Abstract
Aims
Clinical studies comparing nebulized drug delivery systems could be flawed because of the high doses used. We have compared lung and total systemic delivery of salbutamol from a nebuliser with that from a metered dose inhaler by measuring urinary recovery of drug and its sulphate metabolite.
Methods
Twelve healthy volunteers provided urine samples at 0, 0.5, 1, 2, 4, 8, 12 and 24 h after the start of dosing. Formulations and doses were 5 × 100 µg oral solution (ORAL), 5 × 100 µg from a metered dose inhaler (MDI), 2.5 mg using a nebuliser (NEB) and NEB with 25 g oral charcoal (NEBC). Each study phase was separated by 7 days and the order of dosing was randomized.
Results
Mean (s.d.) 30 min urinary salbutamol excretion after ORAL, MDI, NEB and NEBC was 0.4 (0.7), 12.1 (3.7), 15.0 (3.9) and 18.2 (5.7) µg, respectively (all P<0.001 compared with ORAL). When normalized for the dose available for inhalation from MDI, NEB and NEBC, the mean (s.d.) 30 min urinary excretion of salbutamol was 2.4 (0.7), 2.9 (0.6) and 2.7 (0.6)%, respectively, with a mean ratio (90% confidence interval) between NEB and NEBC, of 95.3 (91.1, 99.5)%. The mean (s.d.) excretion of salbutamol plus its metabolite over 24 h post ORAL, MDI, NEB and NEBC dosing was 297.9 (38.3), 290.3 (41.4), 266.5 (44.6) and 151.7 (40.9) µg, respectively. The mean ratio (90% confidence interval) between MDI and ORAL, and NEB and ORAL were 97.5 (94.1, 101.0) and 90.7 (81.2, 100.2)%, respectively. The NEBC data indicate that 6.07 (1.04)% of the nominal nebulized dose was delivered to the lungs.
Conclusions
The 30 min urinary recovery of salbutamol, an index of the relative systemic bioavailability of salbutamol following inhalation, can be used to compare the lung deposition of nebulized systems. Similarly, the urinary 24 h recovery of salbutamol plus its metabolite, an index of the relative systemic bioavailability of salbutamol following inhalation, can be used to compare the delivery of nebulized drug to the systemic circulation.
Keywords: charcoal, metered dose inhalers, nebulisers, oral, urinary salbutamol
Introduction
Nebulisers are used widely in the management of acute and chronic asthma and chronic obstructive pulmonary disease. The cost per treatment is much more expensive than via a metered dose inhaler (MDI), because the time to administer the drug is considerably longer and the doses higher using a nebuliser. High doses are used to ensure maximal response [1] because nebulisers are an inefficient method to deliver drugs to the lungs [2]. Studies comparing MDIs to nebulisers are limited. Newnham et al. found that in eight patients with moderately severe asthma, nebulised terbutaline therapy produced the same bronchodilation as drug delivered with an MDI [3]. Plasma terbutaline concentrations were lower when using the nebuliser, suggesting that less was delivered to the airways than following inhalation [4]. However, the lower plasma terbutaline concentrations after nebulisation could also be due to less drug being swallowed. The similar responses from the two methods of administration could be explained by the attainment of maximal bronchodilation. This highlights a problem when clinical studies use doses at the top of the dose–response relationship to compare different methods of inhalation or inhaled drugs. The measurement of plasma salbutamol concentrations during the lag phase of oral absorption has been shown to be useful for the comparison of the relative lung deposition using different inhalation methods [4, 5]. Such plasma salbutamol concentration data have shown that a Ventsteam system delivers more drug to the airways than a Hudson nebuliser [5]. We have shown that the amount of salbutamol excreted in the urine over the first 30 min after the commencement of an inhaled dose is a useful index of the relative lung deposition of the drug [6], but we have not extended our studies to nebulized therapy. A linear relationship between salbutamol urinary recovery and inhaled dose from a metered dose inhaler has been established [7]. Furthermore, the 30 min urinary salbutamol recovery has been shown to correlate with the dose of methacholine required to reduce the FEV1 by 20% (PD20) in asthmatic patients following inhalation of one and two doses of salbutamol from an MDI [8]. In addition it has recently been shown that this urinary measurement can be used when the inhalation period is 8 min, and thus could be applied to drug delivery by nebulisers [9]. Accordingly we have therefore carried out an evaluation of the feasibility of using urinary salbutamol excretion to assess the performance of nebulisers. The nebuliser system chosen was one commonly used in local hospitals at the time of the study.
