Abstract
Aims
Many drugs belonging to different therapeutic classes appear to share a potentially fatal side-effect: ventricular tachyarrhythmias associated with QT prolongation. The aim of this study was to assess the relevance and the magnitude of the problem in seven countries by grouping all nonantiarrhythmic drugs according to the type of evidence on QT prolongation and analysing their sales data.
Methods
We divided all nonantiarrhythmic QT-prolonging agents into the following categories (in increasing order of clinical relevance): group A, drugs with published clinical or preclinical evidence on QT prolongation or with relevant official warnings; group B, drugs with published clinical or preclinical evidence; group C, drugs with published clinical evidence; group D, drug with published clinical evidence on torsades de pointes or ventricular arrhythmias associated with QT prolongation; group E, drugs belonging to group D with official warnings. We retrieved 1998 sales data from community pharmacies in seven countries (Australia, Denmark, England, Germany, Greece, Italy and Sweden). Data for individual agents were expressed as defined daily doses per 1000 inhabitants per day (DDD/1000/day). Overall use in each country was calculated for each drug group. Groups D and E were considered as the most clinically relevant.
Results
Among the 102 nonantiarrhythmic agents meeting at least one of the inclusion criteria, 33 drugs had sales data ≥1 DDD/1000/day and 71 drugs had a use ≥0.1 DDD/1000/day in at least one country. Among the 37 nonantiarrhythmic agents with published reports of ventricular arrhythmias associated with QT prolongation, 12 compounds had sales data ≥1 DDD/1000/day. Total consumption in each country ranged: from 51.9 to 94.7 DDD/1000/day for group A; from 51.6 to 92.7 DDD/1000/day for group B; from 37.1 to 76.6 DDD/1000/day for group C; from 12.9 to 29.1 DDD/1000/day for group D; and from 5.8 to 15.3 DDD/1000/day for group E.
Conclusions
In spite of wide variations in the sales of individual agents, the overall extent of use of nonantiarrhythmic QT-prolonging drugs was of the same order of magnitude in all countries. The significant use of drugs belonging to categories D and E should prompt careful risk/benefit assessment of each agent.
Keywords: adverse drug reactions, drug utilization, QT prolongation, torsades de pointes
Introduction
Drug-induced prolongation of the QT interval is used as a surrogate marker of cardiotoxicity, since it is associated with an increased likelihood of occurrence of torsades de pointes (TdP), a life-threatening ventricular tachyarrhythmia [1–5].
The list of nonantiarrhythmic drugs having the potential to prolong the QT interval is rapidly growing. In the past few years, several of these agents were withdrawn as a result of periodic safety reviews or following case reports to the pharmacovigilance system [3]. Since, to our knowledge, no studies of the overall use of QT-prolonging drugs are available, we decided to use an exhaustive list of QT-prolonging drugs [6] in order to:
analyse sales data of individual agents dispensed through community pharmacies in seven countries (Australia, Denmark, England, Germany, Greece, Italy and Sweden);
assess the magnitude of the problem in each country by analysing overall consumption of drugs grouped according to the strength of evidence on the proarrhythmic risk (published clinical evidence, published nonclinical evidence, official warnings in the summary of the product characteristics, etc.).
To this end, we retrieved 1998 sales data of drugs dispensed through community pharmacies, expressed in defined daily doses per 1000 inhabitants per day (DDD/1000/day). Since data of in-hospital use for individual drugs are not easily available in all countries, we decided to limit our investigation to community pharmacies. On the other hand, since QT-associated proarrhythmic risk is less well controllable outside of a hospital setting, it is important to assess the magnitude of the problem in the community by using homogenous criteria.
Methods
List of QT prolonging drugs: inclusion criteria
In a previous publication [6], we performed a systematic review of the available information on drugs capable of prolonging the QT interval and proposed a comprehensive list of such drugs. Briefly, for each nonantiarrhythmic drug, the type of evidence supporting an effect on the QT interval was structured according to the following inclusion criteria:
-
Published clinical evidence:
clinical studies and/or case reports associating the drug with the occurrence of TdP or ventricular tachyarrhythmias associated with QT prolongation
clinical studies reporting only QT prolongation (with no mention of TdP or tachyarrhythmias).
