Abstract
Aims
Levocetirizine (R-cetirizine), is the active enantiomer of cetirizine, an antihistamine indicated in the treatment of allergic rhinitis and chronic idiopathic urticaria. The purpose of this trial was to analyse the effects of levocetirizine single and multiple doses on CNS using integrated measures of cognitive and psychometric performance.
Methods
A battery of psychometric tests was used: critical flicker fusion (CFF), choice reaction time (CRT), body sway (BS), learning memory test (LMT) and subjective assessments of alertness compared with placebo. Nineteen (19) healthy male volunteers received either levocetirizine 5 mg (therapeutic dose), diphenhydramine 50 mg or placebo once daily for 5 consecutive days (3-way cross-over). Diphenhydramine was used as a positive control. CFF tests were performed on days 1 and 5 at baseline and up to 24 h following drug intake. Subjects used the Bond-Lader visual analogue scales (VAS) to assess their mood and vigilance.
Results
In contrast to diphenhydramine, when compared with placebo, levocetirizine did not modify the CFF (primary endpoint), regardless of the dosing scheme (−1.62 Hz [−2.61, −0.64] and −0.81 Hz [−1.80, 0.19], respectively, 3 h after dosing on day 1). CRT was decreased with both levocetirizine and placebo up to 5 h after dosing on day 1 and up to 3 h after dosing on day 5. Body sway data were similar with levocetirizine and placebo but increased with diphenhydramine. LMT was similar in all three groups. No relevant difference between placebo and levocetirizine was recorded by the subjects on their assessment of alertness using the VAS, whilst decreased alertness was reported following diphenhydramine 50 mg.
Conclusions
This study showed that levocetirizine does not produce any deleterious effect on cognitive and psychometric functions compared with placebo in healthy male volunteers.
Keywords: antihistamine, critical flicker fusion, levocetirizine, psychomotor performance, reaction time
Introduction
Recently developed antihistamine drugs have an increased benefit-risk ratio compared with first generation antihistamines. As a matter of fact, the efficacy of new antihistamines is not correlated with sedation, which has been demonstrated to be minimal [1]. However, it remains that the effect of a given drug on the CNS has to be explored specifically, especially when this drug could be administered to people involved in potentially dangerous activities. Methodological aspects are central to this exploration, and some study designs were sometimes controversial in appreciating CNS effects of a given drug [2, 3]. Therefore, a battery of psychometric tests is essential to characterize the level of CNS impact of a new drug.
Cetirizine is a chiral molecule with potent antiallergic effects and clinically insignificant sedative effects with minimal impact on routine daily activities [2–5].
Levocetirizine (R-cetirizine) is the active enantiomer of cetirizine. This molecule has been shown to have both important affinity and selectivity for H1-receptors [6]. Its affinity is 2 times and 25 times higher compared with cetirizine and dextrocetirizine (S-cetirizine), respectively. Levocetirizine 5 mg has at least equivalent inhibitory effects on cutaneous wheal and flare responses as cetirizine 10 mg. Conversely, dextrocetirizine does not differ from placebo [7]. Like cetirizine, levocetirizine is indicated for the treatment of allergic rhinitis (seasonal and perennial) and chronic idiopathic urticaria.
The purpose of this trial was to study the effect of single and repeated doses of levocetirizine on integrated measures of cognitive and psychometric performances in healthy volunteers. Both a negative (placebo) and a positive control (diphenhydramine) were used, in order to assess both the sensitivity of the tests used and the effects of levocetirizine.
Methods
Drugs profile
Levocetirizine is rapidly absorbed and has a fast onset of action, peak plasma concentrations are reached about 1 h after dosing and plasma half-life in adults is between 8 and 10 h [6]. More than 85% of levocetirizine is excreted unchanged in the urine; the remaining fraction being excreted in the faeces.
When compared with cetirizine 10 mg, levocetirizine 5 mg demonstrates similar absorption properties, a smaller volume of distribution (0.4 l compared with 0.6 l), a lower total body clearance, and a higher fraction of unchanged compound in the urine. Clinical studies have shown that levocetirizine 5 mg has similar effects to cetirizine 10 mg on inhibition of histamine induced cutaneous wheal and flare and histamine induced increased nasal resistance and pressure [7, 8].
