Abstract
Avian song learning involves memorizing and reproducing song material produced by conspecifics. In several species song repertoire size correlates with the overall volume of two song-related brain regions, the HVc (acronym used as the proper name) and the robust nucleus of the archistriatum (RA). We raised male zebra finches with two adult tutors and found that individual differences in HVc volume and neuron number correlated positively with differences in the number of tutor syllables accurately copied. These results were replicated in a second study. The relationship between RA volume and song learning was similar, but less robust. Importantly, total repertoire size (number of song syllables) did not correlate significantly with anatomical measures of either the HVc or RA. Because previous work suggests that the volume and neuron number of these regions are not regulated by song learning, it is possible that naturally occurring variation in neuron number constrains how much song material can be copied or reproduced.
Keywords: birdsong, zebra finch, brain size
Comparative analyses of neuroanatomical organization imply that an expansion of neural network space permits the development of more complex behavior. However, within species there is little evidence that individual differences in learning capacity relate to differences in brain space. Perhaps such relationships are elusive because the neural circuitry involved in learning a specific skill often participates in many different behaviors. However, in oscine songbirds a network of brain regions appears to be dedicated to the learning, production, and perception of song, and there are several indications that the sizes of these regions correlate with the capacity for song learning and production. Song is a male-typical behavior, and across species, sexual dimorphisms in the anatomy of song-related brain nuclei relate to the degree of sex difference in vocal behavior (1–3). Also, the volume of one song region, the HVc, tends to be larger in males of those species with larger song repertoires than in those that produce simpler songs (4–6). This relationship seems not to reflect species differences in perceptual demands because population differences in repertoire size of marsh wrens correlate significantly with the size of the HVc in males (who produce the songs) but not in females (7). Finally, in at least two species individual differences in HVc size correlate with individual differences in song complexity. Male canaries that produce a large number of song syllables have a larger HVc than do those that have a smaller repertoire (8), and a similar relationship is evident within two different populations of marsh wrens (4). Despite these strong brain–behavior relationships, a causal relationship between the size of individual song regions and song learning has not been established.
If such correlations between brain space and song complexity reflect a causal relationship, it could be that HVc growth is influenced by how much song material is learned. Song learning is tightly coupled with the addition, growth, and loss of song-related neurons (9–16), and these cellular events could be affected by the complexity of song material processed and acquired. In fact, such experience-dependent neural growth does occur in the hippocampus of food-storing passerine birds (17). In song learning, however, auditory experience does not appear to regulate the growth of song-control regions. Neuron addition and growth in song nuclei are not attenuated in male zebra finches deafened at an early age to prevent song learning (18). Also, the volume and cellular attributes of song regions are not significantly different between marsh wrens tutored with small repertoires and those tutored with large repertoires, despite large behavioral differences in the number of song types produced (19). Taken together, these findings discredit the view that variation in neuron number or song region volume stem from individual differences in the amount of song material learned.
An alternative view is that individual and species differences in the growth of song-related brain nuclei affect how much song material can be memorized, stored, and/or reproduced. This hypothesis suggests that the number of circuit components places computational constraints on the size of the song repertoire. We tested the relationship between brain space, repertoire size, and song learning in the Australian zebra finch (Peophilla guttata). Zebra finches memorize song material from social tutors between 20–60 days after hatching and begin song-like vocalizations at 35–40 days of age. Auditory feedback is used to match their vocalizations to the song material memorized earlier in life and by 90 days of age, a stereotyped song pattern emerges. We find that in groups of young males raised with a common set of song material, the amount of song material learned, but not the total number of syllables produced, correlates positively and significantly with HVc volume and neuron number.
METHODS
This study was performed as two independent replications. The first study consisted of eight males from five clutches, and the replication involved six males from four clutches. Within each study, the chicks were born within a span of 10 days, isolated from male song at or before eight days posthatch, and raised by their mothers until fledged. In this species only males sing, and song acquisition does not begin until after 20 days of age (20–22). At 30 days of age, the juveniles were moved to a large cage occupied by two adult zebra finch tutors and their mates. The tutors were implanted with testosterone to promote singing. In the first study, one tutor produced a song consisting of nine syllables (including introductory notes), the other tutor’s song consisted of six syllables. The 15 tutor syllables were distinct from one another, and all were potential models because zebra finches can copy song material from more than one adult male (23). In the replication, one tutor from the previous experiment was used (nine-syllable song) along with a second tutor whose song contained seven syllables. Again, all 16 syllables were distinctly identifiable. At 90 days of age, the female siblings were removed from the study. Males remained with their tutors until 120 days of age.
