Abstract
Aims
To investigate the pharmacokinetics (PK) and pharmacodynamics (PD) of betaine in the treatment of classical homocystinuria due to cystathionine β-synthase (CβS) deficiency with a view to optimizing the dosage regimen.
Methods
Betaine was given as a single oral dose of 100 mg kg−1 to six patients (age range 6–17 years) who normally received betaine but whose treatment had been suspended for 1 week prior to the study. Plasma betaine and total homocysteine concentrations were measured by high performance liquid chromatography (h.p.l.c.) at frequent intervals over 24 h. The best-fit PK model was determined using the PK-PD program Win-Nonlin and the concentration-time-effect data analysed by an indirect PD model. Using the PK and PD parameters, simulations were carried out with the aim of optimizing betaine dosage.
Results
Betaine PK was described by both mono- and bi-exponential disposition functions with first order absorption and a lag time. The correlation coefficient between betaine oral clearance and body weight was 0.6. Mean betaine clearance was higher in males than in females (P = 0.03). PK-PD simulation indicated minimal benefit from exceeding a twice-daily dosing schedule and a 150 mg kg−1 day−1 dosage for betaine.
Conclusions
PK-PD modelling allows recommendations for optimal dosage of betaine in the treatment of homocystinuria, that have the potential for improved patient compliance and both therapeutic and pharmacoeconomic benefit.
Keywords: betaine, dose optimization, homocystinuria, pharmacokinetic-pharmacodynamic modelling
Introduction
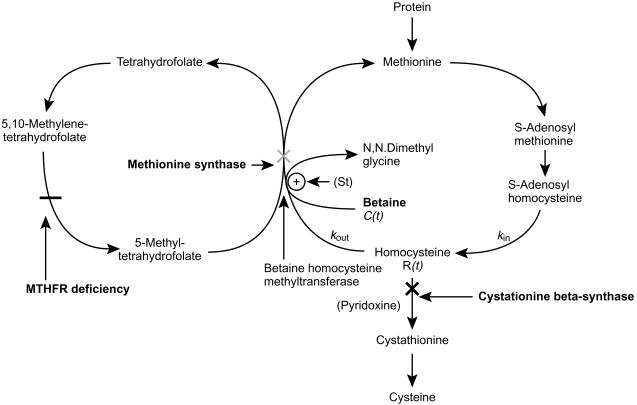
A number of inherited metabolic disorders affect homocysteine (HCY) metabolism resulting in an increase in its plasma concentration and urinary excretion. These include classical homocystinuria due to CβS deficiency [1], remethylation defects due to functional methionine synthase deficiency [2] and methylenetetrahydrofolate reductase (MTHFR) deficiency [3] (Figure 1). An elevated plasma HCY concentration is associated with mental retardation, osteoporosis, premature development of vascular disease and ocular defects [4–6]. Treatment is aimed at reducing the HCY concentration in plasma.
Figure 1.
The metabolic cycle of methionine and the possible enzyme defects in homocystinuria (cross signs: bold cross indicating patients in this study). The mechanism of action of administered betaine is indicated. R(t) is the concentration of homocysteine at a given time, C(t) is the concentration of betaine at a given time, kin and kout are rate constants for the production and loss of homocysteine, respectively, S(t) is a stimulation function for the betaine induced increase in homocysteine elimination.
The worldwide incidence of CβS deficiency is estimated to be 1 : 344 000 live births [1]. Of these, between 25 and 50% of patients, dependent upon the population, respond to large doses of pyridoxine which acts as a cofactor for CβS [7]. For those not responding to the latter treatment, restriction in dietary methionine can also lead to a marked reduction in HCY plasma concentrations [8, 9] although compliance with such a diet can be difficult [7]. An alternative or supplementary treatment strategy is to use oral betaine. This acts as a methyl donor and promotes the conversion of HCY to methionine via the enzyme, betaine-homocysteine methyltransferase, thereby, decreasing high plasma concentrations of the amino acid [10, 11].
Clinical responses to betaine have been encouraging in both classical homocystinuria and remethylation defects [10–15]. In classical homocystinuria the average decrease in plasma HCY concentration is reported to be between 74 and 92% following doses of 6–20 g day−1 of betaine [11, 16, 17]. However, some studies have found no clinical benefit using 6 g day−1 of betaine [15, 18]. Despite widespread usage there is little consensus on betaine dosage and frequency of administration. Case studies reported to date have used doses of 150–250 mg kg−1 day−1 or a standard dose of 2–3 g day−1 in children and 5–20 g day−1 in adults usually given two or three times daily [10–15].