Diphenhydramine is a first generation antihistamine with central sedative properties and anticholinergic effects. It is extensively metabolized; tmax and elimination half-life are 1.7 h and 9.2 h, respectively [9].
The most frequently reported side-effects of diphenhydramine are related to CNS depressing properties of the drug (sedation, drowsiness, lassitude and motor incoordination), making it a good candidate for a positive verum. The dose used, 50 mg, is considered to be the lowest producing a change in performance test scores [10].
Subjects and study design
As required by the protocol, no volunteers were found to consume alcohol abusively, nor did they smoke more than 10 cigarettes a day. They did not consume more than six cups of xanthine-containing beverages a day and none had any history of illicit drugs of abuse. No medication was allowed 2 weeks prior to recruitment, nor during the study, except for the study drugs.
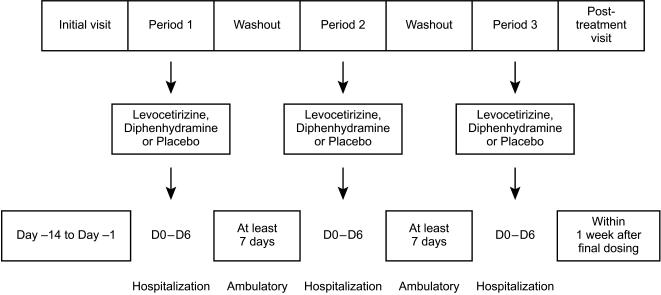
Three treatment periods of 5 days separated by a wash-out period of at least 7 days were planned (Figure 1). The last visit was a follow-up visit that occurred within the week following the last study drug administration. Subjects were hospitalized in the Study Unit during the 5 day treatment periods. No blood samples were taken except for safety assessments during screening and at the end of the study. Meals were standardized and subjects had to refrain from intense physical activity, smoking, alcohol and grapefruit juice. Study drugs were assigned according to a Latin-square based randomization list. During each of the three treatment periods study drugs were administered every morning to the subjects, after breakfast, from day 1 to day 5. Capsules were identically matched in size, colour and shape, to respect the double-blind nature of the study.
Figure 1.
Study design.
Every subject gave his written consent and complied with the French law Huriet related to biomedical research. The study protocol, the Subject Information Sheet and the Informed Consent Form were approved by the Independent Ethics Committee (Comité Consultatif de Protection des Personnes se prêtant à la Recherche Biomédicale – CCPPRB) of Brest (France).
Study objectives
The primary objective was to assess the effect of levocetirizine 5 mg after both single and repeated doses, on psychometric and cognitive functions compared with placebo, using the critical flicker fusion test.
Secondary objectives included assessment of effects on a battery of tests including choice reaction time, body sway, and on learning memory. Besides, subjective perception of mood changes and vigilance were also measured through visual analogue scales.
Cognitive and psychometric tests
Critical flicker fusion (CFF)
The critical flicker fusion test is a widely recognized and validated tool for measuring an integrated index of CNS activity. The CFF threshold is an integrated index of CNS activity, i.e. alertness and cortical arousal particularly sensitive to impairment [4, 11, 12]. The frequency at which a light source must oscillate before it appears flicker-free is called the critical fusion frequency or critical flicker frequency. Subjects were asked to indicate when a red-light-emitting flickering source increasing in frequency, is perceived to become a continuous signal. They were also required to distinguish the threshold at which a flickering signal was perceived from a continuous signal, when frequency decreased. This fusion and flicker are a reliable measure of cortical, alertness and arousal and reasonably stable in a given subject. Decrease in thresholds are indicative of altered CNS function.
Choice reaction time (CRT)
The CRT requires that the subject makes a decision and choose a response. CRT is based on a memory search paradigm [11, 13], where subjects are asked to react as quickly and accurately as possible in matching responses to a given stimuli. This procedure tests the time (in ms) taken by a subject to make a motor response to a sensory stimulus. The subject has to operate the correct button to switch off one of six light-emitting diodes located on a panel. The scores used for analysis are the mean values of the various times (CRT) obtained from a maximum of 50 stimuli. An increase in CRT values would be indicative of impaired alertness.