Methods
Local Ethics Research Committee approval was granted and all volunteers gave written consent. On different study days each volunteer received 5 × 100 µg oral doses (each dissolved in 20 ml of water) of salbutamol (ORAL) or 5 × 100 µg doses of salbutamol from a Ventolin (Glaxo Wellcome plc, UK) metered dose inhaler (MDI). Using the latter all volunteers exhaled slowly to attain residual lung volume, put the inhaler (immediately after shaking it) into their mouth, sealed their lips tightly round the mouthpiece, and started to inhale slowly. At the commencement of inhalation they activated the inhaler and continued to inhale slowly (through their mouth) to total lung capacity. This took 5–10 s to complete. Subjects then held their breath for 10 s before breathing normally. Each 100 µg dose was separated by 1.25 min. Subjects also received 2.5 mg (in 2.5 ml) of salbutamol solution (Ventolin Nebules, GlaxoWellcome, UK) from a nebuliser (NEB). This was repeated, on a separate day, with the oral administration of 25 g activated charcoal (NEBC). The latter (Carbomix, Penn Pharmaceuticals, UK) was given as 10 g in 50 ml before dosing with salbutamol, 10 g immediately after dosing and 5 g 5 min later. The nebuliser system used was a Micro Neb III chamber (Lifecare Hospital Supplies, UK) attached to a Medix AC 2000 Hi-Flow Compressor (Medix Ltd, UK). For the nebulised dose each volunteer wore a nose clip to prevent nasal breathing and subjects inhaled via a mouthpiece using normal tidal breathing. All exhaled air passed through a filter to retain the expired salbutamol. Nebulisation continued for 5 min. Afterwards each chamber, mouthpiece and exhalation filter were washed to recover all the salbutamol. The fraction of drug not inhaled was determined using high performance liquid chromatography [6]. The mean (s.d.) static and dynamic flow rates for this system were 13.50 (0.53) l min−1 and 9.06 (0.35) l min−1, respectively. The mean (s.d.) MMAD (mass median aerodynamic diameter) of the aerolized dose from this system was 3.18 (0.27) µm with a respirable dose of 77 (2)% of the amount available for inhalation.
The order of the four study doses was randomized and there was a 7 day washout between each study day. Immediately before dosing, volunteers voided their bladder and then provided a urine sample at 30 min after the start of the first dose/nebuliser switch on. Urine samples were then provided at 1, 2, 4, 8, 12 and 24 h. Amounts of salbutamol and its sulphate metabolite in the urine were determined using high performance liquid chromatography [6]. The limit of quantification was 0.5 µg l−1 and all measured concentrations were above 50 µg l−1. The intra- and inter-day precision analysis for the 50 µg l−1 of salbutamol in urine were 4.1 and 10.3%, respectively.
One way analysis of variance was used to determine any statistically significant differences in the 30 min and 24 h salbutamol urinary excretions between the methods of drug delivery. Bonferroni corrections were applied with the one-way analysis of variance to determine the difference between each pair of methods. The mean ratio (90%) confidence intervals for paired comparisons was determined as appropriate.
Results
Twelve (six females) healthy volunteers completed the study. Their mean (s.d.) age, height and weight was 33.6 (10.9) years, 1.75 (0.1) m and 72.4 (9.5) kg. The pH of all the urine samples was <6.5. Thus, pH-dependent renal clearance of salbutamol via passive tubular reabsorption was unlikely [6]. The mean (s.d.) excretion rates of salbutamol plus its sulphate metabolite, indicating a longer time to peak excretion following oral dosing are shown in Figure 1. Table 1 shows the amounts of salbutamol excreted in the urine between 0 and 30 min. The amounts excreted in the MDI, NEB and NEBC phases were all greater (P<0.001) than after oral administration. The ratios of the amount of drug recovered using MDI, NEB and NEBC with respect to ORAL are 30.3, 37.4 and 45.5, respectively. The mean ratio (90% of confidence interval) for NEB to NEBC was 83.9 (76.7, 91.1)% and for NEB to MDI was 135.0 (106.9, 163.1)%.
