Abstract
Aims
To determine whether postjunctional α1- and α2-adrenoceptors mediate vasoconstrictor responses in the cutaneous vasculature of the human forearm.
Methods
Drugs were administered transdermally by iontophoresis in the forearm of 20 healthy participants. Phenylephrine and clonidine were administered at sites pretreated with the relevant antagonist (terazosin and rauwolscine), and at additional untreated sites and sites pretreated with saline. To enhance the contrast between sites, the forearm was heated to 42° C before flow was measured with the laser Doppler technique.
Results
After the iontophoresis of phenylephrine, blood flow at the site pretreated with terazosin was 24 ± 37% (±95% confidence interval) greater than flow at the reference site, whereas flow was 53 ± 24% lower than reference flow at the previously untreated site and 77 ± 10% lower than reference flow at the site pretreated with saline (P < 0.001). After the iontophoresis of clonidine, blood flow at the site pretreated with rauwolscine was 25 ± 21% greater than flow at the reference site, whereas flow was 56 ± 15% lower than reference flow at the previously untreated site and 72 ± 8% lower than reference flow at the site pretreated with saline (P < 0.001). The saline pretreatment enhanced vasoconstriction to phenylephrine (P < 0.05) and clonidine (P = 0.05).
Conclusions
Pre-treatment with the appropriate antagonist blocked vasoconstrictor responses to phenylephrine and clonidine, consistent with the presence of both α-adrenoceptor subtypes in cutaneous vessels of the human forearm. In addition, iontophoretic pretreatment with saline facilitated vasoconstrictor responses, suggesting that a nonspecific effect of iontophoresis may enhance drug penetration through the stratum corneum.
Keywords: adrenoceptors, iontophoresis, skin, vasoconstriction
Introduction
Postjunctional α1- and α2-adrenoceptors mediate adrenergic vasoconstriction in many different species, including humans [1, 2]. In general α1-adrenoceptors congregate around the neuromuscular junction and respond to noradrenaline released from sympathetic varicosities. In contrast, most postjunctional α2-adrenoceptors are located elsewhere in the vessel wall, and are thus more likely to respond to circulating catecholamines than to neurally liberated noradrenaline. The concentration of the two α-adrenoceptor subtypes differs between various vascular regions [3, 4] and even across different sites in the same region. For example α1-adrenoceptors predominate in arterioles of the rabbit ear, whereas both α-adrenoceptor subtypes appear to mediate constriction of arteriovenous anastomoses in this tissue [5]. In humans α2-adrenoceptors were found in subcutaneous resistance vessels but not in large muscular arteries that supply internal organs [6]. Directly cooling the finger augments α2- but not α1-adrenergic vasoconstriction [7, 8], indicating that α2-adrenoceptors mediate vascular adjustments to local cooling.
In vitro studies have established that human cutaneous arteries have a mixed population of postjunctional α1- and α2-adrenoceptors [4, 9]. Injection of α1- and α2-agonists and antagonists into the brachial artery changes total forearm blood flow, indicating that both subtypes of α-adrenoceptor mediate vasoconstriction in the forearm vascular bed [2]. This clearly applies to digital blood vessels [10], but the presence of postjunctional α2-adrenoceptors in more proximal cutaneous vessels is less certain. For example, iontophoresis of the α2-antagonist yohimbine in the skin of the human forearm only partly antagonized vasoconstriction induced by clonidine, an agonist that is more selective for α2- than α1-adrenoceptors [11]. This finding fits with the observation that α2-adrenoceptors are more prominent in distal than proximal arteries of the limbs [4]. On the other hand, systemic pretreatment with yohimbine reduced cutaneous vasoconstriction in the human forearm during the first few minutes of exercise and during graded local heating, suggesting that α2-adrenoceptors are involved in tonic adrenergic vasoconstriction and sympathetic vasoconstrictor reflexes [12]. However, the likelihood of central and prejunctional α2-adrenoceptor blockade with systemically administered yohimbine complicates the interpretation of these results.
