Abstract
Aims
To determine whether bolus doses of methylprednisolone affect the steady-state trough concentrations of sirolimus.
Methods
Fourteen renal transplant recipients received concentration-controlled sirolimus therapy in combination with azathioprine and steroids (n = 8) or mycophenolate mofetil and steroids (n = 6). Bolus doses of methylprednisolone (mean total dose over 1–5 days, 1694 mg; range, 500–3000 mg) were given for the treatment of acute rejection. For each patient, the sirolimus dose (mean, 24.1 mg; range, 3.3–52.5 mg) was the same before and during methylprednisolone therapy.
Results
Mean sirolimus whole blood trough concentrations before and after treatment with methylprednisolone were 28.8 ng ml−1 (range, 13.9–45.3 ng ml−1), and 28.5 ng ml−1 (range, 13.0–47.9 ng ml−1), respectively (P = 0.85; 95% confidence interval on the difference −3.3, 4.0 ng ml−1).
Conclusions
Bolus methylprednisolone treatment does not affect steady-state sirolimus trough concentrations.
Keywords: drug interaction, methylprednisolone, renal transplantation, sirolimus
Introduction
Sirolimus (rapamycin, Rapamune®) has a mechanism of action distinct from that of all other commercially available immunosuppressive agents used in organ transplantation [1]. It has been tested in renal transplantation, given in association with cyclosporin and steroids [2–4], and administered with a purine antagonist and steroids [5, 6]. More recently, treatment with sirolimus has permitted early withdrawal of cyclosporin from the treatment regimen, resulting in lower blood pressure and improved renal function [7]. Sirolimus has been approved in the United States, the European Union, and many other countries for the prophylaxis of organ rejection in renal transplantation. Product labelling in the European Union specifies that optimal therapy with sirolimus requires therapeutic drug concentration monitoring in all patients [8].
Sirolimus is a substrate for the CYP3A-metabolizing enzymes and P-glycoprotein [9]. Its blood concentration is increased by ketoconazole, cyclosporin (given 4 h before sirolimus), and diltiazem by 90%, 80%, and 60%, respectively [10]. The oral bioavailability of sirolimus was approximately 14% in renal transplant recipients when given with cyclosporin and corticosteroids. The true elimination half-life of sirolimus is approximately 63 h, although the effective half-life is shorter with patients reaching steady state in 5–7 days.
Bolus corticosteroids, in particular high-dose methylprednisolone given intravenously, are the initial treatment for acute rejection in renal transplantation. Methylprednisolone is also a substrate of the CYP3A-metabolizing enzymes [11] and has been reported to increase cyclosporin trough concentrations when it was administered intravenously for the treatment of rejection episodes [12, 13]. Sirolimus did not affect prednisolone pharmacokinetics when given to renal transplant patients receiving stable doses of prednisone and cyclosporin [14]. However, the effect of steroids on the disposition of sirolimus has not been previously studied.
In summary, there is a theoretical basis to suspect that high-dose methylprednisolone therapy could increase systemic exposure to sirolimus, particularly as both drugs are substrates of the CYP3A-metabolizing enzymes. The present paper examines the effect of methylprednisolone therapy on sirolimus trough concentrations during the treatment of acute rejection episodes in renal transplant recipients.
Methods
Patients
Adult patients receiving a primary renal allograft from a cadaveric donor were enrolled in two multicentre European trials comparing sirolimus with cyclosporin in which the drugs were used in triple-therapy regimens with azathioprine and corticosteroids [5], or with mycophenolate mofetil and corticosteroids [6]. Approvals were obtained from local ethics committees and written informed consent was obtained from each patient enrolled. The studies were carried out according to the Declaration of Helsinki.
Sirolimus was administered once daily in the morning as an oral solution (5 mg ml−1) mixed with either water or orange juice. Dosing was concentration-controlled, targeting whole blood sirolimus trough concentrations of 30 ng ml−1 during the first 2 months following transplantation, and 15 ng ml−1 thereafter. Methylprednisolone was administered as daily intravenous doses over 1–5 days for the treatment of a suspected or biopsy proven acute rejection. Sirolimus trough concentration data were included in the study if 1) the patient received a stable dose of sirolimus for at least 3 days before the initial trough concentration and until the first trough concentration following the last methylprednisolone bolus and 2) there was no change in concomitant therapy dosage other than methylprednisolone.
Measurement of sirolimus trough concentrations
Blood samples were collected into tubes containing solid or solubilized sodium or potassium EDTA. Tubes were shipped to a central laboratory (Analytical Unit, Cardiological Sciences, St George's Hospital Medical School, London) where whole blood sirolimus concentrations were determined by high-performance liquid chromatography with ultraviolet detection [15]. For the three concentrations of control samples, precision was ≤4% and between assay reproducibility was ≤6.6%.
