Abstract
Aims
To evaluate the potential pharmacokinetic interaction between the HIV protease inhibitor saquinavir and rifabutin.
Methods
Fourteen HIV-infected patients provided full steady-state pharmacokinetic profiles following administration of rifabutin alone (300 mg once daily) or saquinavir soft-gel formulation (1200 mg three times daily) plus rifabutin (300 mg once daily) in this open label, partially randomized study.
Results
Coadministration of saquinavir and rifabutin resulted in a reduction in saquinavir AUC(0,8 h) and Cmax(0,8 h) of 47% (95% CI 30, 60%) and 39% (95% CI 11, 59%), respectively. Rifabutin AUC(0,24 h) and Cmax(0,24 h) was increased by an average of 44% (95% CI 17, 78%) and 45% (95% CI 14, 85%), respectively. Saquinavir in combination with rifabutin was well tolerated. Gastrointestinal intolerance and asymptomatic increases in liver enzymes were the only adverse events of note.
Conclusions
Administration of rifabutin with saquinavir may decrease the efficacy of this HIV protease inhibitor.
Keywords: CYP3A, HIV, interaction, pharmacokinetics, rifabutin, saquinavir
Introduction
Disseminated infection with Mycobacterium avium complex (MAC) is a well-recognized opportunistic infection in HIV-positive individuals. The incidence of MAC infection increases as the CD4 cell count drops below 50 cells ml−1 [1]. Primary prophylaxis for MAC using either a macrolide antibiotic (azithromycin, clarithromycin) or rifabutin continues to be widely used although there are increasing data supporting the discontinuation of prophylaxis in persons whose CD4 cell count rises with antiretroviral therapy [2–5]. However, current guidelines recommend that individuals treated for disseminated MAC disease should continue full therapeutic doses of anti-MAC agents for life [3], generally with at least two drugs. Rifabutin is a moderate inducer of cytochrome P450 3A (CYP3A) [6] and is itself primarily metabolized by the same isozyme [7]. Furthermore, since all of the approved HIV protease inhibitors (saquinavir [SQV-SGC], ritonavir, indinavir, nelfinavir, amprenavir and lopinavir [coformulated with ritonavir]) are substrates and inhibitors of CYP3A it is not surprising that significant pharmacokinetic interactions have been reported with the coadministration of rifabutin and protease inhibitors. Several reports have suggested that in addition to inducing drug metabolizing enzymes such as CYP3A the related antituberculosis drug, rifampicin, induces the expression of P-glycoprotein (pgp) [8, 9]. This efflux pump has been implicated in decreasing oral bioavailability or modulating clearance of a number of drugs including protease inhibitors [10]. Considering the similarities between rifampicin and rifabutin it cannot be excluded that part of the effect of rifabutin on protease inhibitors is mediated through an effect of rifabutin on expression of pgp.
Ritonavir (500 mg twice daily), which is a potent inhibitor of CYP3A, increases the area under the concentration-time curve (AUC) of rifabutin (150 mg once daily) by 4-fold. When indinavir (800 mg three times daily) is coadministered with rifabutin (300 mg once daily) indinavir exposure is reduced by 32% and rifabutin exposure increased 204%. When nelfinavir (750 mg three times daily) is coadministered with rifabutin (300 mg once daily), nelfinavir exposure is reduced 32% and rifabutin exposure increased 207%. The dose of rifabutin is recommended to be reduced to 150 mg once daily when given with both indinavir and nelfinavir [11, 12]. Co-administration of rifabutin and protease inhibitors leads to an increased risk of both suboptimal protease inhibitor exposure hence reduced antiviral effect, and higher rifabutin exposure with an associated risk of uveitis. Indeed there have been published case reports of uveitis in patients receiving rifabutin and the protease inhibitors indinavir and ritonavir and with the combination of ritonavir and saquinavir [13].
The objective of the present investigation was to study the tolerability and pharmacokinetics of SQV-SGC at steady state when dosed alone and in combination with rifabutin. The study also evaluated the effect of SQV-SGC on the pharmacokinetics of rifabutin.
This study was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, California, September 1999 (abstract 339).