Few studies have been undertaken on the PK of betaine in man and no proper link has been established between its concentration and effect. Sakura et al. [19] studied the PK of betaine in three healthy adult patients and in a single patient with MTHFR deficiency. All subjects were given a 100-mg kg−1 test dose of betaine. Peak betaine concentrations were noted between 1 and 2 h after dosing and showed marked interindividual variability (148–258 µmol l−1), with an apparent elimination half-life of 1 h. Consequently, Sakura et al. [19] recommend that betaine doses be given repeatedly at short intervals to achieve a concentration at which HCY is maximally methylated to methionine [19]. In a dose ranging study (20–120 mg kg−1 betaine) in the patient with MTHFR deficiency, total HCY did not decrease further once a threshold betaine concentration of 400 µmol l−1 was achieved.
Schwahn et al. [20] studied betaine PK in 12 healthy male subjects following a single 50 mg kg−1 dose of betaine and during continuous intake of 50 mg kg−1 twice daily. Following the single dose, betaine was rapidly absorbed and distributed with mean peak plasma concentrations of 940 µmol l−1 reached at 0.9 h and a mean elimination half-life of 14 h. Plasma dimethylglycine (DMG) levels increased significantly after betaine administration, thus providing evidence that catabolism of betaine is an important route of elimination in healthy adults. Schwahn et al. [20] conclude that further studies are required on betaine PK in patients with homo cystinuria, with emphasis on betaine dosage interval and monitoring.
This study aims to clarify the PK and PD characteristics of betaine in patients with pyridoxine unresponsive classical homocystinuria due to CβS deficiency. Individual plasma betaine concentration – time data were fitted by an optimal PK model, and individual concentration-time-effect data were analysed by an indirect response PD model. Both models were then linked in an algorithm to simulate the effects of betaine dose and dosage interval on plasma HCY concentrations. This was then applied to determine the optimal regimen of betaine to control plasma total HCY in the individual CβS deficient patients.
Methods
Patients and sample collection
Ethical approval for the study was obtained from the Salford and Trafford Research Ethics committee. A total of six patients (age 6–17 years) with homocystinuria due to pyridoxine-unresponsive CβS deficiency were recruited and written informed consent was given in all cases. Response to pyridoxine was assessed in vivo by analysis of amino acid profiles. No patients included in this study were taking pyridoxine. Patients were asked to stop their betaine therapy 1 week before the study but to continue on a methionine restricted diet and maintain a food diary during this period. To ensure that all patients had an adequate protein intake just prior to the study they were given a protein load of 30% of their daily allowance. Each patient had a wide-bore intravenous cannulae inserted in to the arm to facilitate blood sample collection. Baseline blood samples were collected prior to administration of a single 100 mg kg−1 dose of betaine. Subsequent blood samples were then collected at 20 min intervals for the first 2 h and then every hour for a further 6 h and finally at 24 h after dosage. All blood samples were collected into EDTA tubes, placed on ice and the plasma separated within 20 min Plasma samples were frozen at −20° C prior to assay.
Materials
Betaine was obtained from Orphan Medical Inc (Minnetonka, USA). All other chemicals were from Sigma (Poole, Dorset) and were of analytical grade.
Measurement of plasma betaine
The method was based on that described by Laryea et al. [21], with slight modifications. Fifty microlitres of 100 mmol l−1 KH2PO4 was added to 50 µl of plasma. After vortex-mixing, 900 µl of derivatizing solution (2.5 mmol 18 Crown-6 ether, 50 mmol 4-bromophenacyl bromide in acetonitrile) was added. Tubes were capped, vortex mixed, heated to 80° C for 60 min and then allowed to cool to room temperature. Following centrifugation at 1000 g, 15 µl of the supernatant containing the phenylacyl esters of DMG and betaine was injected directly onto the h.p.l.c. system.