Songs recorded at 90 and 120 days of age in the first study confirmed that song structure had stabilized by the latter age. Songs produced at 120 days of age in the presence of a female were recorded through Fender P2 unidirectional condenser microphones using Marantz PMD201 tape recorders. Sonograms produced by a Kay Elemetrics digital sound spectrograph were scored independently by two observers according to a visual method of comparison used routinely in our laboratory (24–28). Because zebra finch songs contain syllables with complex, frequency-modulated, harmonic structure, we have found that this method generates more reliable results than computer-based autocorrelation programs (see also ref. 29). Individual syllables were defined as acoustic events lasting at least 20 ms and surrounded by intervals of baseline energy at least 10 ms in duration, except where frequency changed abruptly (>1 kHz), in which case the intersyllable interval could be as short as 5 ms. For each pupil, every syllable was compared with the tutor syllable that it most closely resembled, and was scored on a 0–3 scale (0, no resemblance; 1, slightly similar; 2, highly similar; and 3, matched). Introductory notes were included in this analysis as they are frequently learned from adult song models. We did not score learning of tutor call notes because we could not be confident that all call notes were recorded. Learned syllables were defined operationally as those that either matched or were highly similar to (score = 3 or 2) a tutor’s syllable. For each bird the number of learned syllables produced was averaged between the two observers (agreement was >90%). Also for each bird, the total syllable number (repertoire size) and the number of syllables that did not match tutor syllables (score = 0 or 1) were calculated. These measures were used to evaluate the relationship between song learning and repertoire size. Finally, the percentage of available tutor syllables learned was determined for each bird by calculating the number of tutor syllables accurately reproduced (average of two observers) and dividing by the sum of the two tutor’s song syllable repertoires. This value usually reflected accurately the number of learned syllables produced by a pupil. However a few birds produced two different syllables (distinguished from one another based on positioning within the song phrase and subtle differences in morphology) that were each scored as learned from the same tutor syllable. In these cases, the number of learned syllables produced by a pupil was slightly greater than the number of tutor syllables copied. Because the percentage of available material learned seemed a more accurate measure of learning capacity, we used this measure, along with total repertoire size, for comparison to anatomical measures. However, the brain–behavior relationships reported were evident using either behavioral measure.
Between 121–123 days of age, birds were given a lethal injection of equithesin and were transcardially perfused with 0.75% saline followed by 10% formalin in a 0.1 M phosphate buffer (pH = 7.4). The brains were removed, postfixed in formalin, and embedded in paraffin. Serial frontal sections (10 μm) were mounted onto slides and stained with thionin.
All anatomical analyses were done without knowledge of how much song material had been copied by each bird. Because previous studies have failed to detect systematic right–left differences in the volume of forebrain song nuclei (1, 4, 8), we measured only the right hemisphere. This side was chosen because zebra finches exhibit right-side dominance for song production (30). Total nuclear volume and neuron density were measured for the HVc, RA, and area X. For the HVc, drawings excluded the thin dorsomedial strip of cells that extends from the medial margin of caudal HVc. In zebra finches, this region cannot be reliably traced in Nissl-stained material. Histological artifact precluded accurately measuring HVc volume in two birds, and area X volume in one bird in the first study. Based on the results of this first study, analyses in the replication were limited to the HVc and RA. Among this second group of birds, histological artifact precluded accurately measuring RA in two birds. The perimeter of each song region was traced at regular intervals (10–70 μm) throughout its rostrocaudal extent, and an image analysis system (image, National Institutes of Health) was used to calculate the area encompassed by these drawings. Using the formula for a cone frustum (31), area measurements were used in conjunction with the sampling interval to reconstruct the volume of song nuclei. For each song region, neuron density was determined by averaging across fields (81,000 μm3 per field) sampled at regular anterior-posterior intervals. Neurons were identified by their darkly staining cytoplasm, pale nucleus, and distinct nucleolus. Sample fields were chosen at low magnification to avoid biased selection and included rostro-caudal, medial-lateral, and dorsal-lateral locations within each nucleus. Fewer fields were sampled in area X (n = 8–10) than in the HVc (n = 36) or the RA (n = 36) because neuron density in area X was relatively high and homogeneous. This number of sample fields generated within animal standard errors that averaged 3.9% of the mean for HVc neuron density (range = 3.3–5.9%), 6.6% of the mean for RA (range = 5.1–8.1%), and 3.9% of the mean for area X (range = 3.4–6.0%). For each animal, neuron number was calculated by multiplying nuclear volume by average neuron density. We focused on the measure of neuron number because it is likely a more accurate measure of network space, and shrinkage produced by histological processing will effect volume but not final neuron number estimates. The Konigsmark modification of the Abercrombie correction factor (32) was applied to adjust for split nucleoli in the HVc, RA, and area X.