The goal of the present study was to more clearly delineate the local vascular response to α-adrenoceptor agonists and antagonists in the skin of the human forearm. Drugs were administered by iontophoresis to ensure a high local concentration throughout the epidermis and upper dermis, but at the same time minimizing systemic responses and upstream effects on perfusion pressure and flow. In particular, the aim was to determine whether local pretreatment with the α1-antagonist terazosin would block vasoconstrictor responses to the α1-agonist phenylephrine, and whether local pretreatment with the α2-antagonist rauwolscine would block vasoconstrictor responses to the α2-agonist clonidine. Phenylephrine and clonidine were administered at sites pretreated with the relevant antagonist, and at additional sites in the forearm for comparison. Cutaneous vascular responses were monitored with the laser Doppler technique.
One major advantage of iontophoresis over systemic or intra-arterial administration is that multiple sites can be investigated simultaneously in the same vascular bed (i.e. the subject acts as their own control). However, a limitation of iontophoresis is that nonspecific aspects of the procedure influence vascular responses. For example, Grossmann et al. [13] reported that skin blood flow increased in a dose-related manner during and after the iontophoresis of a series of anions (nitrite, chloride, acetate and bicarbonate) and cations (sodium, lithium and acetylcholine), suggesting that current-mediated dilatation contributed to cutaneous vascular responses. Since nonspecific current-mediated vasodilatation might inhibit vasoconstriction to α-adrenergic agonists, some of the skin sites were pretreated with saline in the present study. If current-mediated vasodilatation inhibits α-adrenergic vasoconstriction, blood flow may be greater at sites of saline pretreatment than at previously untreated sites. Nevertheless, if α-adrenoceptors mediate vasoconstriction in the cutaneous vasculature, α-adrenergic vasoconstriction should be greater at sites of saline pretreatment than at sites of α-adrenergic blockade.
Method
Subjects
The sample consisted of two healthy men and 18 healthy women aged between 17 and 25 years (mean age 21.1 years). None of the participants was taking antihypertensive drugs or other medications that might affect the response to α-adrenergic agonists or antagonists. Each subject gave their informed consent for the procedures, which were approved by the Murdoch University Human Research Ethics Committee.
Procedures
The experiment was carried out in a temperature-controlled laboratory maintained at 20° C. Four or five sites on the dorsal aspect of the right or left forearm were cleaned with alcohol. The sites were separated by at least 5 cm. To facilitate the attachment of iontophoresis chambers and blood flow transducers, hair was removed from the sites with a disposable razor. Care was taken not to touch the skin with the razor.
Drug administration
The drugs consisted of terazosin hydrochloride (an α1-adrenoceptor antagonist, Sigma Chemical Company, St Louis, MO), rauwolscine hydrochloride (an α2-adrenoceptor antagonist, ICN Biomedicals, Costa Mesa, CA), phenylephrine hydrochloride (an α1-adrenoceptor agonist, Sigma Chemical Company), and clonidine hydrochloride (an α2-adrenoceptor agonist, Sigma Chemical Company). Stock solutions (10 mm in distilled water) were prepared fortnightly and stored in airtight containers in a refrigerator at 4° C. To administer the drugs, an iontophoresis capsule (internal diameter 2.0 cm) was attached to the prepared site with an adhesive washer. The capsule was filled with the drug solution, and a weak direct current was passed through the solution to introduce positively charged ions into the skin. The ground electrode was a silver plate measuring 3 cm by 5 cm, covered in electrode paste and attached to the volar aspect of the forearm near the wrist. The stock solutions of phenylephrine and clonidine were diluted on the day of use to 0.5 mm with distilled water. The 10 mm solutions of terazosin and rauwolscine were not diluted to ensure that a sufficient supply of cations was available during 10 min of iontophoresis.
The drug treatment at each site is summarized in Table 1. In the first eight subjects, terazosin was administered to one of the prepared sites by passing 200 µA through the drug solution for 10 min. For comparison, the same current strength was passed through 0.9% saline at another site for 10 min (the nonspecific effects of iontophoresis were investigated with saline instead of distilled water to ensure that an abundant supply of vaso-inactive cations was available for iontophoresis). Next, phenylephrine was administered to the two treated sites and to another untreated site by passing 100 µA through the drug solution for 30 s. In the other 12 subjects, rauwolscine was administered to one of the prepared sites (200 µA, 10 min) and 0.9% saline was administered to two other sites (200 µA, 10 min). Clonidine was then administered to the rauwolscine site, one of the saline pretreated sites, and another untreated site (100 µA, 30 s). For comparison, the same current strength (100 µA, 30 s) was passed through 0.9% saline at the second saline pretreated site.