An isocratic reverse-phase Waters hplc system (Waters Corp, USA) was used with a Supelcosil LC-SCX, 5 µm, 25 cm×4.6 cm bonded silica reverse phase column, with detection at 254 nm using a Waters photodiode array detector. The mobile phase was acetonitrile 90%–water 10% containing 22 mmol l−1 choline at a flow rate of 1.5 ml min−1. Retention times were 12.7 min and 14.8 min for DMG and betaine, respectively. The percentage recovery of betaine was 99–103%. The limit of determination was 20 µmol l−1 betaine, and linearity was maintained over the concentration range 20–1000 µmol l−1 (r2 = 0.99). The within batch coefficients of variation for the assay were 7.4% and 2.5% at concentrations of 20 and 200 µmol betaine, respectively.
Measurement of total plasma HCY
This method was based on that described by Moat et al. [22]. Briefly, α-mercapto-propionylglycine was used as the internal standard. Plasma (200 µl) was reduced using a 10% solution of tri-n-butylphosphine in dimethylformamide to reduce disulphide bonds between HCY and plasma proteins and between mixed disulphides. Plasma proteins were then removed by deproteinization with 10% trichloroacetic acid. Samples were then derivatized using 7-fluorobenzo-2-oxa- 1,3-diazole-4-sulphonic acid to permit subsequent fluorimetric detection. Aliquots (10 µl) were injected on to a Waters 600E h.p.l.c. system, with a Chromsep Microsphere C18, 100 mm×4.6 mm, 5 µm particle size column and a Waters 474 fluorescence detection system with excitation at 385 nm and emission at 515 nm. The mobile phase was gradient elution at a flow rate of 1.5 ml min−1 with 0.15 mmol l−1 potassium phosphate buffer (pH 1.75) and acetonitrile. The limit of detection was 0.5 µmol l−1. The inter- and intra-batch coefficients of variation were less than 5% at plasma total homocysteine concentrations of 11 µmol l−1 and 70 µmol l−1.
Pharmacokinetic analysis
The concentration-time data for each subject were entered into the PK-PD program Win-Nonlin (Scientific consulting Inc, USA). The profiles were described by different models of varying complexity including mono- or bi-exponential disposition functions. Assessment of ‘best fit’ was based on the Akaike Information Criteria (AIC) and the visual inspection of residual errors.
Pharmacodynamic analysis
It was assumed that the delayed betaine effect on suppressing total HCY concentration was only related to its methyl donation, which accelerated the elimination of HCY. Thus, the concentration-time-effect data for each subject were analysed by the most physiologically appropriate indirect response PD model incorporating a simulation function [23]. Consistent with the biochemical model for a decrease in plasma total HCY concentration by betaine shown in Figure 1, the change in response with time is given by equation 1:
| (1) |
where kin and kout are rate constants for the production of HCY (zero-order) and for the loss of HCY (first-order), respectively. R(t) is the plasma HCY concentration at a given time and S(t) is the stimulation function given by equation 2:
| (2) |
EMax is the maximum betaine effect, EC50 the betaine concentration producing 50% of maximum effect and C(t) the plasma concentration of betaine at a given time.
The model was fitted to the data using the Microsoft Excel worksheets developed by Piotrovsky [23].
Using the individual PK and PD parameters obtained in this study, an algorithm was developed within a Microsoft Excel worksheet to simulate the effect of different betaine doses and dosing regimens on the 24 h plasma HCY concentration profile. The simulation included a graphical representation of both expected betaine and plasma total HCY concentrations over time and also a calculated total daily and hourly reduction in HCY concentration. Various dosing regimens were simulated to find both the optimum dose and dosing schedule.
Results
Betaine pharmacokinetics
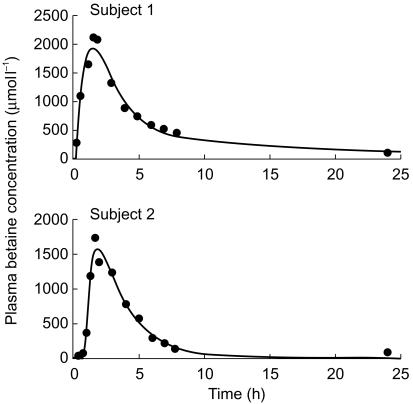
The plasma concentration-time profile of betaine in three individuals was best described by a mono-exponential disposition model with first-order absorption and a lag time (subjects 2, 3 and 5). However, the data for the three other individuals were best fitted by bi-exponential disposition functions with first-order absorption and a lag time (subjects 1, 4 and 6). Representative concentration-time profiles are shown in Figure 2. The clinically important PK parameters are listed in Table 1. The correlation coefficient between betaine clearance and body weight was 0.6, which is consistent with the practice of weight adjustment of dosage. Nevertheless, there were too few individuals in the study to show a statistically significant relationship (P = 0.2). The female patients appeared to have a lower weight adjusted oral clearance of betaine compared with males (P = 0.03, unpaired t-test). None of the other parameters in Table 1 was significantly different between the two sexes.