A version of the optical dissector method (33) was also used to estimate HVc and RA neuron number in the first study. In this technique, nucleoli in focus at the surface of the section are considered split and are not included in the density measurement for that field. The two quantitative methods yielded near identical results in the HVc, the optical dissector giving estimates that were higher (2.9% on average) in every animal than those generated by the Abercrombie method. In the RA, however, the optical dissector estimates were significantly lower (17% ± 1.6%; P < 0.001) than the counts generated by the Abercrombie correction. The low cell density characteristic of the RA and the relatively thin sections used in the present study (10 μm) may have artificially lowered neuron number estimates yielded by the optical dissector method. That is, low cell density decreases the accuracy with which the slice surface can be identified, thus leading to overestimates in the number of nucleoli split during sectioning. Because of the agreement between the counting methods in the HVc and our concerns about the effect of cell density in RA, we present the data for the Abercrombie estimates only. However, all the statistically significant brain–behavior relationships reported are also evident if one uses the optical dissector estimates of neuron number.
A software program (gb-stat, Dynamic Microsystems, Silver Spring, MD) was used to perform statistical analysis. Simple regressions were done to calculate the r and P values reported below. P values are for two-tailed tests.
RESULTS
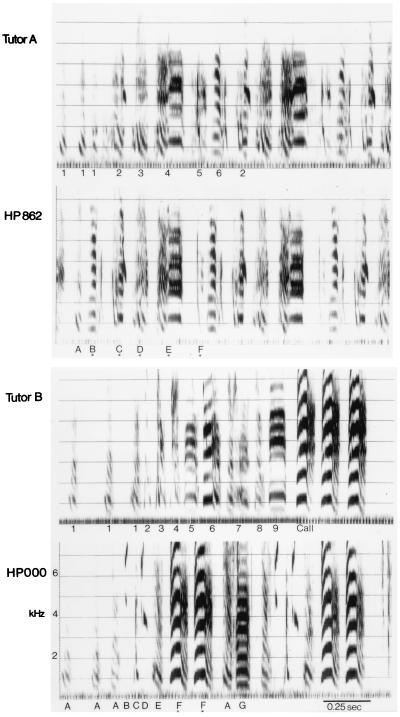
In the initial study each bird copied song material from only one of the two tutors: three accurately reproduced syllables sung by the tutor with a nine-syllable song, and five copied material from the tutor with a six-syllable song. Examples of the tutors’ songs and copies produced by the pupils are shown in Fig. 1. Neither of the tutors’ songs was copied in its entirety, suggesting that learning was not limited by the availability of song material. When the combined total of 15 distinct tutor syllables are considered, the percentage of tutor syllables copied ranged from 3% (0.5 syllables) to 33% (5 syllables). (The percentage of an individual tutor’s song that was learned ranged from 6–86% across the two experiments.) Noninteger values stem from averaging across scorers (see Materials and Methods). Clutchmates did not always copy material from the same tutor, and variability in the amount learned was as great within a clutch as across clutches. Total repertoire size in the pupils ranged from 3 to 8 syllables, with 0.5–5.5 syllables produced scored as learned. In one individual from this group the number of learned syllables produced (5.5 syllables) differed from the number of tutor syllables accurately copied (5 syllables).
Figure 1.
Sonograms of each tutor’s song and two pupils’ songs (HP862 and HP000) demonstrating learning of tutor syllables in the first study. Asterisks indicate syllables scored as learned by one investigator. For HP862, both investigators scored syllables B–F as learned from Tutor A and one also scored syllable A as learned from Tutor A. For HP000, only one experimenter scored syllable F as learned from Tutor B (both experimenters also scored syllable F as a copy of the tutor’s call note, but call notes were not included in the final analysis; see Materials and Methods).
In the replicate experiment all six males copied from the tutor with seven syllables and one also copied material from the tutor producing nine syllables. Again, no bird learned either tutor’s entire song. The percentage of available material learned ranged from 6% to 44% (1–7 syllables out of 16 tutor syllables), and the total repertoire size of birds in this replication ranged from 5 to 10 syllables, with 1–7.5 syllables scored as learned. In three members of this group the number of learned syllables produced (3.5, 4.5, and 7.5 learned syllables) differed slightly from the number of syllables accurately copied (3, 4, and 7 syllables, respectively).