-
Published nonclinical evidence on effects on cardiac repolarization:
in vitro studies showing HERG K+ channel blockade (i.e. inhibition of the human repolarizing current IKr, which leads to the prolongation of the cardiac action potential [4])
in vitro studies showing IK inhibition or prolongation of the action potential duration, or in vivo studies in animals showing prolongation of the QT interval.
-
Official warnings in the labelling (including official warnings issued by the EMEA, the FDA or included in the British National Formulary, March 2000 Edition, or in the Physician Desk Reference, 2000 Edition) on:
QT prolongation or occurrence of TdP
cardiac arrhythmias (not otherwise specified); to avoid inclusion of drugs causing arrhythmias through different mechanisms, compounds that met only criterion IIIb (and none of the preceding criteria) were not considered.
Since the present investigation assessed sales through community pharmacies, we excluded a few QT prolonging drugs intended for in-hospital use (e.g. general and local anaesthetics) and some ‘nontherapeutic’ substances such as cocaine.
Retrieval of sales data through community pharmacies
The drugs meeting the inclusion criteria were arranged according to the 1999 version of the Anatomic Therapeutic Chemical (ATC) classification [7]. If a drug had more than one ATC code (a different route of administration may imply a different ATC code), these were added, provided that a systemic effect could be expected on the basis of the literature. Topical preparations for dermatological use were excluded, whereas topical preparations involving mucosal absorption (ophthalmic, gynaecological) were included. Fixed dose combinations containing QT prolonging drugs were not considered, except sulfamethoxazole/trimethoprim (published evidence on the proarrhythmic risk refers to the combination).
We retrieved 1998 sales data of drugs supplied through community pharmacies in Australia, Denmark, Greece, Italy, Sweden (prescription data), England and Germany (drugs reimbursed through the respective health services). Sales data were expressed as defined daily doses per 1000 inhabitants per day (DDD/1000/day) in order to normalize the data and allow comparisons among countries.
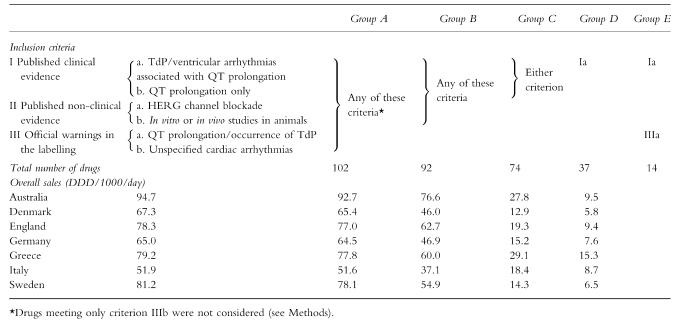
To assess overall drug use in each country, compounds were grouped on the basis of the strength of evidence about their proarrhythmic potential (in increasing order of severity):
group A:drugs meeting at least one of the subcriteria detailed in the preceding paragraph;
group B:drugs with published evidence on QT prolongation in human or nonhuman models (i.e. meeting one of the four criteria Ia or Ib or IIa or IIb);
group C:drugs with published clinical evidence on QT prolongation (criteria Ia or Ib);
group D:drugs with published reports of TdP or ventricular arrhythmias associated with QT prolongation in humans (criterion Ia);
group E:drugs belonging to group D with official warnings on QT prolongation (criteria Ia and IIIa).
Finally, total DDD/1000/day dispensed through community pharmacies in 1998 (i.e. sales of all drugs) were also retrieved, except for England, where the database does not contain this information (total prescription items were used instead).
Results
The list of nonantiarrhythmic agents meeting at least one of the criteria included 102 compounds (Tables 1 and 2). Of these, 31 drugs showed values of DDD/1000/day equal to zero in all countries; 18 were no longer available (old drugs) or not yet marketed in 1998, and the others had negligible use (<0.05 DDD/1000/day). Of the remaining 71 drugs with a use ≥0.1 DDD/1000/day, 33 compounds had sales data ≥1 DDD/1000/day in at least one country (Table 1). The most used drugs were salbutamol, glibenclamide, verapamil, diltiazem and citalopram (means among all countries: 13.9, 6.0, 4.6, 4.4 and 4.1 DDD/1000/day, respectively), with wide variations among countries. Salbutamol reached 31.6 DDD/1000/day in England and 29.1 in Australia, but was much lower in the other countries (4.5–11 DDD/1000/day); similarly, citalopram use was highest in Sweden and in Denmark (13.8 and 10.8 DDD/1000/day, respectively).