Body sway (BS)
BS is a technique enabling the measurement and recording of involuntary anterior-posterior and left-right postural oscillations using a vertical force platform. Both subject's positioning and equipment are standardized, in accordance with recommendations from the International Society of Posturography [14, 15]. The computerized measures used for analysis reflects the total displacement distance (cm from the centre of gravity and its corresponding surface (cm2)). The test is performed with both eyes open and closed. Body sway is increased in case of alcohol consumption; this is an indirect measure of alertness.
Learning memory test (LMT)
This test assesses short and long-term memory [16]. The subjects are asked first to freely recall the 15 words presented to them; then, to recall again the memorized words after a delay of 3 min during which he or she performs a digit symbol substitution test (DSST, used as a prolonged distraction task). The number of accurate words recalled determines the scores of immediate and delayed free memory recall.
Bond and Lader's visual analogue scale (VAS)
These subjective assessments of mood and vigilance are recorded using 16 horizontal 100 mm scales. Drugs effects on three parameters were calculated by factorial analysis, namely: alertness, contentedness and calmness [17].
CFF, CRT, BS and VAS were performed at the initial visit and on day 0 of period 1 to familiarize the subject with the procedures. They were further performed on day 1 and day 5 of each treatment period 1, 2, 3, 5 and 12 h after study drug administration, then again on day 2 and day 6, 24 h after dosing.
LMT and DSST were performed at the initial visit and on day 0 of period 1 to familiarize the subject with the procedures. They were also performed on day 1 and day 5 of each treatment period, before and 2 h after drug administration.
Safety assessments
A physical examination was performed by a physician at the initial visit, on day 6 of each study period and at each post-treatment visit. Cardiovascular safety was monitored by means of standard 12-lead ECGs at the initial visit, on day 1 and day 5, both before and 1 h after study drug administration, of each period and at the post-treatment visit. Vital signs (blood pressure and heart rate) were recorded at the initial visit and on day 6 of each period and at the post-treatment visit. All adverse events and undesirable experiences occurring during the study were reported.
Statistical analysis
The primary variable was expressed as the change in CFF score between the baseline score (predrug administration) and the one obtained at the predefined time points on day 1.
This was an exploratory study for which sample size was based on experience in similar studies [4, 21]. Sufficient volunteers had to be selected by the investigator in order to have a total number of 18 evaluable subjects for the analysis. Withdrawn subjects were replaced for this reason.
A repeated measures analysis of variance was used to compare treatment changes from baseline for this three-way crossover study [18, 19]. Statistical tests were carried out two-tailed at the 5% level of significance using SAS® software.
The fixed effects included treatment effect, period effect, time-point effect and the interaction of treatment × time-point effect. The baseline value was included into the model as a covariate. In presence of a significant treatment × time–point interaction, the treatment was evaluated at each time-point in the same model. The 95% confidence intervals of the least-squares means treatment differences were calculated for pairwise comparisons. Some model checks were performed: normality of residuals, absence of outliers (residual vs predicted values), and correlation between subject's effects and their predicted values.
Secondary variables were the change in CFF score between baseline and the predefined time points on day 5 as well as the changes between baseline scores and those obtained on day 1 and day 5 for CRT, BS, LMT and VAS parameters. Descriptive statistics and 95% confidence intervals (CI) of the mean treatment differences were calculated.
Results
Disposition of subjects
Thirty-one subjects were screened, 19 were included out of whom 18 completed the study. Out of the 19 subjects randomized, one withdrew his consent when under diphenhydramine treatment. Therefore, 19 received diphenhydramine, 18 received levocetirizine and 18 placebo treatment. They were aged between 20 and 39 years [mean (s.d.): 24.5 (4.2)], weighed between 56 and 82 kg [71.0 (7.3)], and were between 166 and 189 cm in height [178.3 (6.0)].