Table 1.
Drug treatment at each site.
| Site | First set of iontophoreses (current intensity 200 µA) (current duration 10 min) | Second set of iontophoreses (current intensity 100 µA) (current duration 30 s) |
|---|---|---|
| Group 1 (n = 8) | ||
| 1 | Terazosin | Phenylephrine |
| 2 | Saline | Phenylephrine |
| 3 | None | Phenylephrine |
| 4 | None | Blood flow reference site* |
| Group 2 (n = 12) | ||
| 1 | Rauwolscine | Clonidine |
| 2 | Saline | Clonidine |
| 3 | None | Clonidine |
| 4 | Saline | Saline |
| 5 | None | Blood flow reference site* |
After the second set of iontophoreses, the forearm was warmed to 42° C for 10 min in a water bath, and blood flow was measured at each site.
Vascular responses to drug administration
After the final iontophoresis, plastic probe holders were attached to the iontophoresis sites and one untreated reference site with adhesive washers. To enhance the contrast in blood flow between treated and untreated sites, the subject immersed their forearm in a water bath (66 cm long, 34 cm wide, 30 cm deep and heated to 42° C) with the recording sites just below heart level. Blood flow usually stabilized at the reference site within 5 min of immersion, and measurements at drug-treated sites began 5 min later. Cutaneous blood flow was monitored with a wide surface area probe attached to a Moor MBF3D laser Doppler flowmeter (Moor Instruments, Axminster, UK). The probe consists of a central near-infrared laser beam emitter (wavelength 810 nm) surrounded by eight glass fibres, equally spaced on a 2 mm diameter circle, which collect the reflected light. The signal was integrated by the MBF3D monitor to give an average flux over a surface area of approximately 7 mm2 and a depth of 1–2 mm. Blood flow was sampled at 5 Hz, and was later averaged using Moorsoft software. During the recordings, the bath's heater and circulating jet were switched off to prevent data artefacts caused by agitation of the immersed probe. To ensure uniform probe distance from the skin across recordings, tape was applied to the probe so that it was positioned 1 mm above the skin surface when in its holder. After 30 s of stable flow the probe was moved to the next recording site. The order in which blood flow was measured at the treated sites varied randomly across subjects. Blood flow was measured at the reference site before and after measuring flow at the treated sites.
Data reduction and statistical analysis
Blood flow remained stable at the reference site over the recording period [r(17) = 0.91, P < 0.001; change in flow from the beginning to the end of the recording period, t(18) = 0.61, not significant]. Since the flowmeter detected relative differences between sites but did not measure blood flow in absolute terms, the flow at each site was expressed in relation to mean flow at the reference site.
Paired t-tests were used to compare: (1) blood flow at sites of α-adrenergic blockade with blood flow at sites of saline pretreatment (to investigate the effect of α-adrenergic blockade on vasoconstriction to α-adrenergic agonists); (2) blood flow at sites of saline pretreatment with blood flow at sites of no pretreatment (to investigate the effect of saline pretreatment on vasoconstriction to α-adrenergic agonists); and (3) blood flow at the rauwolscine site with blood flow at the site of repeated saline administration (to investigate the nonspecific effect of iontophoresis on skin blood flow).
Results
As shown in Figure 1, vasoconstriction to phenylephrine was antagonized by pretreatment with terazosin [t(7) = 7.0, P < 0.001]. In addition, the saline pretreatment enhanced vasoconstriction to phenylephrine [t(7) = 2.74, P < 0.05].
Figure 1.
Vascular responses to phenylephrine administered by iontophoresis (mean ± 95% confidence interval). Blood flow lower than flow at the reference site implies vasoconstriction. Blood flow at the site pretreated with saline (open bar) was significantly lower than at the site pretreated with terazosin (filled bar, *P < 0.001) or the previously untreated site (shaded bar, *P < 0.05).