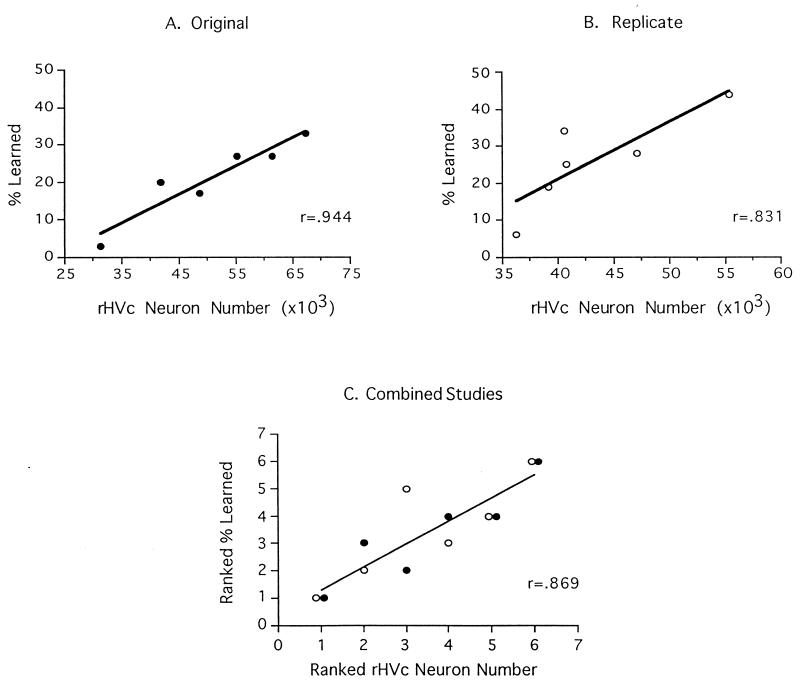
Individual differences in the amount of song material accurately copied from the tutors correlated positively with the anatomy of the HVc. In the first study, birds with more HVc neurons accurately reproduced more tutor song syllables than did birds with fewer HVc neurons (Fig. 2A; r = 0.944, P < 0.01). Similar results were obtained in the replication study (Fig. 2B; r = 0.831, P < 0.05). Also, the percentage of the tutor song material accurately copied was correlated positively with the volume of the HVc. This brain–behavior relationship was statistically significant in the first study (r = 0.897, P = 0.01), but not in the replication (r = 0.769, P = 0.07). Because the absolute amount of song learned may be affected by variables that were not controlled across the two studies (i.e., the amount of tutor song produced, the number of juveniles in each experiment), we believe the data are best interpreted as separate studies. To capture a larger sample size, however, we rank ordered the behavioral and anatomical measures within each study, and then collapsed across the two data sets. As shown in Fig. 2C, the amount learned was significantly correlated with HVc neuron number within this larger data set (r = 0.869, P < 0.001). Within this larger data set, the correlation between learning and the volume of the HVc was also statistically significant (r = 0.810, P = 0.001). Collapsing across the replicates by using raw data rather than ranks yielded similar results for HVc neuron number (r = 0.669, P < 0.05), although the relation between HVc volume and learning was not significant (r = 0.535, P = 0.07).
Figure 2.
Scatterplots showing the percentage of available tutor song material learned and the number of neurons in the right HVc for the original (A) and the replicate study (B). Individual differences in HVc neuron number were large, and significantly correlated with the amount of song material learned. This robust relationship was also evident when the data were converted to ranked scores and combined across studies (C). •, First study; ○, replication.