Critical flicker fusion test (CFF)
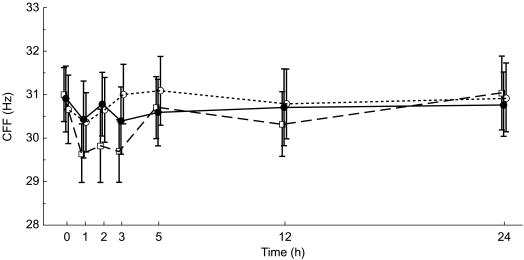
At baseline, mean (±s.d.) CFF baseline values were similar in the levocetirizine (30.9 ± 3.2 Hz), placebo (30.7 ± 3.3 Hz) and diphenhydramine arm (31.0 ± 2.7 Hz). During the first 24 h following drug administration, placebo and levocetirizine mean CFF values fluctuated around the baseline values within a range that never surpassed 0.5 Hz below or above baseline values, which corresponds to expected and acceptable fluctuations. Of note, levocetirizine and placebo results were never statistically significantly different from each other, neither globally over all time points (P = 0.292), nor at any particular time point. By contrast, mean CFF values, after diphenhydramine administration displayed a maximum decrease within 1 h of dosing, with a mean decrease from baseline of 1.35 Hz. The difference between diphenhydramine and placebo was statistically different globally (P = 0.019) and more specifically 1, 2 and 3 h after dosing (P < 0.04). The maximum difference between placebo and diphenhydramine occurred at the third hour with a mean difference of 1.62 Hz (P = 0.002, Table 1). This difference was more than twice the mean difference observed between levocetirizine and placebo (Figure 2).
Table 1.
Mean treatment differences between levocetirizine (or diphenhydramine) and placebo in CFF from baseline on day 1 by time-point.
| Comparisons | Time | Mean difference and 95% CI (Hz) | P values |
|---|---|---|---|
| Levocetirizine vs placebo | 1 h | −0.15 (−1.15, 0.84) | 0.764 |
| 2 h | −0.08 (−1.07, 0.92) | 0.876 | |
| 3 h | −0.81 (−1.80, 0.19) | 0.111 | |
| 5 h | −0.69 (−1.69, 0.30) | 0.168 | |
| 12 h | −0.27 (−1.27, 0.72) | 0.56 | |
| 24 h | −0.34 (−1.34, 0.65) | 0.491 | |
| Diphenhydramine vs placebo | 1 h | −1.04 (−2.03, −0.06) | <0.05 |
| 2 h | −1.14 (−2.13, −0.16) | <0.05 | |
| 3 h | −1.62 (−2.61, −0.64) | <0.005 | |
| 5 h | −0.71 (−1.70, 0.27) | 0.152 | |
| 12 h | −0.78 (−1.76, 0.21) | 0.120 | |
| 24 h | −0.20 (−1.18, 0.78) | 0.685 |
Figure 2.
Effect of levocetirizine 5 mg (•, solid line), diphenhydramine 50 mg (□, dashed line) and placebo (○, dotted line) on CFF thresholds in healthy volunteers: Mean (±s.e. mean) of CFF (Hz) on day 1 – ITT population.
After 4 consecutive treatment days, i.e. on day 5, CFF times evolution were globally similar to those of day 1 for levocetirizine and placebo. Again, diphenhydramine produced a decrease, albeit less pronounced than that on day 1 and no longer achieving a statistically significant difference relative to placebo.
Choice reaction time (CRT)
The baseline mean (±s.d.) CRT values on day 1 were similar for placebo (433.7 ± 33.9 ms), levocetirizine (434.3 ± 50.8 ms) and diphenhydramine (422.5 ± 59.7 ms).