Table 1.
Non-antiarrhythmic, QT-prolonging drugs supplied in 1998 through community pharmacies (DDD/1000/day)*
| Criteria | Countries | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs | Ia | Ib | IIa | IIb | IIIa | IIIb | Australia | Denmark | England | Germany | Greece | Italy | Sweden |
| Gastrointestinal prokinetics | |||||||||||||
| Cisapride | • | • | • | • | • | 3.1 | 0.6 | 0.6 | 0.7 | 2.8 | 3.2 | 1.5 | |
| Domperidone | • | • | • | 0.2 | – | 0.6 | 0.1 | 0.5 | 2.0 | – | |||
| Cardiovascular drugs** | |||||||||||||
| Diltiazem | • | • | 5.3 | 4.3 | 4.5 | 2.0 | 7.2 | 3.9 | 3.3 | ||||
| Indapamide | • | • | • | 9.7 | 0.4 | 1.1 | 0.7 | 7.7 | 3.3 | – | |||
| Isradipine | • | • | – | 0.7 | <0.05 | 0.9 | 0.9 | 0.4 | 1.6 | ||||
| Mibefradil | • | • | • | • | 0.1 | 0.1 | <0.05 | 0.4 | <0.05 | – | – | ||
| Perhexiline | • | • | 0.2 | – | – | – | – | – | – | ||||
| Verapamil | • | • | 7.7 | 4.7 | 1.8 | 9.0 | 1.5 | 4.4 | 3.4 | ||||
| Antibacterials | |||||||||||||
| Clarithromycin | • | • | • | • | • | <0.05 | 0.2 | 0.4 | 0.7 | 2.6 | 2.3 | 0.2 | |
| Clindamycin | • | <0.05 | – | <0.05 | 0.2 | 0.3 | 0.1 | 0.3 | |||||
| Erythromycin | • | • | • | • | • | 1.8 | 1.2 | 1.9 | 1.2 | 0.7 | 0.2 | 0.6 | |
| Grepafloxacin | • | • | • | • | • | – | – | – | 0.1 | – | – | – | |
| Levofloxacin | • | – | – | – | 0.1 | – | – | – | |||||
| Roxithromycin | • | • | 1.8 | 0.4 | – | 0.7 | 1.4 | 0.5 | <0.05 | ||||
| Spiramycin | • | – | – | – | <0.05 | 0.1 | 0.3 | – | |||||
| Sulfamethoxazole and trimethoprim | • | • | • | 0.7 | – | <0.05 | 1.1 | 0.9 | 1.1 | 0.2 | |||
| Antimycotics | |||||||||||||
| Fluconazole | • | <0.05 | 0.1 | <0.05 | 0.1 | 0.1 | 0.2 | 0.1 | |||||
| Ketoconazole | • | • | • | 0.2 | – | <0.05 | <0.05 | 0.3 | 0.1 | <0.05 | |||
| Opioids | |||||||||||||
| Fentanyl | • | • | – | 0.9 | 0.3 | 0.3 | 0.1 | <0.05 | 0.4 | ||||
| Methadone | • | 0.9 | 2.0 | 1.2 | 1.2 | – | <0.05 | – | |||||
| Antimigraine agents | |||||||||||||
| Naratriptan | • | – | – | 0.1 | <0.05 | – | – | <0.05 | |||||
| Sumatriptan | • | 0.1 | 0.7 | 0.2 | 0.1 | 0.2 | 0.1 | 1.4 | |||||
| Zolmitriptan | • | <0.05 | 0.2 | <0.05 | <0.05 | – | – | <0.05 | |||||
| Antipsychotics | |||||||||||||
| Amisulpride | • | • | – | – | <0.05 | – | – | 1.5 | – | ||||
| Chlorpromazine | • | • | • | • | • | • | 0.3 | 0.1 | 0.4 | <0.05 | 0.8 | 0.1 | 0.1 |
| Clozapine | • | • | • | 0.3 | 0.4 | <0.05 | 0.4 | 0.4 | 0.1 | 0.5 | |||
| Haloperidol | • | • | • | • | • | • | 0.7 | 0.5 | 0.6 | 1.2 | 5.6 | 0.9 | 1.0 |
| Olanzapine | • | • | • | 0.7 | 0.6 | 0.3 | 0.2 | – | – | 0.5 | |||
| Pimozide | • | • | • | • | • | <0.05 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | |