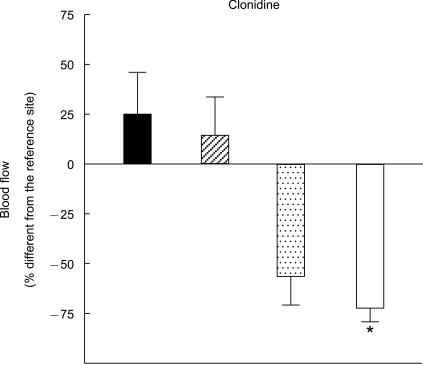
Similarly, vasoconstriction to clonidine was antagonized by pretreatment with rauwolscine [t(11) = 9.51, P < 0.001], and the saline pretreatment enhanced vasoconstriction to clonidine [t(11) = 2.20, P = 0.05] (Figure 2). Blood flow at the rauwolscine site did not differ from blood flow at the site of repeated saline administration [t(11) = 1.01, not significant].
Figure 2.
Vascular responses to clonidine (mean ± 95% confidence interval). Blood flow at the site pretreated with saline (open bar) was significantly lower than at the site pretreated with rauwolscine (filled bar, *P < 0.001) or the previously untreated site (shaded bar, *P = 0.05). Blood flow did not differ significantly between the site of saline iontophoresis (hatched bar) and the site of rauwolscine pretreatment (filled bar).
Discussion
The main finding of this study was that pretreatment with the appropriate antagonist blocked vasoconstrictor responses to phenylephrine and clonidine, consistent with the presence of both α-adrenoceptor subtypes in cutaneous vessels of the human forearm. In addition, iontophoretic pretreatment with saline facilitated vasoconstrictor responses, suggesting that a nonspecific effect of iontophoresis may enhance drug penetration through the stratum corneum. These effects were strong, because they were detected consistently in small groups of subjects.
Cutaneous α-adrenoceptors in the forearm
Lindblad & Ekenvall [10] administered α-adrenergic agonists and antagonists in the human finger by iontophoresis, and measured vascular responses with laser Doppler flowmetry. They reported that blockade of α1-adrenoceptors with doxazosin reduced vasoconstrictor responses to phenylephrine, and blockade of α2-adrenoceptors with rauwolscine reduced vasoconstrictor responses to B-HT 933 (a selective α2-agonist). In the present study, terazosin was used instead of doxazosin because its greater solubility in water made it more suitable for iontophoresis than doxazosin. Terazosin blocked the vasoconstrictor response to phenylephrine, consistent with the presence of α1-adrenoceptors in cutaneous vessels of the human forearm.
The finding that blood vessels constricted to clonidine indicates that stimulation of postjunctional α2-adrenoceptors overshadowed any prejunctional inhibitory influence on the release of noradrenaline. Clonidine stimulates both adrenoceptor subtypes, but is more selective for α2- than α1-adrenoceptors. There was no evidence that clonidine stimulated α1-adrenoceptors in the present study, because the vasoconstrictor response was blocked completely by the selective α2-adrenoceptor antagonist rauwolscine. In summary, the findings support the view that cutaneous forearm vessels contain a mixed population of α1- and α2-adrenoceptors. Thus, circulating catecholamines may augment sympathetic neural vasoconstriction by interacting with vascular α2-adrenoceptors, as in more distal cutaneous regions.
Effect of saline pretreatment on adrenergic vasoconstriction
The nonspecific vasodilatation that develops during iontophoresis interferes with an accurate assessment of cutaneous responses to vasodilators [13], and might also mask cutaneous responses to vasoconstrictors. In the present study, saline was administered to investigate the nonspecific effects of iontophoresis. Blood flow was slightly but not significantly greater at the site of repeated saline iontophoresis than at the reference site, indicating that nonspecific vasodilatation was minimal. Since cutaneous blood flow reaches a ceiling in skin heated to 42° C [14, 15], current-mediated vasodilatation may not have been detectable against the background of high flow.