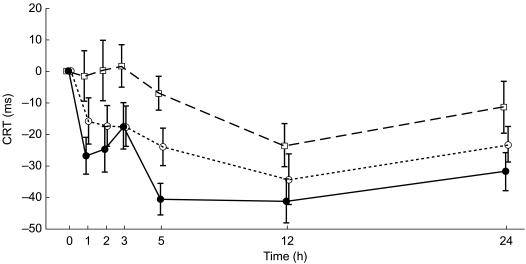
However, a decrease in mean CRT scores was observed during the study, both after levocetirizine and placebo administration from the first to the 24th hour after dosing (with a maximum of −34.4 ms and −41.1 ms at the 12th hour for placebo and levocetirizine, respectively). Levocetirizine was not statistically different from placebo at any time point. The reduction in CRT was much less with diphenhydramine with mean scores of −23.6 ms at the 12th hour. Mean CRT values on day 1 are shown in Figure 3. Mean CRT scores were comparable over time for the three treatments, with no significant differences for groups on day 5.
Figure 3.
Effect of levocetirizine 5 mg (•, solid line), diphenhydramine 50 mg (□, dashed line) and placebo (○, dotted line) on CRT on day 1: mean time changes from baseline, in ms (±s.e. mean).
Body sway
Results on distance and surface displacement from the centre of gravity measured eyes open or closed, were similar for levocetirizine and placebo, whereas an increase in total displacement distance was recorded up to 3 h after dosing with diphenhydramine on day 1. This increase reached statistical significance when the test was performed with eyes closed: at 3 h the mean difference between diphenhydramine and placebo was 16.35 cm (95% CI: 5.61, 27.10).
A similar pattern was observed with the mean difference in surface displacement between diphenhydramine and placebo: 3.08 cm2 (95% CI: 0.76, 5.39) at the 3rd hour after dosing on day 1.
Bond & Lader's Visual Analog Scales (VAS)
Alertness
Scores of alertness increased both after levocetirizine and after placebo treatment, with a maximum at the 12th hour after dosing. These increases were more marked for placebo arm compared with levocetirizine, leading to a statistical difference between the two groups, at the 3rd and 12th hour after dosing, i.e. a mean VAS score difference of −7.87 (95% CI: −15.15, 0.60) and −8.47 (14.05, −2.88), respectively.
A major and significant decrease in alertness was observed after diphenhydramine administration, on day 1, at the 2nd and 3rd hour after dosing, when compared with placebo. These mean differences (−13.51; 95% CI: −26.09, −0.92 and −17.09; 95% CI: −28.89, −4.28, respectively) were expected, diphenhydramine being a positive control.
Contentedness
Results pointed to a similar evolution of contentedness in all three treatments on days 1 and 5.
Calmness
No marked or consistent decreases in calmness were observed with any treatment.
Learning memory test (LMT)
Immediate memory
The results observed were not a decrease of performance, but rather an effect of time with a decrease from baseline to 2 h following the administration of all drugs including placebo on both day 1 and day 5. The decrease was the most marked with diphenhydramine on day 1, 2 h after administration, when compared with placebo and to levocetirizine on immediate first recall. However, these differences when compared with placebo did not reach statistical significance. Levocetirizine was not significantly different from placebo either on day 1 or on day 5.
Delayed memory
Results on delayed memory displayed the same characteristics as those on immediate memory: a decrease of performance for all study treatments when compared to baseline, greatest performance fall observed under diphenhydramine and finally, performance less affected on day 5 than on day 1.
Safety assessment
No serious adverse events were reported. Sixteen (16) subjects out of 19 exposed to study drugs experienced at least one adverse event (AE), of mild to moderate intensity. They consisted mainly in somnolence, asthenia, headache and diarrhoea episodes. However, both the incidence of AEs and the number of subjects who experienced at least one AE, were greater under diphenhydramine when compared with levocetirizine and placebo (occurrences/number of subjects: 20/9, 15/7, 15/8, respectively).
No apparent difference was observed between the three treatments with regard to the number of adverse events related to the study drug. The main adverse event, somnolence, was chiefly reported after the diphenhydramine (7 episodes reported) and placebo (6 episodes reported). Only 4 somnolence episodes were reported during levocetirizine treatment. The number of subjects suffering from somnolence was, respectively, 2 with levocetirizine, 2 with placebo and 4 with diphenhydramine.
No clinically significant changes in laboratory parameters or in vital signs, or in ECG parameters were observed after either treatment.