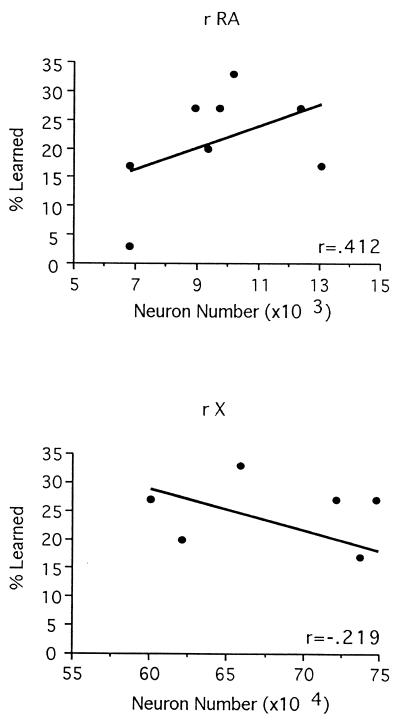
As shown in Fig. 3 (Top), the percentage of available material copied in the initial study was not significantly correlated to either the number of RA neurons (r = 0.412, P = 0.31) or the volume of the RA (r = 0.669, P = 0.07). In the second study, the RA could be measured accurately in only four birds, thus these data were not analyzed as an independent replication. Among these birds, the range of RA neuron number (5,859–6,716 neurons) and volume (0.105–0.152 mm3) was small compared with that observed in the first set of animals (6,808–13,049 neurons, 0.125–0.0207 mm3), even though the proportion of tutor material copied (6–34%) was similar to that in the first study (3–33%). Ranking and collapsing across data sets did not reveal a significant relationship between RA neuron number and song learning, although it did render significant the relationship between RA volume and the percentage of available material copied (r = 0.749, P = 0.005). Because the variance and sample size in these two data sets differed, it is difficult to interpret this collapsed data set confidently. Collapsing the data without ranking each group yields similar results, with only the relationship between RA volume and learning being statistically significant (r = 0.602, P < 0.05).
Figure 3.
Scatterplots showing that the percentage of available song material learned in the first study was not proportional to the number of neurons within the RA (Top) or area X (Bottom).
The overall size of the RA correlated significantly with the size of the HVc. As has been reported for several other species (8, 34, 35), birds in the first data set with a large HVc tended to have a large RA (r = 0.850, P < 0.05). Similarly, neuron number within the HVc and RA were positively related, although this trend did not reach significance with a two-tailed test (r = 0.732, P = 0.098). In the replicate, there were similar trends that were nonsignificant, most likely because of the small sample size. In the data set encompassing both replications, both volume and neuron number were significantly correlated between the HVc and RA (volume: r = 0.800, P < 0.01; neuron number: r = 0.811, P < 0.01).
Although area X volume and neuron number were extremely variable across individuals, neither of these measures related systematically to the amount of song material copied (Fig. 3, Bottom). There also was no significant relationship between our anatomical measures of area X and any measure of the HVc or RA.
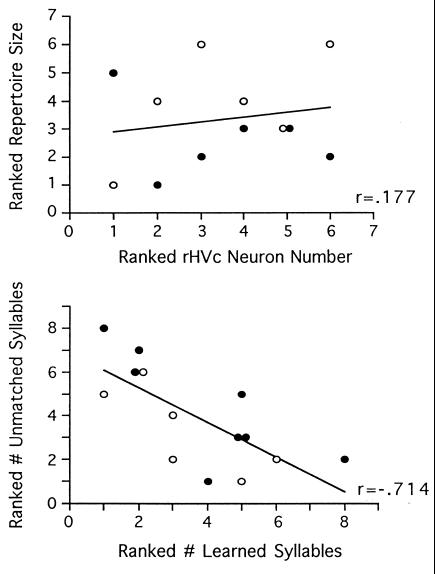
Total repertoire size did not correlate significantly with any of the anatomical measures taken. In neither study did the total number of syllables produced relate obviously to HVc neuron number or volume. Even with the larger sample size generated by collapsing across experiments, no correlation was evident between repertoire size and either HVc neuron number (Fig. 4, Top; r = 0.177) or HVc volume (r = 0.177). This appears paradoxical given the striking relationship between these same neuroanatomical variables and measures of learning. However, total repertoire size was not predicted by the number of learned syllables produced (r = 0.228, P = 0.45). This appears to be due to the fact that birds that imitated fewer tutor syllables produced more unmatched syllables than did those birds that imitated many tutor syllables. Thus, the number of unmatched syllables produced in individual birds’ songs was inversely proportional to the number of learned syllables produced. This relationship was statistically significant in the combined data set (Fig. 4, Bottom; r = −0.714, P < 0.01) and in the initial experiment (r = −0.813, P = 0.01). In the replication, a similar trend was not statistically significant (r = −0.587, P = 0.22), possibly due to the smaller number of data points.
Figure 4.
Song repertoire size was not correlated with HVc neuron number (Top). Also, repertoire size was not predicted by the number of syllables scored as learned because this latter measure correlated inversely to the number of unmatched syllables (Bottom). •, First study; ○, replication.