Figure 2.
Representative best fit model (line) to plasma betaine concentrations(•) in subject 1 and subject 2. Biexponential and monoexponential disposition with first-order absorption were assumed for subjects 1 and 2, respectively.
Table 1.
Individual demographic and pharmacokinetic parameters.
| Sex | Age (years) | Weight (kg) | PK model | t½ (absorption) (h) | t½ (elimination) (h) | Oral clearance (ml min−1 kg−1) | |
|---|---|---|---|---|---|---|---|
| 1 | F | 17 | 113 | 2 comp+tlag | 0.8 | 11.4 | 1.0 |
| 2 | F | 11 | 33 | 1 comp+tlag | 0.3 | 8.0 | 2.6 |
| 3 | M | 17 | 64 | 1 comp+tlag | 0.4 | 10.5 | 3.3 |
| 4 | M | 15 | 111 | 2 comp+tlag | 0.8 | 12.4 | 3.5 |
| 5 | M | 6 | 22 | 1 comp+tlag | 1.4 | 12.2 | 3.2 |
| 6 | F | 14 | 70 | 2 comp+tlag | 0.8 | 26.3 | 0.9 |
Betaine pharmacodymamic effect on plasma total HCY concentration
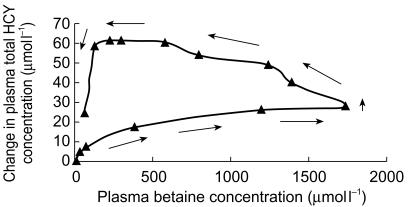
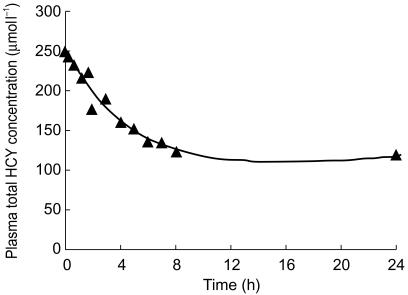
When changes in plasma total HCY concentration following betaine administration were analysed as a function of plasma betaine concentration, a counterclockwise hysteresis was observed for all subjects. A representative plot is shown in Figure 3. The time courses of observed plasma total HCY concentrations for subject 1 and those predicted by the PK-PD model are shown in Figure 4. There was good agreement between observed and predicted plasma total HCY concentrations for all of the subjects, indicating that the model was adequate to describe the acceleration of homocysteine clearance by betaine. Summary statistics for HCY pharmacodynamic parameters are given in Table 2.
Figure 3.
Representative change in plasma total homocysteine as a function of plasma betaine concentration in subject 2 showing counter-clockwise hysteresis.
Figure 4.
A representative model fit (solid line) to homocysteine-time data (▴) following administration of 100 mg kg−1 day−1 of betaine to subject 1.
Table 2.
Individual betaine PD parameters.
| Subject | kin (h−1) | kout (h−1) | EC50 (µmol l−1) | EMax (% extra HCY elimination) |
|---|---|---|---|---|
| 1 | 19.7 | 0.07 | 99 | 180 |
| 2 | 52.2 | 0.43 | 98 | 120 |
| 3 | 52.0 | 0.16 | 98 | 110 |
| 4 | 60.9 | 0.44 | 101 | 60 |
| 5 | 31.9 | 0.20 | 387 | 240 |
| 6 | 12.2 | 0.13 | 296 | 250 |
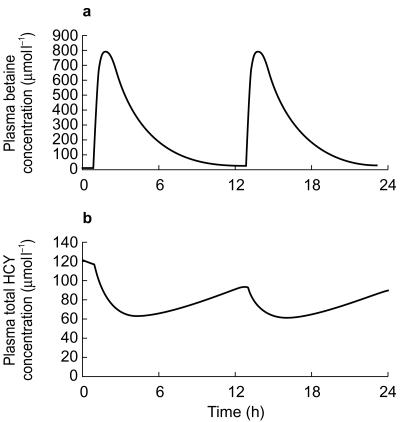
A linked PK-PD simulation was undertaken for each patient, to predict plasma total HCY concentrations in relation to the dose of betaine administered. A representative plot for simulated betaine and plasma total HCY concentrations over a 24 h period in subject 2 following 100 mg kg−1 day−1 of betaine administered in two doses is shown in Figure 5.
