method for the assay of artemotil and artenimol in plasma of children with moderate-to-severe malaria receiving i.m. artemotil. Although artenimol could be quantified in these samples, its concentrations were consistently less than 10% of the artemotil concentrations [21]. This was also the reason why artenimol was not quantified in the assay for the present study.
PCT99% was chosen as the pharmacodynamic parameter to be correlated with artemotil pharmacokinetics since its value is relatively close to PCT, but less dependent than PCT on initial parasite counts in a patient. An important finding of the present study is that the rate of parasite clearance is determined largely by the plasma concentrations of artemotil during the first 2 days after the start of treatment (Figure 2). It appears that a certain minimum exposure in terms of plasma concentration or area under the curve is required to trigger the onset of parasite clearance. When exposure during the beginning of treatment is too low, the onset of parasite clearance is delayed, resulting in high values for PCT99% (Figure 2). Up to 4000 ng ml−1 h, the onset of parasite clearance becomes faster, and PCT99% becomes shorter. Thereafter, the PCT is not reduced further by higher exposure to artemotil, suggesting that the maximum rate of parasite clearance is limited by the parasite clearance mechanism rather than by artemotil plasma concentrations. The choice for the AUC up to 51 h after the first artemotil dose was related to the limited blood sampling schedule in this study.
The findings that artemotil is more slowly absorbed from the intramuscular injection site than artemether, and that early systemic availability may be insufficient for an immediate onset of parasite clearance contributed to the decision to choose a higher loading dose of artemotil (divided over two injection sites) and to omit the fifth dose in later studies. This is also the recommended dose regimen in the labelling of artemotil. With this optimized dosing schedule, the more pronounced depot characteristics of i.m. artemotil can be an advantage, since it allows shorter hospitalization.
RI recrudescence was poorly correlated to overall exposure to artemotil since RI recrudescence may also occur with high exposure to artemotil. This has been confirmed in a subsequent study in adults with severe malaria [17]. Also in clinical studies of other artemisinin derivatives, a high incidence of RI recrudescence has often been reported. A possible explanation has been suggested by Kyle & Webster who observed that early ring stage P. falciparum parasites assume a quiescent state in which they are able to survive exposure to dihydroartemisinin (metabolite of artemotil) and to resume growth after 6–8 days [30]. This would imply that treatment with artemotil should be followed with standard prophylactic treatment if recrudescence is to be fully excluded.
Recrudescence can be proven only if re-infection of patients is prevented during a study or if the identity of parasites is determined by genotyping. No genotyping of parasites was performed during this study, however, the chance of re-infection was considered unlikely, because the patients were kept in hospital for 28 days or remained in the close vicinity of the hospital.
With respect to safety, this study shows that artemotil, when given as a dose of 3.2 mg kg−1 i.m. and followed by either 0.8 mg kg−1 or 1.6 mg kg−1 by intramuscular injection once daily for 4 days, is safe and well tolerated.
Neurological and audiometric assessments gave no indication of adverse effects at the dose levels used during these studies. These findings agree with observations made in healthy volunteers and other clinical studies with artemotil [14–18]. The observations in healthy volunteers and patients with uncomplicated malaria are important because in severely ill patients it is difficult to determine whether neurological adverse reactions are due to the drug or are a result of the coma.
Conclusions
Artemotil was safe and effective in the treatment of acute malaria, based on parasite and fever clearance. The optimum artemotil dose regimen in this study was identical to the standard dose regimen of artemether, and showed similar efficacy. However, the onset of parasite clearance tended to be slower for artemotil than for artemether, which is probably due to a lower absorption rate of artemotil from the injection site. These findings have contributed to the decision for later studies to choose a higher loading dose of artemotil, divided over two injection sites, and to omit the dose on the fifth day.
Acknowledgments
The development of this drug has been performed in collaboration with the World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases, the Walter Reed Army Institute of Research, Washington, D.C., and the Dutch pharmaceutical company, ARTECEF BV. We also wish to acknowledge the technical assistance of A. Aguilar and K. Trotman of the WRAIR.
We thank the nursing and laboratory staff of the Bangkok Hospital of Tropical Diseases for their support.