Methods
Study design
The study was open label and partially randomized. Each patient received three treatment regimens in a three-way crossover. Stopping and starting antiretroviral therapy in HIV infected patients may represent a small increased risk of developing drug resistant strains of HIV. Consequently in order to prevent this risk all patients received treatment A first (rifabutin 300 mg once daily for 10 days) followed, after a 14 day washout, then in a randomized fashion, treatment B (SQV-SGC 1200 mg three times daily for 10 days) and treatment C (SQV-SGC 1200 mg three times daily plus rifabutin 300 mg once daily for 10 days). Patients continued to receive SQV-SGC 1200 mg three times daily between the second and third treatment periods. The use of other agents known to be metabolized by cytochromes P450, including other protease inhibitors and non-nucleoside reverse transcriptase inhibitors, was excluded during the study.
Ethics
The study was performed in accordance with the Declaration of Helsinki and its amendments, and the protocol approved by the Riverside Research Ethics Committee (UK). Written informed consent was obtained from all subjects.
Sample size
Prior to the start of the study a sample size calculation was made based on the intrasubject coefficient of variation for AUC for both saquinavir (59%) and rifabutin (36%) derived from previous studies (Roche, data on file). Assuming the same variability as in the previous studies, a sample size of 15 would ensure that the 95% confidence limits for the ratio of AUC of saquinavir would be included within the limits 50–200% of the true ratio (100%) with a probability of about 0.80 at the 5% level of significance. For rifabutin a sample size of 12 would be sufficient. It was intended that 15 subjects would be enrolled. By the close of the study 16 subjects were enrolled with 14 completing all evaluations.
Bioanalysis
At the end of each treatment period, a drug plasma concentration-time profile was obtained for interaction testing. Concentrations of rifabutin were measured using a sensitive r.i.a. assay (BAS Analytics, data on file). Within 20 successful sample batches the mean interassay precision (CV) for the standards was ±4.9% (n = 305) and for the QC samples ±9.5% (n = 158). The lower limit of quantification was 1.0 ng ml−1 which was measured with an interassay accuracy of 100.0% and a precision of 4.3% (n = 39).
Saquinavir was measured using a sensitive HPLC assay [18]. Within 10 successful sample batches (n = 136), the interday precision for the standards varied from ±1.9% (499 ng ml−1 standard) to ±10.6% (7.49 ng ml−1 standard). The lower limit of quantification was 4.99 ng ml−1 which was measured with an interassay accuracy of 100.1% and a precision of 9.5% (n = 20). The interday precision for nondiluted QC samples (n = 59) ranged from ±4.4% to ±8.1%. The accuracy of the mean was 98.9% for the high concentration (402 ng ml−1) QC sample, 100.7% for the middle (80.4 ng ml−1) QC sample and 94.3% for the low concentration (10 ng ml−1) QC sample.
Safety monitoring
Adverse events were documented throughout the study. All 16 patients randomized to the protocol were evaluable for safety reporting. Fourteen patients were eligible for inclusion in the pharmacokinetic analyses.
Pharmacokinetic and statistical analysis
The primary pharmacokinetic parameters were the AUC(0,8 h) (up to 8 h following the dose) and Cmax(0,8 h) (up to 8 h following the dose) of saquinavir and the AUC(0,24 h) and Cmax(0,24 h) for rifabutin. For both drugs the relative bioavailability of the combined treatments to treatment with the single agent were estimated and the 95% confidence limits were calculated using contrasts from the anova on the log transformed variable.
The primary statistical analysis to estimate interaction effects of saquinavir was the main effect anova model with factors patient, period and treatment applied to the logarithmically transformed AUC(0,8 h) and Cmax(0,8 h) of saquinavir (sequence was initially included in the model then excluded after it was shown not to be significant). The relative bioavailability of the AUC(0,8 h) and of the Cmax(0,8 h) for saquinavir of the combined saquinavir plus rifabutin treatments (Treatment C) to treatment with saquinavir alone (Treatment B) were estimated and 95% confidence limits were calculated using contrasts from anovas on the logtransformed variable. The least squares geometric mean kinetic parameters of Treatment B (reference for saquinavir) were compared with the least square geometric mean kinetic parameters for Treatment C.