Figure 5.
Simulated effects of administering 100 mg kg−1 day−1 of betaine as a twice daily dose on plasma betaine concentration (a) and the predicted effects on plasma total homocysteine concentration (b). Simulation for subject 2.
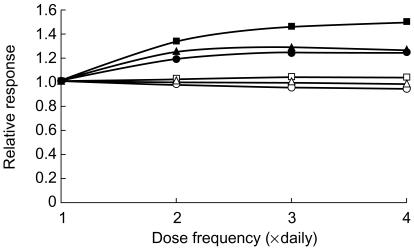
For a given total daily dose, the effects of betaine were maximized by increasing the dose frequency. However, the benefits from exceeding twice daily dosing were minimal (Figure 6) and twice daily dosage appeared to be optimal.
Figure 6.
Simulated effects of increasing dose frequency on the relative decrease in plasma total homocysteine in six patients receiving a fixed dose of 100 mg kg−1 day−1 of betaine (▴ = subject 1, □ = subject 2, ▪ = subject 3, ○ = subject 4, • = subject 5, ▵ = subject 6).
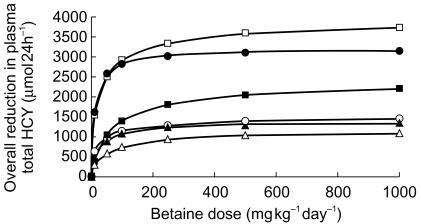
When a twice-daily dosing schedule was used and the daily dose was escalated from 10 to 1000 mg kg−1, there appeared to be only a marginal benefit from exceeding 150 mg kg−1 (a range of 1.05–1.2 fold increase in effect for a 6.6 fold increase in dose; Figure 7).
Figure 7.
Simulated effects of increasing the betaine dose on the overall reduction in Plasma total homocysteine concentration in 6 patients on a fixed twice-daily dosing schedule (▴ = subject 1, □ = subject 2, ▪ = subject 3, ○ = subject 4, • = subject 5, ▵ = subject 6).
Discussion
The mean absorption and elimination half-lives of betaine following a single oral dose were in agreement with those reported by Schwahn et al. [20]. The observed increased in the weight normalized oral clearance of betaine in males compared to females has not been commented on previously.
To our knowledge this is the first time that a PD model has been applied to describe the concentration-time-effect of betaine on plasma total HCY. The indirect response model used in this study describes a delayed PD effect at non steady state conditions and incorporates a stimulation function (S) for the acceleration of HCY clearance by betaine. The usefulness of the model is enhanced as plasma concentration-time profiles for betaine can be approximated by a universal piecewise function and no parametric betaine PK model is needed [23].
The PK-PD simulation allows the plasma total HCY concentration to be predicted for each patient following a single oral dose of 100 mg kg−1 betaine. Therefore, treatment can be optimized on an individual basis and predictive targets can be established. Previous studies have indicated that a ceiling effect is reached with betaine at plasma concentrations between 400 and 500 µmol l−1[19, 24]. The indirect nature of the interaction between plasma betaine and total HCY concentration makes it difficult to define a ceiling concentration for betaine, although at a near optimal dosage (150 mg kg−1 as a twice daily dose) the mean 24 h betaine plasma concentration is 450 µmol l−1. This simulation indicates that there is a ceiling dose for betaine in each patient above which, reduction in plasma total HCY is minimal. This may explain the lack of concordance between dose escalation of betaine and total HCY suppression in a number of patients with homocystinuria who were previously thought to be poor compliers [7].
The results from this study indicated minimal benefit in response from exceeding twice daily dosing with betaine, and in a number of patients once daily dosing would suffice. This contrasts with a recent PK study suggesting that betaine should be taken more frequently than twice daily [19]. Reducing the need to take multiple daily doses has a positive effect on compliance with drug therapy. This may be especially important for teenage patients on betaine who are often resistant to taking medication [25].