DISCUSSION
The results presented in this paper reveal a strong, positive correlation in zebra finches between the amount of song material accurately copied from a social tutor and the number of neurons within the HVc. Furthermore, this correlation was found to be consistent upon replication. These observations are consistent with the hypothesis that neuron number in the HVc limits the ability of individual animals to acquire and/or accurately reproduce song syllables heard during vocal development. Interestingly, because the number of learned syllables in each bird’s repertoire correlated inversely with the number of unmatched syllables, total repertoire size did not correlate with the anatomy of either the HVc or RA.
The hypothesis that HVc size may limit how much song is learned was suggested originally by the observation that individual differences in HVc volume correlate positively with repertoire size in both canaries and marsh wrens (4, 8). However, this hypothesis was challenged by comparative studies that failed to detect a significant relationship between HVc volume and repertoire size in the red-winged blackbird (Agelaius phoeniceus; ref. 34), rufous-sided towhee (Pipilo erythrophthalmus; ref. 36), european starling (Sturnus vulgaris; ref. 35), and zebra finch (unpublished observation). The present experiment offers a possible explanation for these species differences. In distinguishing accurately copied syllables from unmatched syllables we find that, in zebra finches, the overall repertoire size (total number of syllables) is not an accurate measure of how much song material has been faithfully reproduced. This is because birds that mimic fewer syllables tend to produce more unmatched syllables, creating a total repertoire that can be as large as those of individuals that accurately copied more song syllables. We do not know whether unmatched syllables are improvisations, or failures in either acquisition or sensorimotor learning. In any case, HVc size in zebra finches does not vary consistently with total repertoire size, but rather with the proportion of accurately learned song syllables. Because previous studies have not distinguished learned from unmatched song material, species differences in the relationship between repertoire size and brain space may reflect differences in the extent to which repertoire size reflects the amount of conspecific song accurately copied. To test the generality of our observations, it will be important to conduct additional comparative studies in which learning is assessed.
These are the first studies to show that individual differences in HVc neuron number (rather than volume) relate to individual differences in song learning. To understand this relationship between “network space” and learning better, it will be important to determine if a specific subpopulation of HVc neurons is responsible for the correlation observed, or whether all HVc neuronal subtypes contribute equally to this relationship. On a gross level, three classes of neurons reside within the HVc: those that project to the RA, those that project to area X, and interneurons. Retrograde tracing studies in both zebra finches and canaries suggest that RA-projecting neurons account for ≈50% of the neurons in the HVc (11, 12, 37), and are 2–3 times more numerous than area X-projecting HVc neurons (37–39). If only one population is responsible for the correlation observed in the present study, it is likely to be the RA-projecting neurons, because the others each account for only ≈25% of all HVc neurons. It would be exciting if the amount of song material learned relates to the number of HVc-RA neurons because song learning in both zebra finches and canaries is associated with the addition of this neuronal subpopulation (11, 37, 38). While these neurons are involved in song production (they form part of the efferent motor pathway (refs. 40 and 41), there is not yet any direct evidence implicating them in the process of song learning. Thus, understanding which HVc neuronal subtypes are involved in the correlations observed in the present study may provide important insights into the neural substrates for vocal learning.
Because song learning per se does not regulate the development of HVc neuron number (18, 19), we presume that if a causal relationship underlies this brain–behavior correlation, it must be that HVc neuron number influences how much song material is accurately memorized and/or reproduced. All other things being equal, the computational power of a neural network will increase as more neurons are added to it. It is possible, however, that the correlation observed in the present study does not reflect a direct causal relationship, but rather an influence of some third variable on song learning, that itself varies with HVc neuron number and volume. For example, the amount of song produced during vocal practice could affect a bird’s success in mimicking acquired material accurately, and could also be related positively to the size of the HVc. Although the volume of the HVc is not known to correlate significantly with the quantity of song behavior, such a relationship has been identified for several other song-related brain regions. In white-throated sparrows, color morph differences in song production rate correlate positively with the volume of the RA, the tracheosyringeal portion of the hypoglossal nucleus, and several song-related nuclei in the rostral forebrain (42). Also in male European starlings, song bout length correlates significantly with the volume of RA (and to a lesser extent, the HVc) when volume is corrected for overall brain size (35). In the present study, a post hoc analysis failed to detect systematic relationships between any of our anatomical measurements and song bout length. However, it is important to note that all of these studies have compared variation in song production and song region volume only in adults, and it is not known if differences in the amount of song behavior produced in adulthood are related systematically to differences in the extent of vocal practice during song development. Thus, we cannot rule out the possibility that variation in HVc neuron number relates to individual differences in early song production, and further, that such differences in production affect learning (e.g., through increased vocal practice).