Statistical analysis
Sirolimus trough blood concentrations measured before and after intravenous methylprednisolone therapy were compared by a two-tailed paired t-test. Linear regression was used to determine if there was any relationship between total methylprednisolone dose and the change in sirolimus trough concentration.
Results
Fourteen patients, eight receiving sirolimus in combination with azathioprine (patients 1–8) and six in combination with mycophenolate mofetil (patients 9–14), were evaluated for the effect of bolus methylprednisolone doses on sirolimus trough concentrations (Table 1). The mean total methylprednisolone dose was 1694 ± 833 mg administered over 1–5 days (mean 3.3 days). Sirolimus dosing was constant for each patient between trough concentration sampling and averaged 24.07 ± 11.73 mg. The mean time between the last methylprednisolone bolus and the post-treatment trough concentration was 2.9 ± 3.1 days.
Table 1.
Demographic characteristics and dosing information.
| Patient | Sex | Age (years) | Weight (kg) | Total methyl-prednisolone dose (mg) | Daily sirolimus dose (mg) |
|---|---|---|---|---|---|
| 1 | M | 52 | 76.4 | 750 | 39.5 |
| 2 | M | 62 | 55.7 | 2000 | 23.0 |
| 3 | M | 54 | 110.5 | 1250 | 28.5 |
| 4 | F | 60 | 80.5 | 500 | 14.0 |
| 5 | M | 37 | 80.1 | 3000 | 23.5 |
| 6 | M | 51 | 65.0 | 1940 | 17.5 |
| 7 | F | 47 | 71.5 | 1730 | 24.7 |
| 8 | M | 30 | 49.2 | 2000 | 11.0 |
| 9 | M | 51 | 85.6 | 3000 | 23.5 |
| 10 | M | 52 | 63.2 | 3000 | 3.3 |
| 11 | M | 50 | 88.0 | 1550 | 18.0 |
| 12 | M | 47 | 64.4 | 1500 | 25.5 |
| 13 | M | 56 | 79.0 | 750 | 52.5 |
| 14 | M | 38 | 79.0 | 750 | 32.5 |
| Mean | 49.1 | 74.9 | 1694 | 24.1 | |
| s.d. | 8.6 | 14.7 | 833 | 11.7 |
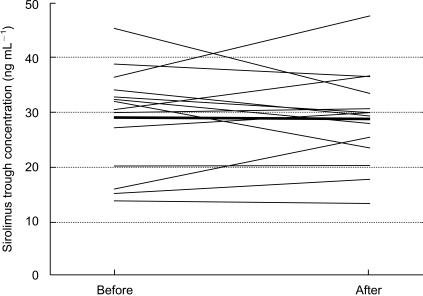
The mean sirolimus trough concentrations before and after treatment with bolus methylprednisolone were 28.8 ± 11.7 ng ml−1 (range, 13.9–45.3 ng ml−1), and 28.5 ± 9.1 ng ml−1 (range, 13.0–47.9 ng ml−1), respectively. The mean observed difference in sirolimus trough concentrations before and after methylprednisolone treatment was 0.3 ng ml−1, with a 95% confidence interval of −3.3, 4.0 ng ml−1 (P = 0.85). This test would have been significant if the difference would have been ≥3.7 ng ml−1, or about 12% of the observed mean concentration. Individual data are plotted in Figure 1. The correlation coefficient between the change in sirolimus trough concentration and the total methylprednisolone dose was 0.203, which is not significant (P = 0.486).
Figure 1.
Sirolimus trough concentrations (ng ml−1) before and after intravenous methylprednisolone therapy in renal transplant recipients. Thin lines represent data from individual patients and the thick line represents the mean data.
Discussion
Therapeutic drug monitoring is routinely employed to manage therapy of the immunosuppressive drugs: cyclosporin, tacrolimus and sirolimus. Amongst other benefits, monitoring minimizes the impact of drug interactions. Although high-dose methylprednisolone is not given chronically, a transient rise in sirolimus trough concentrations could have an impact in sirolimus dosing. A decision by the clinician to reduce the dose of sirolimus in the event of an elevated sirolimus trough concentration, which if only transiently increased by an acute steroid therapy, could ultimately result in low sirolimus concentrations. This could compromise the management of patients experiencing acute rejection. The present study did not find any evidence that bolus methylprednisolone therapy affects sirolimus trough concentrations. Consequently, other aetiologies should be explored if sirolimus trough concentrations increase during or immediately following methylprednisolone therapy for the treatment of acute rejection.