Betaine has been granted orphan drug status for the treatment of homocystinuria in the USA and is awaiting an authorization as such in Europe. Orphan drug status is given to encourage pharmaceutical companies to develop treatments for rare diseases that affect fewer than 5 people in every 10 000. Because betaine is only used in a small number of patients its associated production costs are high. Currently 5 g of betaine costs £45.35. Considering the cost of treating homocystinuria with betaine, optimizing individual dosage regimens has significant pharmacoeconomic as well as therapeutic benefit. For instance, the current data suggest that increasing the daily dose from 150 to 250 mg kg−1 would result in negligible clinical benefit for an additional average cost of £2100 per annum for every patient. Our current estimates are that around 70 patients in the UK and 700 in the USA and Europe are currently receiving betaine therapy.
Schwahn et al. [20] have demonstrated an increase in betaine plasma elimination half-life on repeat administration of the drug to healthy subjects, but this has not been shown in patients with elevated plasma total HCY. Owing to the indirect nature of the response any changes in half-life might not be important. Both this and other possible complications with our approach can be incorporated into the simulation as more information becomes available.
The approach of optimizing betaine therapy based on a single dose PKPD simulation requires further validation in the clinical setting and especially in patients who are at steady state in respect to plasma betaine concentrations. Nevertheless this study has potential application for the optimal management of patients with homocystinuria and currently suggests that there is little benefit in exceeding 150 mg kg−1 day−1 of betaine given as two doses.
Because betaine is an orphan drug with limited clinical usage and without the commercial backing of a large pharmaceutical company there is limited scope for undertaking PK-PD studies. Therefore, clinical trial simulations [26] are the most logical way to optimize future clinical studies for this drug and avoid costly inconclusive results.
References
- 1.Mudd SH, Levy HL, Skovby F. Disorders of transsulphation. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 7. New York: McGraw-Hill; 1995. pp. 1279–1327. [Google Scholar]
- 2.Fenton WA, Rosenberg LE. Inherited disorders of cobolamin transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 7. New York: McGraw-Hill; 1995. pp. 3129–3149. [Google Scholar]
- 3.Rosenblatt DS. Inherited disorders of folate transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 7. New York: McGraw-Hill; 1995. pp. 3111–3128. [Google Scholar]
- 4.Mudd SH, Skovby F, Levy HL, et al. The natural history of homocystinuria due to cystathionine β-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcken DEL, Wilcken B. The natural history of vascular disease in homocystinuria and the effects of treatment. J Inherit Metab Dis. 1997;20:295–300. doi: 10.1023/a:1005373209964. [DOI] [PubMed] [Google Scholar]
- 6.Burke JP, O’Keefe M, Bowell R, Naughten ER. A closer look in the eye in homocystinuria: a screened population. J Inherit Metab Dis. 1988;11(Suppl 2):237–239. doi: 10.1007/BF01804245. [DOI] [PubMed] [Google Scholar]
- 7.Walter JH, Wraith JE, White FJ, Bridge C, Till J. Strategies for the treatment of cystathionine β-synthase deficiency: the experience of the Willink Biochemical Genetics Unit over the past 30 years. Eur J Pediatr. 1998;157(Suppl 2):S71–S76. doi: 10.1007/pl00014308. [DOI] [PubMed] [Google Scholar]
- 8.Komrower GM, Lambert AM, Cusworth DC, Westall RG. Dietary treatment of homocystinuria. Arch Dis Child. 1966;41:666–671. doi: 10.1136/adc.41.220.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry TL, Hansen S, Love DL, Crawford LE, Tischler B. Treatment of homocystinuria with a low-methionine diet, supplemental cysteine, and a methyl donor. Lancet. 1968;ii:474–478. doi: 10.1016/s0140-6736(68)90646-6. [DOI] [PubMed] [Google Scholar]