Figure 1.
Mean (s.d.) urinary excretion of salbutamol and its sulphate metabolite following administration orally (ORAL, ▴), by metered dose inhaler (MDI, ▪), nebuliser (NEB, ♦) and nebuliser with oral charcoal (NEBC, ○).
Table 1.
The amount of salbutamol (µg) excreted in the urine over the first 30 min after dosing orally (ORAL)), by metered dose inhaler (MDI), nebuliser (NEB) and nebuliser with oral charcoal (NEBC).
| Subject | ORAL | MDI | NEB | NEBC |
|---|---|---|---|---|
| 1 | 0 | 14.57 | 10.84 | 17.36 |
| 2 | 0 | 5.95 | 14.12 | 14.48 |
| 3 | 0 | 16.39 | 12.51 | 15.77 |
| 4 | 0.68 | 14.72 | 21.61 | 25.94 |
| 5 | 0 | 9.95 | 14.80 | 16.26 |
| 6 | 0 | 16.87 | 16.38 | 14.20 |
| 7 | 0 | 11.05 | 19.34 | 28.77 |
| 8 | 0 | 14.04 | 11.80 | 14.56 |
| 9 | 0 | 9.81 | 8.47 | 9.46 |
| 10 | 2.39 | 8.40 | 13.78 | 16.57 |
| 11 | 1.04 | 8.22 | 17.13 | 22.64 |
| 12 | 0.63 | 15.60 | 18.91 | 23.35 |
| Mean | 0.40 | 12.13 | 14.97 | 18.20 |
| (s.d.) | (0.72) | (3.66) | (3.85) | (5.69) |
Table 2 shows the total urinary excretion of salbutamol and its metabolite over 24 h postdose. Statistical analysis revealed no difference between recoveries using MDI and ORAL or NEB and ORAL, with mean ratios (90% confidence intervals) of 97.5 (94.1, 101.0)% and 90.7 (81.2, 100.2)%, respectively. Similarly, there was no difference between recoveries using NEB and MDI, with a mean ratio (90% confidence interval) of 93.9 (81.9, 105.8)%. The mean (s.d.) fraction of metabolite recovered expressed as a percentage of the total urinary excretion over 24 h after ORAL, MDI, NEB and NEBC was 55.7 (8.0), 48.1 (8.9), 56.4 (6.5) and 24.4 (4.7)%, respectively. The mean (s.d.) 24 h urinary excretion of salbutamol and metabolite following NEBC, when expressed as a percentage of the nominal dose, was 6.07 (1.64)%, which is equivalent to the pulmonary absorbed fraction following nebulization.
Table 2.
Total urinary salbutamol and its sulphate metabolite (µg) in the 24 h post dose. See legend to Table 1 for the definition of abbreviations.