Discussion
The purpose of this study was to assess the impact of a new H1-receptor antagonist, levocetirizine, on psychomotor and sensorimotor performance using tests that are validated surrogate markers of CNS impairment and performance of daily activities.
Cetirizine, the parent compound of levocetirizine, has demonstrated convincing evidence in verum and placebo controlled studies that objective measures of the incidence of somnolence and CNS impairment at therapeutic doses were similar to that produced by placebo [2]. As a result, the therapeutic index of cetirizine is much wider than the one of the first generation of H1-receptor antagonists.
Levocetirizine is the eutomer of cetirizine and has been shown to have equivalent efficacy and tolerability at half the cetirizine dose. We did not expect that levocetirizine would have a deleterious effect on CNS. However as impact on potentially dangerous daily activities (e.g. driving) remained of concern, it seemed appropriate to measure objectively any potential effect after single and repeated active doses of levocetirizine.
The well recognized positive control used in this study is an antihistamine of the first generation and clearly induced CNS impairment in the tests performed, therefore validating the methodology used. Diphenhydramine exhibited its negative effect in the tests used, specifically on day 1.
CFF is the most commonly used task in studies investigating the central effects of antihistamines and has proved sensitive to a wide range of compounds. CFF has consistently demonstrated the reduction in cognitive capacity following traditional antihistamines, as well as detecting changes following other antihistamines, e.g, loratadine and cetirizine, where other tests have failed to detect any impairment [11].
CFF was used as an index of global cortical arousal [12] and as the primary endpoint of this study. This sensitive and validated test did not elicit any clinically relevant or statistically significant difference between levocetirizine and placebo. These observations, indeed, point to the anticipated conclusion that levocetirizine does not induce any reduction in vigilance.
All effects were less marked on day 5, reflecting the development of tolerance to the treatments [13]. The consistent increase in performance of CFF on day 5 is a sign of tolerance to the sedative action of diphenhydramine after multiple dosing (tachyphylaxis).
As to the CRT test, positive results would have indicated impairment of psychomotor speed. The data in this study pointed to negative values, i.e. an increase in performance for both placebo and levocetirizine, even more so for the latter. This may be explained not by a learning effect as subjects were already trained in the method, but by a slight increase in alertness over the course of the study day, which is consistent with results obtained with the visual analogue scales. This type of result with significant effect of time justifies the necessity of using a verum and a placebo control group for evaluating CNS effect of antihistamines [20]. In comparison, the close to null effect after diphenhydramine treatment would tend to signify, then, impairment of reaction speed.
The remaining, more ancillary tests had their sensitivity also validated, with diphenhydramine showing a negative impact on performance at least on day 1. The results accumulated a body of evidence showing that levocetirizine was never different from placebo on day 1 or on day 5 (Table 2).
Table 2.
Mean treatment differences between levocetirizine (or diphenhydramine) and placebo with 95% CI at 1, 2 or 3 h time point for the main trial tests, at day 1 and day 5.
| Levocetirizine 5 mg | Diphenhydramine 50 mg | |||||
|---|---|---|---|---|---|---|
| Test | 1 h | 2 h | 3 h | 1 h | 2 h | 3 h |
| Day 1 | ||||||
| CFF (Hz) | −0.15 | −0.08 | −0.81 | −1.04 | −1.14 | −1.62 |
| (−1.15, 0.84) | (−1.07, 0.92) | (−1.80, 0.19) | (−2.03, −0.06) | (−2.13, −0.16) | (−2.61, −0.64) | |