| Prochlorperazine | • | • | • | 0.2 | 0.1 | 0.3 | – | – | – | <0.05 | |||
| Risperidone | • | • | 0.5 | 0.2 | 0.3 | 0.2 | 1.0 | 0.3 | 0.6 | ||||
| Sertindole | • | • | • | • | – | – | <0.05 | <0.05 | 0.1 | – | – | ||
| Thioridazine | • | • | • | • | • | • | 0.6 | 0.1 | 0.5 | 0.2 | 0.7 | 0.3 | 0.6 |
| Trifluoperazine | • | • | 0.3 | – | 0.4 | 0.05 | 0.3 | – | – | ||||
| Zotepine | • | – | – | – | 0.2 | – | – | – | |||||
| Antidepressants | |||||||||||||
| Amitriptyline | • | • | • | • | • | 2.8 | 1.8 | 3.9 | 2.8 | 1.6 | 1.3 | 1.9 | |
| Citalopram | • | • | 0.8 | 10.8 | 0.9 | 0.5 | 1.3 | 0.5 | 13.8 | ||||
| Clomipramine | • | • | • | 0.4 | 0.7 | 0.6 | 0.4 | 1.0 | 0.9 | 1.4 | |||
| Desipramine | • | • | 0.1 | – | <0.05 | <0.05 | – | <0.05 | – | ||||
| Doxepin | • | • | • | • | 1.7 | 0.3 | 0.2 | 1.9 | 0.1 | – | – | ||
| Fluoxetine | • | • | 4.0 | 3.0 | 6.5 | 0.6 | 1.8 | 1.1 | 2.7 | ||||
| Imipramine | • | • | • | • | 0.7 | 0.3 | 0.4 | 0.2 | – | – | 0.1 | ||
| Mianserin | • | • | • | 0.4 | 0.7 | 0.1 | 0.2 | 0.6 | 0.4 | 0.6 | |||
| Nortriptyline | • | • | • | 0.2 | 1.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | |||
| Trazodone | • | • | – | – | 0.25 | 0.1 | <0.05 | 0.4 | – | ||||
| Venlafaxine | • | 1.9 | 1.0 | 1.0 | 0.2 | 1.2 | 0.2 | 1.7 | |||||
| Antimalarials | |||||||||||||
| Chloroquine | • | • | • | 0.1 | 0.3 | <0.05 | 0.2 | – | 0.1 | 0.5 | |||
| Quinine | • | 1.0 | 0.5 | 0.7 | 0.6 | – | – | 0.2 | |||||
| Antiasthmatics | |||||||||||||
| Fenoterol | • | • | <0.05 | 0.8 | – | 0.8 | 0.2 | 1.2 | 0.4 | ||||
| Procaterol | • | – | – | – | – | – | 0.1 | – | |||||
| Salbutamol (albuterol) | • | • | 29.1 | 9.1 | 31.6 | 5.4 | 11.0 | 4.5 | 6.3 | ||||
| Salmeterol | • | • | 1.7 | 4.2 | 3.7 | 1.3 | 2.0 | 2.9 | 5.3 | ||||
| Antihistamines | |||||||||||||
| Astemizole | • | • | • | • | • | 0.1 | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 | – | |
| Cetirizine | • | 0.2 | 3.5 | 2.5 | 2.7 | 4.9 | 3.7 | 5.1 | |||||
| Chlorpheniramine | • | • | – | – | <0.05 | – | – | 0.1 | – | ||||
| Clemastine | • | • | – | – | <0.05 | 0.2 | – | <0.05 | 0.9 | ||||
| Cyproheptadine | • | 0.5 | 0.1 | <0.05 | – | – | 0.1 | <0.05 | |||||
| Diphenhydramine/dimenhydrinate | • | • | • | • | <0.05 | – | <0.05 | 0.1 | 0.4 | – | 0.1 | ||
| Ebastine | • | • | • | – | 0.3 | – | – | <0.05 | – | 0.8 | |||
| Fexofenadine | • | 0.3 | 0.4 | 0.5 | 0.6 | – | – | 0.5 | |||||
| Loratadine | • | • | 0.5 | 3.6 | 3.3 | 2.0 | 2.8 | 2.0 | 9.3 | ||||
| Mizolastine | • | • | – | – | <0.05 | 0.4 | 0.2 | – | – | ||||
| Oxatomide | • | – | – | – | – | 0.1 | 0.8 | – | |||||
