Abstract
Organization of the inner ear into auditory and vestibular components is dependent on localized patterns of gene expression within the otic vesicle. Surrounding tissues are known to influence compartmentalization of the otic vesicle, yet the participating signals remain unclear. This study identifies Sonic hedgehog (Shh) secreted by the notochord and/or floor plate as a primary regulator of auditory cell fates within the mouse inner ear. Whereas otic induction proceeds normally in Shh−/− embryos, morphogenesis of the inner ear is greatly perturbed by midgestation. Ventral otic derivatives including the cochlear duct and cochleovestibular ganglia failed to develop in the absence of Shh. The origin of the inner ear defects in Shh−/− embryos could be traced back to alterations in the expression of a number of genes involved in cell fate specification including Pax2, Otx1, Otx2, Tbx1, and Ngn1. We further show that several of these genes are targets of Shh signaling given their ectopic activation in transgenic mice that misexpress Shh in the inner ear. Taken together, our data support a model whereby auditory cell fates in the otic vesicle are established by the direct action of Shh.
Keywords: Shh, Pax2, Ngn1, otic vesicle, cochlea, cochleovestibular ganglia
The mammalian inner ear is a complex sensory organ comprised of auditory and vestibular structures that serve to coordinate the senses of hearing and balance, respectively. The inner ear develops over a protracted period originating from a thickening of surface ectoderm, the otic placode, which forms at the level of the prospective hindbrain upon inductive influences from neighboring tissues (Groves and Bronner-Fraser 2000; Ladher et al. 2000). Once induced, the otic placode invaginates to form the otic cup and shortly thereafter pinches off from the surface ectoderm to give rise to the otic vesicle. Over the next several days the otic vesicle undergoes an intense period of proliferation, differentiation, and morphogenesis culminating in the establishment of the ventrally derived auditory component of the inner ear, the cochlea, as well as the more dorsally derived vestibular apparatus, comprising the semicircular canals, utricle, and saccule (for review, see Torres and Giraldez 1998).
Grafting and lineage tracing experiments performed in the chick, in addition to mutational analyses performed in the mouse, have confirmed that the fate of inner ear progenitors is specified early in development (Baker and Bronner-Fraser 2001). By the otic vesicle stage, numerous genes showing restricted patterns of expression compartmentalize the otic epithelium along its three major axes (Fekete and Wu 2002). With respect to the auditory component of the inner ear, the expression of several genes in the ventral and ventromedial regions of the otocyst, including the overlapping expression of the homeobox transcription factors Otx1 and Otx2 as well as the paired-box gene Pax2 mark the location of cochlear duct outgrowth (Fekete and Wu 2002). For vestibular development, the homeobox transcription factors Hmx2, Hmx3, and Dlx5 in the dorsolateral region of the otocyst mark the territory contributing to semicircular canal formation (Fekete and Wu 2002). Loss-of-function studies in the mouse confirm that each of these genes participates actively in establishing regional identity within the inner ear (Acampora et al. 1996, 1999; Torres et al. 1996; Hadrys et al. 1998; Wang et al. 1998, 2001; Depew et al. 1999; Morsli et al. 1999).
In addition to the establishment of regional identity, a number of genes have also been identified that have an impact on the specification of distinct cell fates within the otocyst. The inner ear is a self-contained organ in that the majority of cell types contributing to its development including sensory, nonsensory, and neurogenic are derived from the otic epithelium (Torres and Giraldez 1998). For instance, within the anteroventral region of the otic vesicle, cells expressing the bHLH transcription factors Neurogenin-1 (Ngn1) and NeuroD form the neuronal lineage, giving rise to the cochleovestibular ganglia (cvg; VIIIth cranial nerve; Ma et al. 1998). A similar domain in more posterior regions of the otic vesicle, where Ngn1 and NeuroD expression is absent, marks cells fated to become sensory in character (Fekete and Wu 2002).