Variation in the number of song-related neurons may reflect differences in cell production, specification, or survival. While the physiological factors producing this variation are unknown, both neurotrophins (43) and gonadal hormones (44–46) can regulate neuron number in the song system and therefore may be valuable tools for manipulating HVc neuron number. Regarding hormones, estradiol can stimulate the incorporation of new HVc neurons in young female zebra finches (47, 48) and estrogen levels during song acquisition are higher among young male swamp sparrows that learn their tutor’s song than among those that do not learn (49). Also, yolk concentrations of testosterone vary greatly in both canary and zebra finch eggs and in canaries, increases with the order in which eggs are laid (50). Anecdotally, our initial study included three male clutchmates, and among these siblings, HVc neuron number (and amount learned) increased systematically with hatch order. Thus, variation in the amount of testosterone deposited in the egg yolk may underlie individual variation in HVc neuron number. Given the robust brain–behavior relationship described in the present study, it will be important to identify what factor(s) regulate HVc neuron number in males. Also, as we learn how to manipulate HVc neuron number nonintrusively, we can test more directly for a causal relationship between HVc neuron number and learning capacity.
Acknowledgments
We are grateful to Beth Harris and Donna Shannon for expert technical assistance. This work was supported by U.S. Public Health Service Grant MH45096.
ABBREVIATIONS
- HVc
(acronym used as the proper name)
- RA
robust nucleus of the archistriatum
References
- 1.Nottebohm F, Arnold A P. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- 2.Brenowitz E A, Arnold A P, Levin R N. Brain Res. 1985;343:102–112. doi: 10.1016/0006-8993(85)91163-1. [DOI] [PubMed] [Google Scholar]
- 3.Brenowitz E A, Arnold A P. J Neurosci. 1986;6:2875–2879. doi: 10.1523/JNEUROSCI.06-10-02875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canady R A, Kroodsma D E, Nottebohm F. Proc Natl Acad Sci USA. 1984;81:6232–6234. doi: 10.1073/pnas.81.19.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVoogd T J, Krebs J R, Healy S D, Purvis A. Proc R Soc London B. 1993;254:75–82. doi: 10.1098/rspb.1993.0129. [DOI] [PubMed] [Google Scholar]
- 6.Kroodsma D E, Canady R A. Auk. 1985;102:439–446. [Google Scholar]
- 7.Brenowitz E, Nalls B, Kroodsma D E, Horning C. J Neurobiol. 1993;25:197–208. doi: 10.1002/neu.480250210. [DOI] [PubMed] [Google Scholar]
- 8.Nottebohm F, Kasparian S, Pandazis C. Brain Res. 1981;213:99–109. doi: 10.1016/0006-8993(81)91250-6. [DOI] [PubMed] [Google Scholar]
- 9.Nordeen E J, Nordeen K W. Trends Neurosci. 1990;13:31–36. doi: 10.1016/0166-2236(90)90060-n. [DOI] [PubMed] [Google Scholar]
- 10.Nordeen K W, Marler P, Nordeen E J. J Neurobiol. 1989;20:651–661. doi: 10.1002/neu.480200705. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla A, Kirn J R, Nottebohm F. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- 12.Kirn J R, Alvarez-Buylla A, Nottebohm F. J Neurosci. 1991;11:1756–1762. doi: 10.1523/JNEUROSCI.11-06-01756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirn J R, Nottebohm F. J Neurosci. 1993;13:1654–1663. doi: 10.1523/JNEUROSCI.13-04-01654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottjer S W, Miesner E A, Arnold A P. Neurosci Lett. 1986;67:263–268. doi: 10.1016/0304-3940(86)90319-8. [DOI] [PubMed] [Google Scholar]
- 15.Bottjer S W, Glaessner S L, Arnold A P. J Neurosci. 1985;5:1556–1562. doi: 10.1523/JNEUROSCI.05-06-01556.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottjer S W, Sengelaub D R. J Neurobiol. 1989;20:609–618. doi: 10.1002/neu.480200702. [DOI] [PubMed] [Google Scholar]