The primary statistical analysis to estimate interaction effects of rifabutin was the main effect anova model with factors patient and treatment applied to the logarithmically transformed AUC(0,24 h) and Cmax(0,24 h) of rifabutin. The relative bioavailability of the AUC(0,24 h) and of the Cmax(0,24 h) for rifabutin of the combined rifabutin plus saquinavir treatments (Treatment C) to treatment with rifabutin alone (Treatment A) were estimated and 95% confidence limits were calculated using contrasts from anovas on the logtransformed variable. The least squares geometric mean kinetic parameters of Treatment A (reference for rifabutin) were compared with the least square geometric mean kinetic parameters for Treatment C.
Model-independent pharmacokinetic parameters for saquinavir and rifabutin were calculated from the plasma concentration-time profiles. Area under the concentration-time curve was estimated using the linear trapezoidal rule. The software used to calculate the pharmacokinetic parameters was WinNonlin Professiona (Pharsight Corporation).
Results
Demographics
All of the randomized patients were male (15 Caucasian, 1 black) with a mean (range) age of 33.8 years (25–54 years) weight of 71.4 kg (58–86 kg) and height 175.9 cm (165–190 cm). Patients received dual nucleoside analogue therapy throughout excluding ddI. All patients had CD4 cell counts >200/mm3 and had no active opportunistic disease or gastrointestinal dysfunction.
Safety
There were no serious adverse events reported in this study. Two patients were prematurely withdrawn because of adverse events. These two patients had a number of adverse events on different days of the study. The first patient reported headache, pyrexia, nausea and vomiting while on treatment. The second patient reported abdominal pain, arthralgia and night sweats while on treatment.
A total of 70 adverse events were reported by the 16 patients, with a higher incidence in treatment C. Of these, 53 were judged by the investigator to be related to treatment (49 possibly, 3 probably and 1 remotely). All adverse events resolved without sequelae or were considered by the investigator to be manifestations of the underlying disease (lymphadenopathy and lethargy). The most commonly reported adverse events during all treatments were headache (8 occurrences for 6 patients), diarrhoea (8 occurrences for 6 patients) and nausea (7 occurrences for 6 patients). The majority of adverse events reported were considered to be mild (32 events) or moderate (34 events). Four events were considered to be severe, migraine, transaminase increase and nausea possibly related to treatment and night sweats probably related to treatment.
There were no findings of clinical relevance with regard to blood pressure or pulse rate. Although several laboratory values were outside of the investigator's normal ranges, none of these was considered to be of potential clinical relevance by the investigator.
Saquinavir pharmacokinetic variability
The results indicated that saquinavir pharmacokinetic variability in the present study was generally lower than in the previous studies used to estimate sample sizes. Based on AUC(0,8 h) the observed intersubject variability (%CV) for the saquinavir reference treatment was slightly greater (53%) compared with when coadministered with rifabutin (46%). The within patient variability was approximately 34%. Based on Cmax(0,8 h) the observed intersubject variability (%CV) for the saquinavir reference treatment was slightly lower (58%) compared with when coadministered with rifabutin (60%). The within patient variability was approximately 49%.
Rifabutin pharmacokinetic variability
The results indicated that rifabutin pharmacokinetic variability in the present study was generally lower than in the previous studies used to estimate sample sizes. Based on AUC(0,24 h) the observed intersubject variability (%CV) for the rifabutin reference treatment was somewhat greater (28%) compared with when coadministered with saquinavir (20%). The within patient variability was approximately 25%. Based on Cmax(0,24 h) the observed intersubject variability (%CV) for the rifabutin reference treatment was greater (33%) compared to when coadministered with saquinavir (21%). The within patient variability was approximately 29%.
Effects of rifabutin on saquinavirpharmacokinetics
The mean (%CV) AUC(0,8 h), Cmax and C8 for saquinavir when administered alone were 2447 ng ml−1 h (53.2%), 950 ng ml−1 (57.7%) and 49.9 ng ml−1 (66.6%), respectively. When coadministered with rifabutin the mean (%CV) AUC(0,8 h), Cmax and C8 for saquinavir decreased to 1276 ng ml−1 h (46.1%), 594 ng ml−1 (59.7%) and 35.2 ng ml−1 (71.6%), respectively. No statistically significant period effect was found (P values ≥0.52).