| Promethazine | • | • | 0.8 | 1.2 | 0.3 | 1.2 | 0.3 | 0.2 | 1.3 | ||||
| Terfenadine | • | • | • | • | • | 0.1 | 0.8 | 0.8 | 0.4 | – | – | 0.5 | |
| Miscellaneous | |||||||||||||
| Famotidine | • | 6.1 | – | 0.1 | 0.5 | 1.2 | 0.4 | 1.2 | |||||
| Glibenclamide | • | • | 4.1 | 3.4 | 2.2 | 13.2 | 8.5 | 3.3 | 7.3 | ||||
| Hydroxyzine | • | • | • | <0.05 | 0.1 | 0.2 | 0.1 | 1.3 | 0.1 | 0.9 | |||
| Sildenafil | • | • | – | 0.1 | <0.05 | – | – | – | 0.2 | ||||
| Tacrolimus | • | • | • | • | <0.05 | – | <0.05 | 0.1 | – | – | 0.1 | ||
| Tamoxifen | • | • | 1.7 | 0.3 | 2.6 | 1.9 | 1.9 | 1.8 | 1.5 | ||||
Thirty-one compounds met at least one criterion, but their DDD values were equal to zero: antiemetics (dolasetron, granisetron, ondansetron), cardiovascular drugs (bepridil, indoramin, ketanserin, lidoflazine, methoxamine, prenylamine, probucol, triamterene), antimicrobics (gatifloxacin, moxifloxacin, sparfloxacin), opioids (levacetylmethadol), antiepileptics (felbamate, fosphenitoine), antipsychotics (droperidol, mesoridazine, quetiapine, sultopride) antidepressants (protriptyline, zimeldine), antimalarials (halofantrine, mefloquine), antihistamines (emedastine, epinastine), miscellanea (dexfenfluramine, pentamidine, ritanserin, terodiline).
The table excludes only those drugs having the main indication as antiarrhythmics. Verapamil and diltiazem are indeed used as antiarrhythmics, but this is not their main indication.
Table 2.
Overall sales of drugs grouped according to the strength of evidence on QT prolongation or occurrence of ventricular arrhythmias associated with QT prolongation.
When drugs with sales data ≥0.1 DDD/1000/day were grouped according to the strength of evidence on their proarrhythmic risk, overall use in each country ranged from 51.9 to 94.7 DDD/1000/day for group A, from 51.6 to 92.7 DDD/1000/day for group B, from 37.1 to 76.6 DDD/1000/day for group C, from 12.9 to 29.1 DDD/1000/day for group D and from 5.8 to 15.3 DDD/1000/day for group E (Table 2). Group D included 37 drugs: 12 of these (cisapride, domperidone, indapamide, sulfamethoxazole-trimethoprim, erythromycin, clarithromycin, haloperidol, clomipramine, amitriptyline, doxepin, fluoxetine, promethazine) had a use >1 DDD/1000/day in at least one country (Table 1). Group E included 14 drugs: 5 of these (cisapride, clarithromycin, erythromycin, haloperidol, amitriptyline) had a use >1 DDD/1000/day in at least one country (Table 1). Overall sales of the 39 drugs with official warnings on QT prolongation (criterion IIIa) were (DDD/1000/day): 14.7 (Australia), 13.7 (Denmark), 15.3 (England), 15.4 (Germany), 20.2 (Greece), 14.9 (Italy), 16.4 (Sweden).