| Subject | ORAL | MDI | NEB | NEBC |
|---|---|---|---|---|
| 1 | 225.9 | 240.7 | 207.9 | 147.2 |
| 2 | 267.4 | 266.1 | 296.5 | 129.2 |
| 3 | 257.4 | 219.3 | 319.3 | 146.8 |
| 4 | 332.7 | 317.3 | 301.5 | 224.4 |
| 5 | 302.2 | 304.0 | 208.0 | 151.4 |
| 6 | 285.9 | 257.2 | 259.4 | 98.2 |
| 7 | 292.2 | 268.7 | 303.8 | 173.0 |
| 8 | 297.7 | 286.7 | 193.1 | 99.6 |
| 9 | 370.3 | 351.1 | 252.1 | 145.6 |
| 10 | 336.0 | 351.9 | 255.7 | 113.7 |
| 11 | 314.5 | 309.2 | 318.8 | 171.6 |
| 12 | 292.0 | 311.6 | 282.1 | 219.7 |
| Mean | 297.9 | 290.3 | 266.5 | 151.7 |
| (s.d.) | (38.3) | (41.4) | (44.6) | (40.9) |
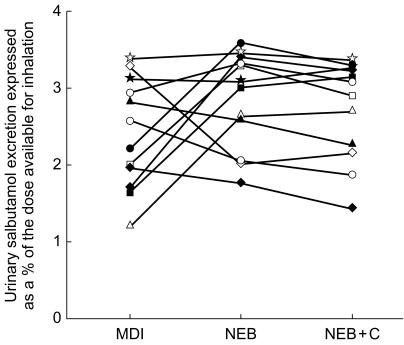
Table 3 shows that the amount available for inhalation during NEBC was greater than that during NEB, which explains the difference between the 0–30 min urinary salbutamol excretion between these two study designs (Table 1). The coefficient of variation for the amounts available for inhalation after NEBC and NEB were 5.0 and 11.0%, respectively. Normalization of these values for the dose available for inhalation is shown in Figure 2 (the MDI dose was assumed as the nominal dose). The mean (s.d.) normalized values are 2.4 (0.7), 2.9 (0.6) and 2.7 (0.6)% after MDI, NEB and NEBC, respectively (Figure 2). The coefficents of variation for these values are 30.0, 22.1 and 23.5%, respectively. Statistical analysis revealed no significant difference between these normalized values. The mean ratio (90% confidence intervals) of the value after NEB to that after NEBC was 104.7 (100.5, 108.9)%. Similar ratios of values after MDI to those after NEB and NEBC were 89.0 (70.4, 107.6)% and 94.0 (74.6, 113.5)%, respectively. When normalized for the amount available the mean (s.d.) amounts of salbutamol plus metabolite excreted 24 h postdose were 59.6 (7.7), 58.1 (8.3), 50.7 (6.8) and 22.3 (4.6)% after ORAL, MDI, NEB and NEBC, respectively. The coefficients of variation for these 24 h recoveries following MDI, NEB and NEBC were 14.3, 13.4 and 19.6%, respectively.
Table 3.
Mean (s.d.) in vitro nebulisation data (all amounts in µg of salbutamol). The amount available for inhalation is 2.5 mg minus the other three values. See legend to Table 1 for the definition of abbreviations.
| NEB | NEBC | |
|---|---|---|
| Remaining in nebuliser chamber | 1440.7 (72.0) | 1227.2 (135.5) |
| T-mouthpiece | 25.3 (9.0) | 35.8 (20.5) |
| Exhalation filter | 537.8 (54.1) | 556.7 (101.3) |
| Available for inhalation | 527.8 (76.9) | 680.4 (153.9) |
Figure 2.
Individual urinary excretion of salbutamol in the first 30 min after the commencement of dosing, normalized for the amount available for inhalation. See legend to Figure 1 for the definition of abbreviations.
Discussion
The amount of salbutamol excreted into the urine in the first 30 min after dosing will be determined by that available for inhalation and subsequently delivered to the lungs followed by renal clearance. In addition a proportion of a dose available for inhalation will be swallowed, absorbed from the gut and metabolized. Because of these factors, intersubject variability in pharmacokinetics is high even when using healthy volunteers. The smaller intersubject variability in the amount of salbutamol excreted over 24 h following the metered dose inhaler and the nebuliser compared with the recovery after 30 min, could suggest that lung deposition is particularly variable, as this would exert more influence on the amounts excreted during the first 30 min.
Our data indicate that the dose available for inhalation during nebulisation shows substantial variability, which may be explained by differences in such factors as nebuliser type, fill volume and breathing pattern. The amount of drug available to subjects during nebulisation after oral charcoal administration (NEBC) was consistent higher and more variable than without charcoal (NEB), findings which are difficult to explain. The data for oral charcoal administration, are consistent with greater amounts of salbutamol excreted in the urine both over the first 30 min and 24 h and the larger variability in recovery. When normalized for the amounts available for inhalation, the degree of variability in the 30 min and 24 h excretion after nebulisation and with oral charcoal, is decreased.