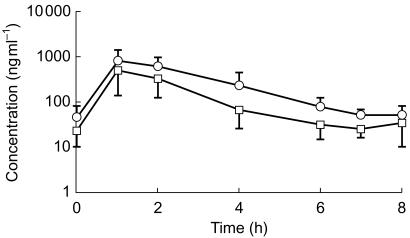
The primary statistical analysis gave an estimate of the reduction in saquinavir exposure when coadministered with rifabutin, as measured by AUC(0,8 h) and Cmax(0,8 h) of 47% (95% CI 30, 60%) and 39% (95% CI 11, 59%), respectively. Figure 1 illustrates the plasma concentration-time profiles for saquinavir when administered alone (Treatment B) and in combination with rifabutin (Treatment C).
Figure 1.
Mean (±s.d.) saquinavir steady state plasma concentration-time profile following administration of SQV-SGC 1200 mg three times daily (Treatment B, ○) or SQV-SGC 1200 mg three times daily plus rifabutin 300 mg once daily (Treatment C, □).
Effect of saquinavir on rifabutin pharmacokinetics
The mean (%CV) AUC(0,24 h), Cmax and C24 for rifabutin when administered alone were 3007 ng ml−1 h (28.0%), 308 ng ml−1 (32.8%) and 46.4 ng ml−1 (26.2%), respectively. When coadministered with saquinavir the mean (%CV) AUC(0,24 h), Cmax and C24 for rifabutin increased to 4337 ng ml−1 h (19.6%), 445 ng ml−1 (20.5%) and 86.6 ng ml−1 (24.7%), respectively.
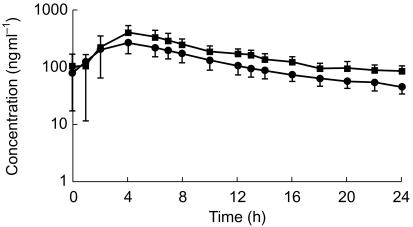
For rifabutin the primary statistical analysis gave an estimate of the increase in rifabutin exposure, when coadministered with SQV-SGC, of 44% (95% CI 17, 78%) and 45% (95% CI 14, 85%) for AUC(0,24 h) and Cmax(0,24 h), respectively. Figure 2 illustrates the plasma concentration-time profiles for rifabutin when administered alone (Treatment A) and in combination with saquinavir (Treatment C).
Figure 2.
Mean (±s.d.) rifabutin steady state plasma concentration-time profile following administration of rifabutin 300 mg once daily (Treatment A, •) or rifabutin 300 mg once daily plus SQV-SGC 1200 mg three times daily (Treatment C, ▪).
Discussion
Data indicate that the extent of the antiviral effect provided by saquinavir is related to plasma concentration of the drug [14]. Additionally, due to variation in CYP activity between individuals, considerable interpatient variability in exposure exists with all protease inhibitors, including saquinavir. Reductions in saquinavir exposure during rifabutin coadministration are likely to influence antiviral activity in at least some individuals. This study therefore indicates that coadministration of saquinavir as the sole protease inhibitor with rifabutin cannot be recommended. Whilst dose adjustment recommendations exist with other PIs when taken with rifabutin, it is unclear if these increases in PI dosage compensate for the drug interaction in all individuals.
Two recent reports have suggested that patients treated with saquinavir and either rifabutin or rifampicin maintain adequate saquinavir exposure when ritonavir was included in the regimen [15, 16]. Therefore, it would seem possible that the potent metabolic inhibitory effects of ritonavir can counteract the metabolic induction mediated by rifabutin and more potently by rifampicin. This may represent a strategy for allowing the coadministration of saquinavir with either rifabutin or rifampicin. However, larger studies are needed to confirm these early findings and make appropriate recommendations on the optimal doses of each agent.
Furthermore, therapeutic monitoring of saquinavir may help to optimize regimes for the coadministration of saquinavir and rifabutin. The success of such an approach relies on establishing a clinically relevant targets for drug exposure. This remains an elusive goal with respect to all antiretrovirals, since effective plasma concentrations may vary depending on the phenotype of the virus, and synergies between antiretrovirals in combination regimens may influence antiviral efficacy. However, based on data from techniques including exposure-response modelling, a trough saquinavir concentration in the region of 50 ng ml−1 appears to be an appropriate target for many antiretroviral or minimally treated patients [14, 17].
Acknowledgments
This study was funded by Roche Products Ltd and carried out independently by the Chelsea and Westminster Hospital, London, UK.
T.G., N.B. and P.S. are employees of Roche.