When DDD/1000/day values for group D and group E were expressed as a percentage of total DDD/1000/day dispensed through community pharmacies, the following values were obtained, respectively: Australia 3.8 and 1.3%, Denmark 1.5 and 0.7%, Germany 1.4 and 0.7%, Greece 3.6 and 1.9%, Italy 1.9 and 0.9%, Sweden 1.3 and 0.6%. In England, overall sales of group D and E represented 3.7 and 1.8% of total prescription items, respectively.
Discussion
The long and growing list of nonantiarrhythmic drugs capable of affecting the QT interval has raised concern on the number of agents that may actually precipitate TdP, especially in case of drug interactions or concomitant risk factors (e.g. hypokalaemia, low heart rate, etc.). This is the first study attempting to quantify overall use of QT prolonging drugs in several countries by implementing a structured list derived from a systematic review [6] of the literature.
In spite of obvious differences in sales of individual agents because of inherent differences in prescription patterns and prevalence of diseases (for instance, use of antidepressants in Sweden and Australia is reported to be higher than in other countries [8]; likewise, the extent of use of antiasthmatics is known to be relatively high in England and Australia [9]), our data indicate that the overall use of nonantiarrhythmic drugs grouped according to the strength of evidence on QT-related proarrhythmic effects is of the same order of magnitude in all countries.
The clinical significance of the present study should be evaluated in the light of some intrinsic limitations of our approach. First, sales data through community pharmacies are an approximate estimation of actual exposures in the community (problems of patient compliance; some databases, namely England and Germany, excluded nonreimbursed medications). Moreover, only when a drug is taken at a dose of 1 DDD per day, can our estimate be considered as a proxy for prevalence of drug use (therapeutic intensity). Second, the strength of evidence on the proarrhythmic potential is based on a systematic review of the literature, which is a possible source of bias. For a few compounds, the published evidence of TdP consists only of isolated case reports (complete safety data on occurrence of TdP are usually disclosed only to regulatory agencies). Moreover, QT prolongation is a surrogate marker of cardiotoxicity and there is no consensus on the degree of QT prolongation that becomes clinically significant [10], since several risk factors for the occurrence of TdP must be considered [3, 11].
Since none of the six criteria is per se sufficient to determine the clinical importance of the arrhythmogenic potential of a given compound, we have tried to subdivide drugs into different categories, from A to E, in increasing order of clinical relevance. Criteria for group A and B are not strict enough to generate clinical concern and considering their DDD values would lead to an overestimation of the magnitude of the problem, whereas categories D and E are more clinically relevant. For instance, the widely used calcium channel blocker verapamil (the prototype of drugs known to block HERG K+ channels, but not associated with TdP) belongs to category A and B, but is excluded from other categories.
Should one worry about the high sales data of group C drugs? At the present state of knowledge, it is difficult to answer this question. On one hand, the fact that QT prolongation (criterion Ib) is a surrogate marker of toxicity (hence, not necessarily associated with a clinically significant risk of TdP) is reassuring; on the other hand, our ignorance of the true incidence of TdP with some drugs is a matter of concern and suggests that measures should be taken to implement active surveillance.
Two notable examples of group C drugs with debatable clinical evidence are glibenclamide and salbutamol. Glibenclamide was reported to prolong the QT interval [12] (although this study had some methodological limitations) and to inhibit HERG K+ channels in vitro at high concentrations [13]. However, in type 2 diabetic patients, a recent retrospective study [14] found that in-hospital mortality after myocardial infarction was not affected by glibenclamide. Salbutamol, which, respectively, represents 40 and 30% of the overall sales of QT prolonging drugs in England and Australia, is reported to prolong the QT interval, but has no recognized risk of occurrence of TdP. Notably, glibenclamide and salbutamol belong to category C, but are excluded from the two remaining categories, D and E, which we regard as the most clinically important for the proarrhythmic risk.