| CRT (ms) | −11.06 | −7.33 | 0.22 | 8.50 | 10.00 | 15.22 |
| (−30.32, 8.21) | (−31.78, 17.11) | (−21.08, 21.53) | (−12.76, 29.76) | (−9.09, 29.09) | (−2.55, 32.99) | |
| BS Distance displ. (cm) | 3.26 | 2.42 | 6.19 | 7.35 | 7.99 | 16.35 |
| Eyes closed | (−1.90, 8.41) | (−9.11, 13.94) | (−1.27, 13.64) | (−0.81, 15.50) | (−4.52, 20.50) | (5.61, 27.10) |
| LMT immediate recall | ND | 0.61 | ND | ND | −0.61 | ND |
| (−1.57, 2.80) | (−1.93, 0.71) | |||||
| VAS Alertness score | −3.78 | −4.47 | −7.87 | −4.88 | −13.51 | −17.09 |
| (−14.04, 6.48) | (−12.19, 3.25) | (−15.15, −0.60) | (−18.04, 8.27) | (−26.09, −0.92) | (−29.89, −4.28) | |
| Day 5 | ||||||
| CFF (Hz) | −0.18 | 0.16 | 0.15 | 0 | −0.17 | −0.63 |
| (−0.91, 0.54) | (−0.74, 1.06) | (−0.56, 0.86) | (−1.09, 1.09) | (−1.27, 0.94) | (−1.69, 0.43) | |
| CRT (ms) | 1.00 | −8.22 | −2.11 | 6.72 | 11.11 | 12.17 |
| (−14.97, 16.97) | (−30.45, 14.00) | (−20.15, 15.93) | (−4.93, 18.38) | (−6.06, 28.28) | (−2.09, 26.42) | |
| BS Distance displ. (cm) | 6.62 | 5.73 | 6.42 | 1.63 | 4.61 | 4.97 |
| Eyes closed | (−2.20, 15.43) | (−3.96, 15.42) | (−2.08, 14.93) | (−6.08, 9.33) | (−2.35, 11.56) | (−3.58, 13.32) |
| LMT immediate recall | ND | −0.06 | ND | ND | −0.56 | ND |
| (−1.38, 1.27) | (−0.94, 2.05) | |||||
| VAS | 0.77 | 7.19 | 5.47 | 0.31 | −5.12 | −4.20 |
| Alertness score | (−4.17, 5.71) | (−1.12, 15.50) | (−2.49, 13.43) | (−8.82, 9.44) | (−13.61, 3.38) | (−12.55, 4.15) |
ND:Not done.
Overall, the smaller effects found on the 5th day in comparison with the first, the results of the tests also replicate the already reported tolerance to the sedative properties of antihistamine drugs as tripolidine [5, 22]. Absence of effect on memory was expected, consistent with the finding in an extensive review of the literature by Hindmarch & Shamsi [20].
Somnolence episodes were the most frequent adverse event reported and were mainly observed after diphenhydramine treatment, as expected. It is important to mention that less episodes were reported with levocetirizine compared with placebo.
In conclusion, single and repeated doses of levocetirizine 5 mg did not induce impairment of CNS function when compared with placebo in healthy volunteers. This is in contrast to the effects of diphenhydramine which served as a verum. The importance of such a positive control for measurement of psychomotor and cognitive function was demonstrated in this study.
It is concluded that levocetirizine is safe when administered orally for 5 days in this population.
Acknowledgments
The authors would like to thank Nadia Cheiab and Diane Kleinermans for their contribution to this study.
References
- 1.Spaeth J, Klimek L, Mosges R. Sedation in allergic rhinitis is caused by the condition and not by antihistamine treatment. Allergy. 1996;51:893–906. doi: 10.1111/j.1398-9995.1996.tb04490.x. [DOI] [PubMed] [Google Scholar]
- 2.Hindmarch I. Psychometric aspects of antihistamines. Allergy. 1995;50:48–54. doi: 10.1111/j.1398-9995.1995.tb04264.x. [DOI] [PubMed] [Google Scholar]
- 3.Rombaut NEI, Hindmarch I. Psychometric aspects of antihistamines: a review. Human Psychopharmacol. 1994;9:157–169. [Google Scholar]
- 4.Patat A, Stubbs D, Dunmore C, et al. Lack of interaction between two antishitamines, mizolastine and cetirizine, and ethanol in psychomotor and driving performance in healthy subjects. Eur J Clin Pharmacol. 1995;48:143–150. doi: 10.1007/BF00192740. [DOI] [PubMed] [Google Scholar]
- 5.Volkerts ER, Van Willigenburg APP, Van Laar MW, Maes RAA. Does cetirizine belong to the new generation of antihistamines? An investigation into its acute and subchronic effects on highway driving, psychometric test performance and daytime sleepiness. Human Psychopharmacol. 1992;7:227–238. [Google Scholar]
- 6. Investigator's brochure UCB Pharma RXCE, 99B0501. Levocetirizine dihydrochloride (UCB 28556) capsules edition number 2. Release date: 21st April 1999.