The present study used trough concentrations as a measurement of exposure to sirolimus. This is an acceptable methodology as there is an excellent relationship between sirolimus trough concentrations and the area under the drug concentration-time curve [16]. Since this was a retrospective study, there was some variability in the time between last methylprednisolone dose and the post-treatment sirolimus trough concentration measurement. However, if one examines the data from the nine patients who had the trough samples drawn on the first or second day following the last methylprednisolone injection, when any effect would be expected to be maximal, sirolimus trough concentrations did not differ before and after methylprednisolone (27.9 ± 8.9 ng ml−1 vs 30.08 ± 9.2 ng ml−1). The relative timing of the steroid dose in relation to the sirolimus dose was not recorded, but clearly could have an influence on the extent of any interaction. Concomitant administration would be expected to produce the maximum increase in drug concentration. All but 1 of the 14 patients had the total methylprednisolone dose administered over more than 1 day; however, it would be standard practice to give sirolimus and the subsequent methylprednisolone doses within 2 h of each other in the morning.
In conclusion, based on data from this study obtained during routine clinical practice, there is no evidence that bolus methylprednisolone therapy for the treatment of acute rejection has any relevant impact on sirolimus exposure.
Acknowledgments
The authors would like to thank the investigational staff of the Sirolimus European Renal Transplant Study Group that enrolled patients in the two trials from which this data was obtained. These studies were supported by grants from Wyeth-Ayerst Research, Philadelphia, PA, USA.
References
- 1.Sehgal SN. Rapamune (sirolimus, rapamycin). An overview and mechanism of action. Ther Drug Monit. 1995;17:660–665. doi: 10.1097/00007691-199512000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y, Neylan J for the Rapamune Study Group. Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in caucasian recipients of mismatched primary renal allografts: a phase II trial. Transplantation. 1999;67:1526–1532. doi: 10.1097/00007890-199911270-00016. [DOI] [PubMed] [Google Scholar]
- 3.Kahan BD The Rapamune US Study Group. Efficacy of sirolimus compared with azathioprine for the reduction of acute renal allograft rejection: a randomised multicentre study. Lancet. 2000;356:194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald AS The Rapamune Global Study Group. A worldwide, phase III, randomised, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2000;71:271–280. doi: 10.1097/00007890-200101270-00019. [DOI] [PubMed] [Google Scholar]
- 5.Groth CG, Bäckman L, Morales JM, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation. Similar efficacy and different toxicity compared with cyclosporine. Transplantation. 1999;67:1036–1042. doi: 10.1097/00007890-199904150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Kreis H, Cisterne JM, Land W, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation. 2000;69:1252–1260. doi: 10.1097/00007890-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RWG, Kreis H, Oberbauer R, et al. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001;72:777–786. doi: 10.1097/00007890-200109150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Rapamune (sirolimus) Summary of Product Characteristics December 2000.
- 9.Lampen A, Zhang Y, Hackbarth I, et al. Metabolism and transport of the macrolide immunosuppressant sirolimus in the small intestine. J Pharmacol Exp Ther. 1998;285:1104–1112. [PubMed] [Google Scholar]
- 10.MacDonald A, Scarola J, Burke JT, Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther. 2000;22(Suppl B):B101–B121. doi: 10.1016/s0149-2918(00)89027-x. [DOI] [PubMed] [Google Scholar]
- 11.Prichard L, Fabre I, Fabre G, et al. Cyclosporine A drug interactions. Screening for inducers and inhibitors of cytochrome P-450 (cyclosporine A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos. 1990;18:595–606. [PubMed] [Google Scholar]
- 12.Klintmalm G, Säwe J. High dose methylprednisolone increases plasma cyclosporin levels in transplant recipients. Lancet. 1984;i:731. doi: 10.1016/s0140-6736(84)92239-6. [DOI] [PubMed] [Google Scholar]
- 13.Ubhi CS, Woodhouse L, Giles GR. Interaction of intravenous methylprednisolone with oral CsA. Nephrol Dial Transplant. 1990;5:376–378. doi: 10.1093/ndt/5.5.376. [DOI] [PubMed] [Google Scholar]
- 14.Jusko WJ, Ferron GM, Mis SM, Kahan BD, Zimmerman JJ. Pharmacokinetics of prednisolone during administration of sirolimus in patients with renal transplants. J Clin Pharmacol. 1996;36:1100–1106. doi: 10.1002/j.1552-4604.1996.tb04162.x. [DOI] [PubMed] [Google Scholar]
- 15.Holt DW, Lee T, Johnson A. Measurement of sirolimus in whole blood using high-performance liquid chromatography with ultraviolet detection. Clin Ther. 2000;22(Suppl B):B38–B48. doi: 10.1016/s0149-2918(00)89021-9. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman JJ, Kahan BD. Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J Clin Pharmacol. 1997;61:416–428. doi: 10.1002/j.1552-4604.1997.tb04318.x. [DOI] [PubMed] [Google Scholar]