Cytochrome P450 2C9 (CYP2C9) is a polymorphic enzyme that catalyses the metabolism of many clinically used drugs [1]. Prediction of CYP2C9 activity in vivo might prove to be of clinical importance in patients treated with CYP2C9 substrates with a narrow therapeutic index, such as warfarin and phenytoin [2–4]. We recently proposed that losartan, a selective angiotensin II receptor antagonist, might be used as a specific probe drug for CYP2C9 in vivo and in vitro [5, 6]. The carboxylic acid metabolite of losartan, E-3174, is produced specifically by CYP2C9 [6, 7]. Losartan oxidation in vivo was found to be decreased 2-, 3-, and 40-fold, in individuals genotyped as CYP2C9*1/*3, *2/*3 or *3/*3, respectively, compared with individuals genotyped as CYP2C9*1/*1 [5]. Moreover, there was a good correlation between the plasma AUClosartan/AUCE-3174 ratio and the losartan/E-3174 recovery ratio in urine collected for 8 or 24 h after drug intake [5]. In the present study, the intraindividual variability of this ratio was investigated.
Twenty-three healthy Swedish subjects (female/male: 12/11) with a mean age of 29 years (24–47 range) and 70 kg mean body weight (49–84 range) participated in the study. The subjects were genotyped with respect to CYP2C9*1, *2, and *3 [8]. Experimental conditions followed the protocol by Yasar et al. [5]. In brief, the procedure included collection of urine samples for 8 h following intake of a single 25 mg oral dose of losartan (CozaarR, Merck Sharp & Dohme). Analysis of losartan and E-3174 in urine was performed as described previously [5]. The coefficients of variation (CVs) of the losartan and E-3174 assays were less than 10% and 8%, respectively. The losartan/E-3174 ratio was calculated from the molar recoveries of losartan and E-3174 in the 0–8 h urine collection. The pH of urine samples was measured with Metrohm 691 pH Meter (Herisau, Switzerland). The procedure was repeated in 13 of the subjects with genotypes CYP2C9*1/*1 (6 subjects), *1/*2 (3), *1/*3 (2), *2/*2 (1) and *2/*3 (1) after 9–12 months. The correlation between urinary losartan/E-3174 ratios after losartan administration on these two separate occasions was determined using linear regression (STATISTICA 4.3 (StatSoft. Inc. Tulsa, OK, USA). A paired t-test was applied for comparison of the two urinary recovery ratios in the same subjects. CV was defined as standard deviation over mean obtained from two separate observations. For the comparison of different genotype groups (if one way anova was significant) the Tukey HSD posthoc test in STATISTICA 4.3 was applied, and P values of <0.05 were accepted as statistically significant.
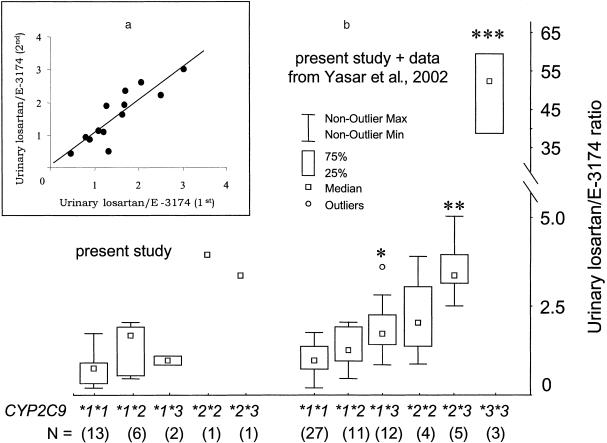
The results show a highly significant correlation (r=0.88, P<0.0001, n=13, slope 1.03) between the individual losartan/E-3174 urinary ratio at two different occasions (Figure 1a). No significant difference between the two measurements was found (P=0.59). There was one outlier (genotype CYP2C9*1/*2) that differed as much as 61% between the two measurements. The CVs were 13% and 9% when the outlier subject was included and excluded, respectively. The average difference was ±10% (8%, 12%, 95% confidence interval). There was no correlation between the urinary losartan/E-3174 ratio and urinary pH (r=0.23, P=0.25).
Figure 1.
(a) The 0–8 h urinary losartan/E-3174 ratio in the same subjects on two different occasions. (b) Box plot of 0–8 h urinary losartan/E-3174 ratios in 23 subjects in the present study and additional to the present data, 39 subjects (17 subjects after 25 mg and 22 subjects after 50 mg single oral dose of losartan) from Yasar et al. 2002 [5]. *P<0.01 compared with *1*1; **P<0.01 compared with *1*1 and *1*2; ***P<0.0001 compared with all other groups.
Figure 1b shows the losartan/E-3174 ratios in different genotypes of CYP2C9. The data from the present subjects in Figure 1a are in accordance with our previous data from 39 healthy Caucasian subjects [5]. Figure 1b also contains a summary of all individuals pheno- and genotyped for CYP2C9 in our laboratory to date, including data from the original study [5]. Importantly, a statistically significant difference in urinary losartan/E-3174 ratios between the different genotypes was found (Figure 1b), where the ratios within each genotype group were very similar in the two studies [5].
In conclusion, the observed good correlation between the metabolic ratios of losartan on two different occasions indicates that individual CYP2C9 activity is stable over time. This suggests that genetic factors governing gene expression and enzyme activity are major determinants of the CYP2C9 phenotype. Furthermore, the low intraindividual variability of this in vivo assay of CYP2C9 makes it suitable for additional studies in larger populations.
Acknowledgments
The authors thank Professor Leif Bertilsson, PhD, for valuable discussions; Lilleba Bohman, and Ulla Pettersson, for the analysis of CYP2C9 variants; Anneli Wahlberg, RN and Katarina Andersson, RN for their excellent assistance in performing the study. Losartan and E-3174 were kindly provided by Merck Sharp Dohme. The study was financially supported by The Swedish Society of Medicine, Swedish Medical Research Council (3902) and Pfizer Ltd. EE is the recipient of a Merck Sharp Dohme fellowship in Clinical Pharmacology.
References
- 1.Streetman DS, Bertino JS, Jr, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 3.Brandolese R, Scordo M, Spina E, Gusella M, Padrini R. Severe phenytoin intoxication in a subject homozygous for CYP2C9*3. Clin Pharmacol Ther. 2001;70:391–394. [PubMed] [Google Scholar]
- 4.Kidd RS, Straughn AB, Meyer MC, et al. Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics. 1999;9:71–80. doi: 10.1097/00008571-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Yasar Ü, Forslund C, Tybring G, et al. Pharmacokinetics of losartan and its metabolite E-3174 in relation to the CYP2C9 genotype. Clin Pharmacol Ther. 2002;71:89–98. doi: 10.1067/mcp.2002.121216. [DOI] [PubMed] [Google Scholar]
- 6.Yasar Ü, Tybring G, Hidestrand M, et al. Role of CYP2C9 polymorphism in losartan oxidation. Drug Metab Dispos. 2001;29:1051–1056. [PubMed] [Google Scholar]
- 7.Stearns RA, Chakravarty PK, Chen R, Chiu SH. Biotransformation of losartan to its active carboxylic acid metabolite in human liver microsomes. Role of cytochrome P4502C and 3A subfamily members. Drug Metab Dispos. 1995;23:207–215. [PubMed] [Google Scholar]
- 8.Yasar Ü, Eliasson E, Dahl ML, et al. Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population [published erratum appears in Biochem Biophys Res Commun 1999; 258: 227] Biochem Biophys Res Commun. 1999;254:628–631. doi: 10.1006/bbrc.1998.9992. [DOI] [PubMed] [Google Scholar]