This investigation received the financial support of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
This project was supported by WHO Steering Committee for the Scientific Working Group on the Chemotherapy of Malaria, and ARTECEF BV, Maarssen, the Netherlands.
References
- 1.Pukrittayakamee S, Supanaranond W, Looareesuwan S, Vanijanonta S, White NJ. Quinine in severe falciparum malaria: evidence of declining efficacy in Thailand. Trans R Soc Trop Med Hyg. 1994;84:324–327. doi: 10.1016/0035-9203(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 2.Price RN. Artemisinin drugs: novel antimalarial agents. Expert Opin Invest Drugs. 2000;9:1815–1827. doi: 10.1517/13543784.9.8.1815. [DOI] [PubMed] [Google Scholar]
- 3.Li GQ, Guo X, Jim R, Wang Z, Jian H. Clinical studies on treatment of cerebral malaria with quinghasosu and its derivatives. J Trad Chinese Med. 1982;2:125–130. [PubMed] [Google Scholar]
- 4.White NJ. The treatment of malaria. N Engl J Med. 1996;335:800–806. doi: 10.1056/NEJM199609123351107. [DOI] [PubMed] [Google Scholar]
- 5.de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- 6.van Agtmael MA, Eggelte TA, van Boxtel CJ. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci. 1999;20:199–205. doi: 10.1016/s0165-6147(99)01302-4. [DOI] [PubMed] [Google Scholar]
- 7.Bunnag D, Viravan C, Looareesuwan S, Karbwang J, Harinasuta TC. linical trial of artesunate and artemether on multidrug resistant falciparum malaria in Thailand: a preliminary report. Southeast Asian J Trop Med Public Health. 1991;22:380–385. [PubMed] [Google Scholar]
- 8.Looareeswuan S, Wilairatana P, Vanijanonta S, et al. A randomised trial of mefloquine, artesunate, and artesunate followed by mefloquine in acute uncomplicated falciparum malaria. Lancet. 1992;339:821–824. doi: 10.1016/0140-6736(92)90276-9. [DOI] [PubMed] [Google Scholar]
- 9.Karbwang J, Bangchang KN, Thanavibul A, Bunnag D, Chongsuphajaisiddhi T, Harinasuta T. Comparison of oral artemether and mefloquine in acute uncomplicated falciparum malaria. Lancet. 1992;340:1245–1248. doi: 10.1016/0140-6736(92)92947-e. [DOI] [PubMed] [Google Scholar]
- 10.Gu HM, Lu BF, Qu ZX. Antimalarial activities of 25 derivatives of artemisinin against chloroquine-resistant Plasmodium berghei. Acta Pharmacol Sinica. 1980;1:48–50. [PubMed] [Google Scholar]
- 11.China Cooperative Research Group on Qinghaosu. Antimalarial efficacy and mode of action of qinghaosu and its derivatives in experimental models. J Trad Chin Med. 1982;2:17–24. [PubMed] [Google Scholar]
- 12.Wu JA, Ji RY. A quantitative structure-activity study on artemisinin analogues (Engl. Abstract) Acta Pharmacol Sinica. 1982;3:55–60. [PubMed] [Google Scholar]
- 13.Davidson DE., Jr Role of arteether in the treatment of malaria and plans for further development. Trans R Soc Trop Med Hyg. 1994;88(Suppl 1):S51–S52. doi: 10.1016/0035-9203(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 14.Shmuklarsky MJ, Klayman DL, Milhous WK, et al. Comparison of beta-artemether and beta-arteether against malaria parasites in vitro and in vivo. Am J Trop Med Hyg. 1993;48:377–384. doi: 10.4269/ajtmh.1993.48.377. [DOI] [PubMed] [Google Scholar]
- 15.Li QG, Peggins JO, Fleckenstein LL, Masonic K, Heiffer MH, Brewer TG. The pharmacokinetics and bioavailability of dihydroartemisinin, arteether, artemether, artesunic acid and artelinic acid in rats. J Pharm Pharmacol. 1998;50:173–182. doi: 10.1111/j.2042-7158.1998.tb06173.x. [DOI] [PubMed] [Google Scholar]
- 16.Brossi A, Venugopalan B, DominguezGerpe L, et al. Arteether a new anti-malarial drug. synthesis and anti-malarial properties. J Med Chem. 1988;31:645–650. doi: 10.1021/jm00398a026. [DOI] [PubMed] [Google Scholar]
- 17.Peeters PAM, van Lier JJ, Oosterhuis B, et al. A placebo-controlled study on the tolerability and pharmacokinetics of multiple dose artemotil in healthy volunteers. in press.