Some sites were pretreated with saline to investigate whether current-mediated vasodilatation inhibited α-adrenergic vasoconstriction. Contrary to expectations, iontophoretic pretreatment with saline facilitated rather than inhibited vasoconstrictor responses to α-adrenergic agonists. This serendipitous finding may have implications for developing iontophoresis as an efficient controlled transdermal drug delivery technique. The transdermal route of drug administration is desirable because it bypasses the gut and hepatic first-pass metabolism. However, the major difficulty with transdermal drug delivery is overcoming the diffusion barrier presented by the stratum corneum. Camel et al. [16] investigated the effect of saline iontophoresis on various measures of skin barrier integrity (transepidermal water loss and capacitance) and inflammation (skin temperature, colour, and visual signs of irritation). Current was delivered at 100 or 200 µA/cm2 to the forearm for 10 min (in contrast, current density was 64 µA/cm2 for the 10 min saline pretreatment in the present study). No damage to the skin barrier in terms of transepidermal water loss or water content was detected at either current, although signs of skin irritation developed transiently [16]. In a subsequent study, saline iontophoresis for 4 h at 200 µA/cm2 was associated with an increase in transepidermal water loss that resolved within 2 h, and local flushing that persisted for 24 h [17]. Higher currents (up to 500 µA/cm2) are associated with increased water permeability, possibly because skin hydration, ion displacement, local heating or electrical field effects disrupt the structural organization of the stratum corneum [18]. However, these effects appear to be minimal at current densities below 100 µA/cm2.
The nonspecific vasodilatation that develops during iontophoresis is proportional to the duration of current flow and to current density (i.e. the strength of the current flowing through the area of contact between the ionized solution and the skin) [13]. It does not seem to develop below anodal currents of around 1 mA s/cm2 (in the present study adrenergic agonists were iontophoresed at 0.95 mA s/cm2 and adrenergic antagonists and saline were iontophoresed at 38.2 mA s/cm2). The mechanism of the vasodilator response is uncertain, but may involve the antidromic release of vasoactive peptides from sensory nerve terminals (neurogenic inflammation) or degranulation of mast cells. The vasodilator response develops at currents lower than that required to disrupt the structure of the stratum corneum [16, 18]. Thus, it is tempting to speculate that substances released during neurogenic inflammation that increase capillary permeability (e.g. histamine or substance P) facilitate the transdermal penetration of drugs by increasing the permeability of the stratum corneum.
Implications
The present findings suggest that transdermal iontophoresis of α-adrenergic agonists and antagonists may potentially be useful for clarifying the function of cutaneous α-adrenoceptors in thermoregulation, metabolism and immune reactions. This may help to clarify the pathophysiology of conditions such as hypertension, diabetes and neuropathic pain, where a cutaneous adrenergic role is suspected. We reported recently that the transdermal iontophoresis of noradrenaline produced dose-related vasoconstriction in skin heated to 42° C [19], indicating that this procedure may be useful in discriminating between the effects of different doses. However, in other situations the administration of drugs by iontophoresis may yield qualitative rather than quantitative information (e.g. where nonspecific vasodilatation interferes with specific vascular responses, or where the dermal penetration or removal of the drug varies unpredictably).
The findings also suggest an association between prior current-induced hyperaemia and penetration of drugs through the stratum corneum. This could be particularly important for efficiently administering small doses of drugs locally with minimal disruption to the skin barrier.
Acknowledgments
This project was supported by the Medical Research Fund of Western Australia. I gratefully acknowledge the research assistance of Mr Darren Hocking.