Owing to a larger dose available for inhalation with nebulisation when oral charcoal was used, excretion data were normalized to the available dose. The mean (90% confidence limit) 30 min urinary excretion ratio following nebulisation compared with nebulisation with oral charcoal was 104.7 (100.5, 108.9)%, indicating it is not necessary to administer charcoal to block oral drug absorption when comparing the relative deposition of nebulisers.
Our data indicate that nebulisers could provide a higher dose of salbutamol to the lung than MDIs. However, usually about 10% of the expelled dose is left in the mouthpiece. Allowing for this, the dose available from the MDI would be almost identical to that delivered by the nebuliser.
The Micro-Neb III is a constant output jet nebuliser such that the dose is released throughout the nebulisation period. However, a high percentage of the aerosol produced may be wasted during expiration [10]. Our results are consistent with this finding, and also show that approximately 50% of the nebulised dose is trapped inside the chamber and thus unavailable to the patient. Similar findings have been reported elsewhere [2]. Overall, only 21–27% of the nominal dose is available for the subject to inhale.
A lower value for the 24 h urinary excretion of salbutamol following nebulisation compared with that using the metered dose inhaler suggests that despite the higher dose available for inhalation with the nebuliser, less is swallowed. The percentage of metabolite with respect to total 24 h excretion following nebulisation and with subjects taking oral charcoal is much lower than the other three methods of drug delivery which suggests that the swallowed fraction is subject to a high first pass effect. Comparison of the 24 h urinary excretion using the nebuliser with and without charcoal suggests that approximately 50% of the dose available for inhalation is deposited in the lung and 50% swallowed.
The 24 h urinary excretion of salbutamol and its metabolite using the nebulised method with oral administration of charcoal would represent the fraction of the pulmonary delivered dose excreted by the kidneys. This values provides an indication of the total lung dose following nebulisation. The mean (s.d.) recovery of 6.07 (1.64)% of the nominal dose compares with gamma scintigraphy values of 10% for an Acorn Nebuliser [11], 9% for a Turret Nebuliser [12], and 3% for an Inspiron [12]. These differences will be due to the different nebuliser systems used. They may also be due to gamma scintigraphy imaging being performed immediately after inhalation and hence before mucociliary clearance which may be up to 20% [13].
Overall the present study has demonstrated that five doses from a metered dose inhaler will deliver similar amounts of drug to the lungs and the systemic circulation as the nebulised system using a dose of 2.5 mg. Other nebulised systems may be more or less efficient. At present there is little data (both in vitro and in vivo) on the different nebulised systems. The choice of a nebuliser is often dependent on availability at the time of prescribing which is determined by cost. Variable output rates, doses and particle sizes could result in large differences in lung deposition [5, 12, 14–16]. Thus, a simple method, as reported here, to evaluate the efficiency of these devices is required. The results of the present study indicate that the simple noninvasive urinary recovery method originally proposed for MDIs [6’ and subsequently used for MDIs attached to spacers [17] and dry powder inhalers [18], can also be used for nebulisers. At present healthy volunteers have been used to compare these devices. The subjects will not show significant intra-individual variability in airway calibre as would patients with obstructive airways disease. A pharmacokinetic study has shown that the greater restriction to airflow, the lower the relative lung deposition following nebulisation [5]. Therefore, patient studies using the urinary salbutamol method are required.
A recent report from the National Institute of Clinical Excellence has concluded that nebulisers provide little or no additional benefit compared to a metered dose inhaler [19]. The present results, indicate that jet nebulisers are inefficient methods to deliver drugs into the airways and thus in stable patients perhaps their use should be discouraged. Nevertheless, for the breathless patient with an acute exacerbation they may be the only available option for stabilizing the patient and thus, should be reserved for this purpose.
Acknowledgments
This study was funded by an internal University of Bradford Studentship.