References
- 1.Horsburgh CR. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 2.Gill J, Moyle G, Nelson M. Discontinuation of Mycobacterium avium complex prophylaxis in patients with a rise in CD4 cell count following highly active antiretroviral therapy. AIDS. 1998;12:680. [PubMed] [Google Scholar]
- 3.USPHS/IDSA. Guidelines for the prevention of opportunistic infections in persons infected with Human Immunodeficiency virus. Clin Infect Dis. 2000. pp. S29–S65. [DOI] [PubMed]
- 4.Dworkin M, Hanson D, Jones J, Kaplan J. Adult/Adolescent Spectrum of HIV Disease Project (ASD). The risk for Pneumocystis carinii pneumonia (PCP) and disseminated non-tuberculous mycobacteriosis (dMb) after an antiretroviral therapy (ART) associated increase in CD4+ T-lymphocyte count [abstract 692] Program and Abstracts of the 6th Conference on Retroviruses and Opportunistic Infections (Chicago) 1999:198. [Google Scholar]
- 5.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 6.Strolin Benedetti M, Dostert P. Induction and autoinduction properties of rifamycin derivatives: a review of animal and human studies. Environ Health Perspect. 1994;102(Suppl 9):101–105. doi: 10.1289/ehp.94102s9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iatsimirskaia E, Tulebaev S, Storozhuk E, et al. Metabolism of rifabutin in human enterocyte and liver microsomes: kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents. Clin Pharmacol Ther. 1997;61:554–562. doi: 10.1016/S0009-9236(97)90135-1. [DOI] [PubMed] [Google Scholar]
- 8.Schuetz EG, Schinkel AH, Relling MV, Schuetz JD. P-glycoprotein: a major determinant of rifampicin-inducible expression of cytochrome P4503A in mice and humans. Proc Natl Acad Sci U S A. 1996;93:4001–4005. doi: 10.1073/pnas.93.9.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147–153. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fromm MF. P-glycoprotein: a defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int J Clin Pharmacol Ther. 2000;38:69–74. doi: 10.5414/cpp38069. [DOI] [PubMed] [Google Scholar]
- 11.Sahai J. Interactions with antiretroviral drugs. AIDS. 1996;10:S21–S25. [PubMed] [Google Scholar]
- 12.Ingrosso A, De Cian W, Narang PK. Update on results from drug-interaction studies with rifabutin [abstract 463] Program and Abstracts of the 6th European Conference on Clinical Aspects and Treatment of HIV-Infection (Hamburg) 1997:76. [Google Scholar]
- 13.Fournier S, Deplus S, Janier M, Poinsignon Y, Decazes JM, Modai J. Anterior uveitis in HIV-infected patients. Three cases in patients treated with an antiprotease. Presse Med. 1998;27:844–848. [PubMed] [Google Scholar]
- 14.Gieschke R, Fotteler B, Buss N, Steimer JL. Relationships between exposure to saquinavir monotherapy and antiviral response in HIV-positive patients. Clin Pharmacokin. 1999;37:75–86. doi: 10.2165/00003088-199937010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Veldkamp AI, Hoetelmans RMW, Beijnen JH, Mulder JW, Meenhorst PL. Ritonavir enables combined therapy with rifampin and saquinavir. Clin Infect Dis. 1999;29:1586. doi: 10.1086/313548. [DOI] [PubMed] [Google Scholar]
- 16.Gallicano K, Khaliq Y, Carignan G, Tseng A, Walmsley S, Cameron DW. A pharmacokinetic study of intermittent rifabutin dosing with a combination of ritonavir and saquinavir in patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 2001;70:149–158. doi: 10.1067/mcp.2001.117612. [DOI] [PubMed] [Google Scholar]
- 17.Hoetelmans RMW, van Heeswijk RPG, Meenhorst PL, Mulder FW, Scheele WA, Beijnen FH. Plasma concentrations of saquinavir (SQV) determine HIV-1 RNA response over a 48-week period [abstract 42261] Program and Abstracts of the 12th World AIDS Conference (Geneva) 1998:825. [Google Scholar]
- 18.Wiltshire HR, Wiltshire BG, Clarke AF, Worth E, Prior KJ, Tjia JF. Chromatographic and immunochemical approaches to the analysis of the HIV protease inhibitor saquinavir in plasma. Anal Biochem. 2000;281:105–114. doi: 10.1006/abio.2000.4545. [DOI] [PubMed] [Google Scholar]