- 18.Thuma TE, Bhat GJ, Mabeza GF, et al. A randomised controlled trial of artemotil (β-arteether) in Zambian children with cerebral malaria. Am J Trop Medical Hyg. 2000;62:524–529. doi: 10.4269/ajtmh.2000.62.524. [DOI] [PubMed] [Google Scholar]
- 19.Moyou-Somo R, Tietche F, Ondoa M, et al. Clinical trial of beta-arteether versus quinine for the treatment of cerebral malaria in children in Yaounde, Cameroon. Am J Trop Med Hyg. 2001;64:229–232. doi: 10.4269/ajtmh.2001.64.229. [DOI] [PubMed] [Google Scholar]
- 20.Looareesuwan S, Oosterhuis B, Schillizi BM, et al. A randomised trial to compare the efficacy of artemotil and artemether in the treatment of adult Thai patients with severe malaria. in press.
- 21.Oosterhuis B, Looaresuwan S, Krudsood S, et al. Comparison of artemotil and artemether for the treatment of moderate-to-severe malaria in Thai children: a double-blind randomised trial. submitted.
- 22.Warrell DA, Molyneux ME, Beales PF. Severe and Complicated Malaria. Trans R Soc Trop Medical Hyg. (2) 1990;84(Suppl 2):1–65. [PubMed] [Google Scholar]
- 23.Melendez V, Peggins JO, Brewer TG, Theoharides AD. Determination of the anti-malarial arteether and its deethylated metabolite dihydroartemisinin in plasma by high-performance liquid chromatograpphy with reductive electrochemical detection. J Pharm Sci. 1991;80:132–138. doi: 10.1002/jps.2600800209. [DOI] [PubMed] [Google Scholar]
- 24.Teja-Isavadharm P, Nosten F, Kyle DE, et al. Comparative bioavailability of oral, rectal and intramuscular artemether in healthy subjects: use of simultaneous measurements by high performance liquid chromatography and bioassay. Br J Clin Pharmacol. 1996;42:599–604. doi: 10.1111/j.1365-2125.1996.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 25.Karbwang J, Na-Bangchang K, Congpuong K, Molunto P, Thanavibul A. Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur J Clin Pharmacol. 1997;52:307–310. doi: 10.1007/s002280050295. [DOI] [PubMed] [Google Scholar]
- 26.Karbwang J, Na-Bangchang K, Tin T, Sukontason K, Rimchala W, Harinasuta T. Pharmacokinetics of intramuscular artemether in patients with severe falciparum malaria with or without acute renal failure. Br J Clin Pharmacol. 1998;45:597–600. doi: 10.1046/j.1365-2125.1998.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokwaro GO. Bioavailability of artemether. Br J Clin Pharmacol. 1997;44:305. [PubMed] [Google Scholar]
- 28.Navaratnam V, >Mansor SM, Sit N-W, Grace J, Li Q, Olliaro P. Pharmacokinetics of artemisinin-like compounds. Clin Pharmacokinet. 2000;39:255–270. doi: 10.2165/00003088-200039040-00002. [DOI] [PubMed] [Google Scholar]
- 29.Murphy SA, Mberu E, Muhia D, et al. The disposition of intramuscular artemether in children with cerebral malaria; a preliminary study. Trans R Soc Trop Med Hyg. 1997;91:331–334. doi: 10.1016/s0035-9203(97)90097-3. [DOI] [PubMed] [Google Scholar]
- 30.Kyle DE, Webster HK. Post-antibiotic effect of quinine and dihydroartemisinin on Plasmodium falciparum in vitro: implication for a mechanism of recrudescence. Abstract XIV International Congress for Tropical Medicine and Malaria; November 17–22; Nagasaki Japan. 1996. [Google Scholar]