- 7.Devalia JL, De Vos C, Hanotte F, Baltès E. A randomized, double-blind, crossover comparison among cetirizine, levocetirizine, and UCB 28557 on histamine-induced cutaneous responses in healthy adult volunteers. Allergy. 2001;56:50–57. doi: 10.1034/j.1398-9995.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang DY, Hanotte F, De Vos C, Clement P. Effect of cetirizine, levocetirizine, and dextrocetirizine on histamine-induced nasal response in healthy adult volunteers. Allergy. 2001;56:339–343. doi: 10.1034/j.1398-9995.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 9.Simons KJ, Watson WT, Martin TJ, et al. Diphenhydramine. Pharmacokinetics and pharmacodynamics in elderly adults, young adults and children. J Clin Pharmacol. 1990;30:665–671. doi: 10.1002/j.1552-4604.1990.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 10.Witek TJ, Canestrari DA, Miller RD, Yang JY, Riker DK. Characterization of daytime sleepiness and psychomotor performance following H1 receptor antagonists. Ann Allergy Asthma Immunol. 1995;74:419–426. [PubMed] [Google Scholar]
- 11.Sherwood N, Kerr JS. The reliability, validity and pharmacosensitivity of four psychomotor tests. In: Hindmarch I, Stonier PD, editors. Human Psychopharmacology. Vol. 4. John Wiley and Sons Ltd; 1993. pp. 1–14. [Google Scholar]
- 12.Schweitzer PK, Muehlbach MJ, Walsh JK. Sleepiness and performance during three-day administration of cetirizine or diphenhydramine. J Allergy Clin Immunol. 1994;94:716–724. doi: 10.1016/0091-6749(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 13.Hindmarch I. Psychomotor function and psychoactive drugs. Br J Clin Pharmacol. 1980;10:189–209. doi: 10.1111/j.1365-2125.1980.tb01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClelland GR. Body sway and the effects of psychoactive drugs: a review. Human Psychopharmacol. 1989;4:3–14. [Google Scholar]
- 15.Kapteyn TS, Bles W, Njiokiktjein CJ, Kodde L, Massen CH, Mol JMF. Standardisation in platform stabilometry being part of posturography. Aggressologie. 1983;24:321–326. [PubMed] [Google Scholar]
- 16.Allain H, Gandon JM. Psychopharmacology of memory components. In: Hindmarch I, Stonier PD, editors. Human Psychopharmacology: measures and methods. Vol. 4. John Wiley and Sons Ltd; 1993. pp. 143–146. [Google Scholar]
- 17.Bond A, Lader M. The use of analogue scales in rating subjective feeling. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- 18.Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester (England): John Wiley and Sons; 1993. p. 266. [Google Scholar]
- 19.Senn S. Cross-over Trials in Clinical Research. Chichester (England): John Wiley and Sons; 1993. p. 266. [Google Scholar]
- 20.Hindmarch I, Shamsi Z. Antihistamines. Models to assess sedative properties, assessment of sedation, safety and other side-effects. Clin Exp Allergy. 1999;29:133–142. doi: 10.1046/j.1365-2222.1999.0290s3133.x. [DOI] [PubMed] [Google Scholar]
- 21.Hindmarch I, Shamsi Z, Stanley N, Fairweather DB. A double-blind, placebo-controlled investigation of the effects of fexofenadine, loratadine and promethazine on cognitive and psychomotor function. Br J Clin Pharmacol. 1999;48:200–206. doi: 10.1046/j.1365-2125.1999.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bye CE, Cooper J, Empey DW, et al. Effects of pseudoephedrine and triprolidine, alone and in combination, on symptoms of the common cold. Br Med J. 1980;281:189–190. doi: 10.1136/bmj.281.6234.189. [DOI] [PMC free article] [PubMed] [Google Scholar]