- 17.Clayton N S, Krebs J R. Proc Natl Acad Sci USA. 1994;91:7410–7414. doi: 10.1073/pnas.91.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burek M J, Nordeen K W, Nordeen E J. J Neurobiol. 1991;22:215–223. doi: 10.1002/neu.480220302. [DOI] [PubMed] [Google Scholar]
- 19.Brenowitz E A, Lent K, Kroodsma D E. J Neurosci. 1995;15:6281–6286. doi: 10.1523/JNEUROSCI.15-09-06281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price P H. J Comp Physiol Psychol. 1979;93:268–277. [Google Scholar]
- 21.Eales L A. Anim Behav. 1985;33:1293–1300. [Google Scholar]
- 22.Eales L A. Anim Behav. 1987;35:1356–1365. [Google Scholar]
- 23.Williams H. Anim Behav. 1990;39:745–757. [Google Scholar]
- 24.Sohrabji F, Nordeen E J, Nordeen K W. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 25.Nordeen K W, Nordeen E J. Beh Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 26.Nordeen K W, Nordeen E J. Behav Neural Biol. 1993;59:79–82. doi: 10.1016/0163-1047(93)91215-9. [DOI] [PubMed] [Google Scholar]
- 27.Aamodt S M, Nordeen E J, Nordeen K W. Neurobiol Learn Mem. 1996;65:91–98. doi: 10.1006/nlme.1996.0010. [DOI] [PubMed] [Google Scholar]
- 28.Basham M E, Nordeen E J, Nordeen K W. Neurobiol Learn Mem. 1996;66:295–304. doi: 10.1006/nlme.1996.0071. [DOI] [PubMed] [Google Scholar]
- 29.Williams H, Kilander K, Sotanski M L. Anim Behav. 1993;45:695–705. [Google Scholar]
- 30.Williams H, Crane L A, Hale T K, Esposito M A, Nottebohm F. J Neurobiol. 1992;23:1006–1020. doi: 10.1002/neu.480230807. [DOI] [PubMed] [Google Scholar]
- 31.Smith G T, Brenowitz E A, Wingfield J C, Baptista L F. J Neurobiol. 1995;28:114–125. doi: 10.1002/neu.480280110. [DOI] [PubMed] [Google Scholar]
- 32.Konigsmark B W. In: Contemporary Research Methods in Neuroanatomy. Nauta W J H, Ebesson S O E, editors. New York: Springer; 1970. pp. 315–340. [Google Scholar]
- 33.Coggeshall R E, Lekan H A. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Kirn J R, Clower R P, Kroodsma D E, DeVoogd T J. J Neurobiol. 1989;20:139–163. doi: 10.1002/neu.480200304. [DOI] [PubMed] [Google Scholar]
- 35.Bernard D J, Eens M, Ball G F. J Neurobiol. 1996;30:329–339. doi: 10.1002/(SICI)1097-4695(199607)30:3<329::AID-NEU2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Brenowitz E A, Nalls B, Wingfield J C, Kroodsma D E. J Neurosci. 1991;11:1367–1374. doi: 10.1523/JNEUROSCI.11-05-01367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordeen K W, Nordeen E J. Nature (London) 1988;334:149–151. doi: 10.1038/334149a0. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez-Buylla A, Theelen M, Nottebohm F. Proc Natl Acad Sci USA. 1988;85:8722–8726. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sohrabji F, Nordeen K W, Nordeen E J. Brain Res. 1989;488:253–259. doi: 10.1016/0006-8993(89)90715-4. [DOI] [PubMed] [Google Scholar]
- 40.Nottebohm F, Stokes T M, Leonard C M. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 41.Simpson H B, Vicario D S. J Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeVoogd T J, Houtman A M, Falls J B. J Neurobiol. 1995;28:202–213. doi: 10.1002/neu.480280207. [DOI] [PubMed] [Google Scholar]
- 43.Johnson F, Hohmann S E, Distefano P S, Bottjer S W. J Neurosci. 1997;17:2101–2111. doi: 10.1523/JNEUROSCI.17-06-02101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlinger B A. J Neurobiol. 1997;33:619–631. [PubMed] [Google Scholar]
- 45.Arnold A P. J Neurobiol. 1997;33:572–584. [PubMed] [Google Scholar]
- 46.Nordeen E J, Nordeen K W. Semin Neurosci. 1994;6:299–306. [Google Scholar]
- 47.Nordeen E J, Nordeen K W. Dev Brain Res. 1989;49:27–32. doi: 10.1016/0165-3806(89)90056-4. [DOI] [PubMed] [Google Scholar]
- 48.Burek M J, Nordeen K W, Nordeen E J. Dev Brain Res. 1995;85:220–224. doi: 10.1016/0165-3806(94)00215-l. [DOI] [PubMed] [Google Scholar]
- 49.Marler P, Peters S, Wingfield J C. J Neurobiol. 1987;18(6):531–548. doi: 10.1002/neu.480180605. [DOI] [PubMed] [Google Scholar]
- 50.Schwabl H. Proc Natl Acad Sci USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]