Concerning group D, the availability of clinical data documenting TdP or ventricular arrhythmias associated with QT prolongation is certainly a strong signal, except in those instances (6 out 37 drugs, namely fexofenadine, fluoxetine, clindamycin, levofloxacin, spiramycin, and fluconazole) where only a few, sometimes questionable case reports exist. These case reports simply generate a signal that needs to be validated and may still be insufficient to generate true clinical concern. A typical example of a drug with a single questionable case report is fexofenadine [15–17]. Concerning fluoxetine, its proarrhythmic potential is at present controversial, although published data now exist on its ability to block HERG K+ channels [18].
In order to identify drugs with the strongest proarrhythmic potential within category D, we also considered group E (drugs having also official warnings on QT prolongation), the category with strongest evidence. The most widely used drugs within group E were cisapride, clarithromycin, erythromycin, haloperidol, amitryptiline.
The fact that compounds belonging to the same therapeutic class widely differ as to proarrhythmic risk [2, 6] demands a careful risk/benefit assessment for individual agents. Antihistamines are perhaps the most extensively studied therapeutic class [19]. Interestingly, one of these compounds, ebastine, was reported to prolong the QT interval but the clinical significance of this finding is debated because the effect depends on the formula used to correct the QT interval for heart rate [20, 21].
We think that identifying those drugs with proarrhythmic potential and high exposures in the general population is an important step to target future pharmacoepidemiological studies aimed at quantifying the proarrhythmic risk. The main limitation of these studies is that rare events (such as drug-induced TdPs) are usually investigated through spontaneous reporting systems or case-control studies, which do not allow to estimate their true incidence. In addition, even with drugs in which the association with QT interval prolongation and arrhythmias has been demonstrated (such as terfenadine), previous epidemiological studies have found it difficult to identify an increased risk of arrhythmias other than in cases of metabolic drug interaction leading to enhanced plasma levels [22, 23].
In conclusion, the present study reported utilization data of QT prolonging drugs grouped according to a structured list and found that the overall extent of use of these compounds was of the same order of magnitude in all countries considered. The significant use of drugs belonging within categories D and E should prompt careful risk/benefit assessment especially of compounds with high exposures.
Acknowledgments
The authors thank Dr Kerstin Burman, Dr Maria Juhasz (the National Corporation of Swedish Pharmacies) and Dr Helen Kendall (Prescription Pricing Authority, Newcastle upon Tyne) for help in retrieving the data.
References
- 1.Woosley RL, Sale M. QT interval. A measure of drug action. Am J Cardiol. 1993;72:36B–43B. doi: 10.1016/0002-9149(93)90039-f. [DOI] [PubMed] [Google Scholar]
- 2.De Abajo FJ, Rodriguez LA. Risk of ventricular arrhythmias associated with nonsedating antihistamine drugs. Br J Clin Pharmacol. 1999;47:307–313. doi: 10.1046/j.1365-2125.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Ponti F, Poluzzi E, Montanaro N. QT-interval prolongation by non-cardiac drugs: lessons to be learned from recent experience. Eur J Clin Pharmacol. 2000;56:1–18. doi: 10.1007/s002280050714. [DOI] [PubMed] [Google Scholar]
- 4.Tamargo J. Drug-induced torsade de pointes: from molecular biology to bedside. Jpn J Pharmacol. 2000;83:1–19. doi: 10.1254/jjp.83.1. [DOI] [PubMed] [Google Scholar]
- 5.Sheridan DJ. Drug-induced proarrhythmic effects. Assessment of changes in QT interval. Br J Clin Pharmacol. 2000;50:297–302. doi: 10.1046/j.1365-2125.2000.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Ponti F, Poluzzi E, Montanaro N. Organising evidence on QT prolongation and occurrence of Torsades de Pointes with non-antiarrhythmic drugs: a call for consensus. Eur J Clin Pharmacol. 2001;57:185–209. doi: 10.1007/s002280100290. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Collaborating Centre for Drug Statistics Methodology (Norway, 1999 ATC index with DDDs, Oslo, Norway): the WHO Collaborating Centre.