References
- 1.Ruffolo RR. Distribution and function of peripheral α-adrenoceptors in the cardiovascular system. Pharmacol Biochem Behav. 1985;22:827–833. doi: 10.1016/0091-3057(85)90535-0. [DOI] [PubMed] [Google Scholar]
- 2.van Brummelen P, van Zwieten PA. α-Adrenergic receptors in human blood vessels. Br J Clin Pharmacol. 1986;21:33S–39S. doi: 10.1111/j.1365-2125.1986.tb02851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruffolo RR, Waddell JE, Yaden EL. Postsynaptic alpha adrenergic receptor subtypes differentiated by yohimbine in tissues from the rat. Existence of alpha-2 adrenergic receptors in rat aorta. J Pharmacol Exp Ther. 1981;217:235–240. [PubMed] [Google Scholar]
- 4.Flavahan NA, Cooke JP, Shepherd JT, Vanhoutte PM. Human postjunctional alpha-1 and alpha-2 adrenoceptors. Differential distribution in arteries of the limbs. J Pharmacol Exp Ther. 1987;241:361–365. [PubMed] [Google Scholar]
- 5.Li Z, Koman LA, Smith BP, Gordon ES, Smith TL. Alpha adrenoceptors in the rabbit ear thermoregulatory microcirculation. Microvasc Res. 1998;55:115–123. doi: 10.1006/mvre.1997.2063. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen H, Thom SM, Hughes AD, Martin GN, Mulvany MJ, Sever PS. Postjunctional α2-adrenoceptors mediate vasoconstriction in human subcutaneous resistance vessels. Br J Pharmacol. 1989;97:829–834. doi: 10.1111/j.1476-5381.1989.tb12022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekenvall L, Lindblad LE, Norbeck O, Etzell BM. α-Adrenoceptors and cold-induced vasoconstriction in human finger skin. Am J Physiol. 1988;255:H1000–H1003. doi: 10.1152/ajpheart.1988.255.5.H1000. (Heart Circ Physiol24) [DOI] [PubMed] [Google Scholar]
- 8.Freedman RR, Sabharwal SC, Moten M, Migaly P. Local temperature modulates α1- and α2-adrenergic vasoconstriction in man. Am J Physiol. 1992;263:H1197–H1200. doi: 10.1152/ajpheart.1992.263.4.H1197. (Heart Circ Physiol32) [DOI] [PubMed] [Google Scholar]
- 9.Borbujo J, Garcia-Villalon AL, Valle J, Gomez B, Diequez G. Postjunctional alpha-1 and alpha-2 adrenoceptors in human skin arteries. An in vitro study. J Pharmacol Exp Ther. 1989;249:284–287. [PubMed] [Google Scholar]
- 10.Lindblad LE, Ekenvall L. Alpha-adrenoceptors in the vessels of human finger skin. Acta Physiol Scand. 1986;128:219–222. doi: 10.1111/j.1748-1716.1986.tb07969.x. [DOI] [PubMed] [Google Scholar]
- 11.Hörnqvist R, Bäck O, Henriksson R. Adrenoceptor-mediated responses in human skin studied by iontophoresis. Br J Dermatol. 1984;111:561–566. doi: 10.1111/j.1365-2133.1984.tb06625.x. [DOI] [PubMed] [Google Scholar]
- 12.Kenney WL, Zappe DH, Tankersley CG, Derr JA. Effect of systemic yohimbine on the control of skin blood flow during local heating and dynamic exercise. Am J Physiol. 1994;266:H371–H376. doi: 10.1152/ajpheart.1994.266.2.H371. (Heart Circ Physiol35) [DOI] [PubMed] [Google Scholar]
- 13.Grossmann M, Jamieson MJ, Kellogg DL, et al. The effect of iontophoresis on the cutaneous vasculature: evidence for current-induced hyperemia. Microvasc Res. 1995;50:444–452. doi: 10.1006/mvre.1995.1070. [DOI] [PubMed] [Google Scholar]
- 14.Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol. 1984;57:191–196. doi: 10.1152/jappl.1984.57.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Savage MV, Brengelmann GL. Reproducibility of the vascular response to heating in human skin. J Appl Physiol. 1994;76:1759–1763. doi: 10.1152/jappl.1994.76.4.1759. [DOI] [PubMed] [Google Scholar]
- 16.Camel E, O'Connell M, Sage B, Gross M, Maibach H. The effect of saline iontophoresis on skin integrity in human volunteers. I. Methodology and reproducibility. Fundam Appl Toxicol. 1996;32:168–178. doi: 10.1006/faat.1996.0120. [DOI] [PubMed] [Google Scholar]
- 17.Singh J, Gross M, Sage B, Davis HT, Maibach HI. Effect of saline iontophoresis on skin barrier function and cutaneous irritation in four ethnic groups. Food Chem Toxicol. 2000;38:717–726. doi: 10.1016/s0278-6915(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 18.Jadoul A, Bouwstra J, Preat V. Effects of iontophoresis and electroporation on the stratum corneum: review of the biophysical studies. Adv Drug Deliv Rev. 1999;35:89–105. doi: 10.1016/s0169-409x(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 19.Lipnicki DM, Drummond PD. Facilitating laser Doppler measurements of cutaneous adrenergic vasoconstriction: a comparison of methods. Clin Auton Res. 2001;11:93–98. doi: 10.1007/BF02322052. [DOI] [PubMed] [Google Scholar]