References
- 1.Yates DY, Peters MJ, Keatings V, Thomas PS, Barnes PJ. Reduced dose salbutamol in comparison with standard dosage for symptom relief in asthma. Eur Respir J. 1995;8:1847–1851. doi: 10.1183/09031936.95.08111847. [DOI] [PubMed] [Google Scholar]
- 2.Clay MM, Newman SP, Clarke SW, Pavia D, Lennard Jones T. Assessment of jet nebulisers for lung aerosol therapy. Lancet. 1983;ii:592–594. doi: 10.1016/s0140-6736(83)90679-7. [DOI] [PubMed] [Google Scholar]
- 3.Newnham DM, Lipworth DJ. Nebuliser performance, pharmacokinetics, airways and systemic effects of salbutamol given via a novel nebuliser delivery system (Ventstream) Thorax. 1994;49:762–770. doi: 10.1136/thx.49.8.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cushley MJ, Lewis RA, Tattersfield AE. Comparison of three techniques of inhalation in the airway response to terbutaline. Thorax. 1983;38:908–913. doi: 10.1136/thx.38.12.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipworth BJ, Clark BJ. Effects of airway calibre on lung delivery of nebulised salbutamol. Thorax. 1997;52:1036–1039. doi: 10.1136/thx.52.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following inhalation. Br J Clin Pharmacol. 1992;34:311–315. doi: 10.1111/j.1365-2125.1992.tb05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomlinson HS, Corlett SA, Chrystyn H. Effect of dose on the relative lung bioavailability of salbutamol. J Aerosol Med. 1995;8:P193. [Google Scholar]
- 8.Chrystyn H, Allen MB, Corlett SA, Tomlinson HS. Simultaneous measurement of pharmacodynamic and pharmacokinetic parameters which can be used to evaluate the equivalence of inhaled salbutamol. Am J Respir Crit Care Med. 1998;157:A636. [Google Scholar]
- 9.Silkstone VL, Corlett SA, Chrystyn H. Relative bioavailablity of salbutamol to the lung when administartion is prolonged. Br J Clin Pharmacol. 2000;50:281–284. doi: 10.1046/j.1365-2125.2000.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax. 1997;52(Suppl 2):S31–S44. doi: 10.1136/thx.52.2008.s31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zainudin BM, Bibbiscombe M, Tolfree SEJ, Short M, Spiro SG. Comparison of bronchodilator response and deposition patterns of salbutamol inhaled from a pressurised metered dose inhaler, as a dry powder, and as a nebulised solution. Thorax. 1990;45:469–473. doi: 10.1136/thx.45.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MA, Newman SP, Bloom R, Talaee N, Clarke SW. Delivery of albuterol and ipratropiom bromide from two nebulized systems in chronic stable asthma. Efficacy and pulmonary deposition. Chest. 1989;96:1–10. doi: 10.1378/chest.96.1.6. [DOI] [PubMed] [Google Scholar]
- 13.Borgstrom L, Newman SP, Weisz AWB, Moren F. Pulmonary deposition of inhaled terbutaline: Comparison of scanning gamma camera and urinary excretion methods. J Pharm Sci. 1992;81:753–755. doi: 10.1002/jps.2600810807. [DOI] [PubMed] [Google Scholar]
- 14.Niven RM, Brain JD. Some functional aspects of air jet nebulisers. Int J Pharm. 1994;104:73–85. [Google Scholar]
- 15.Faurisson F, Dessanges JF, Grimfield A, et al. Nebuliser performance. AFCM study. Respiration. 1995;62:13–18. doi: 10.1159/000196488. [DOI] [PubMed] [Google Scholar]
- 16.Meurs MF. The national use of nebulisers in clinical practice. Eur Respir J. 1997;44:189–197. [Google Scholar]
- 17.Hindle M, Chrystyn H. Relative bioavailability of salbutamol to the lung following inhalation using metered dose inhaler methods and spacer devices. Thorax. 1994;49:549–553. doi: 10.1136/thx.49.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hindle M, Newton DAG, Chrystyn H. Dry powder inhalers are bioequivalent to metered dose inhalers: a study using a new urinary albuterol (salbutamol) assay technique. Chest. 1995;107:629–633. doi: 10.1378/chest.107.3.629. [DOI] [PubMed] [Google Scholar]
- 19.Payne N, Beard S, Brocklebank D, Ram F, Wright J, Taylor R. Clinical and cost effectiveness of inhaler devices for children with chronic asthma. http://server1.nice.org.uk/nice-web/pdf/asthma.pdf.