- 10.Smolin LA, Benevenga NJ, Berlow S. The use of betaine for the treatment of homocystinuria. J Pediatr. 1981;99:467–472. doi: 10.1016/s0022-3476(81)80352-6. [DOI] [PubMed] [Google Scholar]
- 11.Wilcken DEL, Wilcken B, Dudman NPB, Tyrrell PA. Homocystinuria- The effects of betaine in the treatment of patients not responsive to pyridixime. N Engl J Med. 1983;309:448–453. doi: 10.1056/NEJM198308253090802. [DOI] [PubMed] [Google Scholar]
- 12.Holme E, Kjellman B, Ronge E. Betaine for the treatment of homocysteine caused by methylenetetrahydrofolate reductase deficiency. Arch Dis Child. 1989;64:1061–1064. doi: 10.1136/adc.64.7.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wendel U, Bremer HJ. Betaine in the treatment of homocystinuria due to 5, 10-methylenetetrahydrofolate reductase deficiency. Eur J Pediatr. 1984;142:147–150. doi: 10.1007/BF00445602. [DOI] [PubMed] [Google Scholar]
- 14.Bartholomew DW, Batshaw ML, Allen RH, et al. Therapeutic approaches to cobolamin-C methylmalonic acidaemia and homocystinuria. J Pediatr. 1988;112:32–39. doi: 10.1016/s0022-3476(88)80114-8. [DOI] [PubMed] [Google Scholar]
- 15.Berlow S, Bachmann RP, Berry GT, et al. Betaine therapy in homocystinuria. Brain Dysfunction. 1989;2:10–24. [Google Scholar]
- 16.Brens CM, Serra JD, Tomas MLC, Gomez AMG, Monegal MR, Busca AV. Homocistinuria. Eficacia del tratamiento con piridoxina, acido folico y betaina. An Esp Pediatr. 1993;39:37–41. [PubMed] [Google Scholar]
- 17.Kishi T, Kawamura I, Harada Y, et al. Effects of betaine on S-adenosylmethionine levels in the cerebrospinal fluid in a patient with methyltetrahydrofolate reductase deficiency and peripheral neuropathy. J Inher Metab Dis. 1994;17:560–565. doi: 10.1007/BF00711591. [DOI] [PubMed] [Google Scholar]
- 18.Gahl WA, Bernadini I, Chen S, Kurtz D, Horvath K. The effect of oral betaine on vertebral body bone density in pyridixine-non-responsive homocystinuria. J Inher Metab Dis. 1988;11:291–298. doi: 10.1007/BF01800372. [DOI] [PubMed] [Google Scholar]
- 19.Sakura N, Ono H, Nomura S, Ueda H, Fujita N. Betaine dose and treatment intervals for homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. J Inher Metab Dis. 1998;21:84–85. doi: 10.1023/a:1005331902497. [DOI] [PubMed] [Google Scholar]
- 20.Schwahn B, Hafner D, Hohlfeld T, Laryea MD, Wendle U. Pharmacokinetics of oral betaine in healthy subjects. J Inher Metab Dis. 2000;23(Suppl 1):66, 131–O. doi: 10.1046/j.1365-2125.2003.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laryea MD, Steinhagen F, Pawliczek S, Wendle U. Simple method for the routine determination of betaine and DMG in blood and urine. Clin Chem. 1998;44:1937–1941. [PubMed] [Google Scholar]
- 22.Moat SJ, Bonham JR, Tanner MS, Allen JC, Powers HJ. Recommended approaches for the laboratory measurement of homocysteine in the diagnosis and monitoring of patients with hyperhomocysteinaemia. Ann Clin Biochem. 1999;36:372–379. doi: 10.1177/000456329903600311. [DOI] [PubMed] [Google Scholar]
- 23.Piotrovsky VK. Indirect pharmacodynamic response models do not require any parametric pharmacokinetic model to be fitted to effect time data. Meth Find Exp Clin Pharmacol. 1997;19:723–729. [PubMed] [Google Scholar]
- 24.Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related errors of metabolism. Metabolism. 1993;42:1448–1460. doi: 10.1016/0026-0495(93)90198-w. [DOI] [PubMed] [Google Scholar]
- 25.Walson PD. Paediatric clinical pharmacology and therapeutics. In: Speight TM, Holford NG, editors. Avery’s Drug Treatment. 4. Auckland: Adis International; 1997. pp. 127–172. [Google Scholar]
- 26.Bonate PL. Clinical trial simulation in drug development. Pharm Res. 2000;17:252–256. doi: 10.1023/a:1007548719885. [DOI] [PubMed] [Google Scholar]