- 8.McManus P, Mant A, Mitchell PB, Montgomery WS, Marley J, Auland ME. Recent trends in the use of antidepressant drugs in Australia, 1990–1998. Med J Aust. 2000;173:458–461. doi: 10.5694/j.1326-5377.2000.tb139294.x. [DOI] [PubMed] [Google Scholar]
- 9.Janson C, Chinn S, Jarvis D, Burney P. Physician-diagnosed asthma and drug utilization in the European Community Respiratory Health Survey. Eur Respir J. 1997;10:1795–1802. doi: 10.1183/09031936.97.10081795. [DOI] [PubMed] [Google Scholar]
- 10.Malik M, Camm AJ. Evaluation of drug-induced QT interval prolongation: implications for drug approval and labelling. Drug Saf. 2001;24:323–351. doi: 10.2165/00002018-200124050-00001. [DOI] [PubMed] [Google Scholar]
- 11.European Agency for the Evaluation of Medicinal Products (EMEA) Committee for Proprietary Medicinal Products (CPMP): Points to consider: the assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products 1997.
- 12.Ikeda T. QT prolongation in type 2 diabetes mellitus treated with glibenclamide. Diabetes Metab. 1994;20:565–567. [PubMed] [Google Scholar]
- 13.Rosati B, Rocchetti M, Zaza A, Wanke E. Sulfonylureas blockade of neural and cardiac HERG channels. FEBS Lett. 1998;440:125–130. doi: 10.1016/s0014-5793(98)01444-6. [DOI] [PubMed] [Google Scholar]
- 14.Klamann A, Sarfert P, Launhardt V, Schulte G, Schmiegel WH, Nauck MA. Myocardial infarction in diabetic vs non-diabetic subjects. Survival and infarct size following therapy with sulfonylureas (glibenclamide) Eur Heart J. 2000;21:220–229. doi: 10.1053/euhj.1999.1999. [DOI] [PubMed] [Google Scholar]
- 15.Pinto YM, van Gelder IC, Heeringa M, Crijns HJGM. QT lengthening and life-threatening arrhytmias associated with fexofenadine. Lancet. 1999;353:980. doi: 10.1016/s0140-6736(99)01009-0. [DOI] [PubMed] [Google Scholar]
- 16.Dhar S, Hazra PK, Malakar S, Mistri G. Fexofenadine-induced QT prolongation: a myth or fact? Br J Dermatol. 2000;142:1260–1261. doi: 10.1046/j.1365-2133.2000.03576.x. [DOI] [PubMed] [Google Scholar]
- 17.Craig-McFeely PM, Freemantle SL, Pearce GL, Shakir SA. QT lengthening and life-threatening arrhythmias associated with fexofenadine. Br J General Pract. 2000;50:148. [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas D, Gut B, Wendt-Nordahl G, Kiehn J. The antidepressant drug fluoxetine is an inhibitor of human ether-a-go-go-related gene (HERG) potassium channels. J Pharmacol Exp Ther. 2002;300:543–548. doi: 10.1124/jpet.300.2.543. [DOI] [PubMed] [Google Scholar]
- 19.Taglialatela M, Timmerman H, Annunziato L. Cardiotoxic potential and CNS effects of first-generation antihistamines. Trends Pharmacol Sci. 2000;21:52–56. doi: 10.1016/s0165-6147(99)01437-6. [DOI] [PubMed] [Google Scholar]
- 20.Gillen MS, Miller B, Chaikin P, Morganroth J. Effects of supratherapeutic doses of ebastine and terfenadine on the QT interval. Br J Clin Pharmacol. 2001;52:201–204. doi: 10.1046/j.0306-5251.2001.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411–420. doi: 10.1046/j.1540-8167.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 22.Pratt CM, Hertz RP, Ellis BE, Crowell SP, Louv W, Moye L. Risk of developing life-threatening ventricular arrhythmia associated with tefenadine in comparison with over-the-counter antihistamines, ibuprofen and clemastine. Am J Cardiol. 1994;73:346–352. doi: 10.1016/0002-9149(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 23.Hanrahan JP, Choo PW, Carlson W, Greineder D, Faich GA, Platt R. Terfenadine-associated ventricular arrhythmias and QTc interval prolongation. A retrospective cohort comparison with other antihistamines among members of a health maintenance organization. Ann Epidemiol. 1995;5:201–209. doi: 10.1016/1047-2797(94)00039-v. [DOI] [PubMed] [Google Scholar]