Despite our recent understanding of the contribution of individual loci in establishing patterns of growth and differentiation within distinct domains of the otic epithelium, little is known of how these patterning genes are themselves regulated. Surgical manipulations of the otic vesicle in chick, either by altering its location along the anteroposterior neuraxis or rotating its position in situ, have led to a common conclusion that local environment dictates cell fate within the otic epithelium (for review, see Baker and Bronner-Fraser 2001). Further indication that signals from surrounding tissues impact on otic vesicle patterning comes from studies of mouse mutants showing inner ear phenotypes that are a consequence of altered gene function in the neural tube (Mark et al. 1993; McKay et al. 1996; Gavalas et al. 1998; Dupe et al. 1999; Niederreither et al. 2000). Moreover, ablation of the ventral or dorsal neural tube results in an expansion or restriction, respectively, in the expression of Lmx1b, a marker of the dorsal otocyst, supporting the regional segregation of patterning activities within the neural tube (Giraldez 1998).
The notochord and floor plate are sources of the secreted protein Sonic hedgehog (Shh) that functions in both short- and long-range signaling events to promote growth and differentiation of progenitor cells in the ventral neural tube and paraxial structures including the somite (Jessell 2000; Bailey et al. 2001). The juxtaposition of the otic vesicle with respect to the neural tube is similar to that of the somite at more anterior levels of the embryonic axis. Given that the inner ear is reliant on signals from the ventral neural tube and/or notochord for its formation and that notochord-derived Shh signaling is required for patterning paraxial structures, we assessed whether Shh plays an active role in inner ear development by assessing loss- and gain-of-function Shh mutants.
Initiation of otic development proceeded normally in Shh−/− embryos, whereas morphogenesis of the inner ear was greatly perturbed by midgestation. In mouse embryos lacking Shh function, ventral otic derivatives including the cochlear duct and cvg failed to develop. The origin of the inner ear defects in Shh−/− embryos could be traced back to alterations in cell fate specification within the ventral otic epithelium and periotic mesenchyme at earlier stages of inner ear development. Genes previously attributed with required functions in the specification of cochlear (Pax2, Otx1, and Otx2), neuroblast (Ngn1, NeuroD), and chondrogenic (Brn4, Tbx1) lineages were identified in our study as being dependent on Shh. To determine whether Shh is sufficient to induce ventral otic fates, we analyzed a new transgenic mouse line that results in the misexpression of Shh in the otic vesicle. A phenotype reciprocal to that manifested by Shh−/− embryos ensued, namely a loss of dorsal (vestibular) structures at the expense of expanded ventral (auditory) cell fates. Further assessment of this gain-of-function mutant identified Pax2, Ngn1, Brn4, and Tbx1 as bona fide target genes of Shh signaling for inner ear development. Our data thus support a model whereby auditory cell fates in the otic vesicle are established by the direct action of Shh signaling from the notochord and/or floor plate.
Results
Aberrant inner ear morphogenesis in Shh−/− embryos
A simple assay to assess the anatomy of the inner ear during development relies on filling the lumen of the membranous labyrinth with latex paint and observing the three-dimensional structures of the inner ear by conventional light microscopy (Martin and Swanson 1993). To determine the impact of Shh signaling on inner ear morphogenesis, this paint-fill technique was applied to wild-type and Shh−/− embryos at 15.5 days postconception (dpc), a stage when the majority of inner ear structures have completed their development. In embryos lacking Shh, paint-fill analysis revealed multiple irregularities to the morphology of the inner ear (Fig. 1). Instead of the three prominently displayed semicircular canals normally present in wild-type embryos, Shh−/− mutants possess poorly formed posterior and anterior canals, with the lateral canal being absent (Fig. 1a,b). At the gross anatomical level, no distinct development of the five vestibular sensory chambers was evident in Shh−/− embryos including the three ampullae, utricle, and saccule (Fig. 1a,b), even though distinct sensory patches were found within the main membranous chamber (see below). The endolymphatic duct was also noted to be missing in Shh−/− embryos at this stage. A particularly striking feature of the inner ear phenotype in embryos lacking Shh was the complete absence of the cochlear duct. In place of this ventral inner ear derivative was a rudimentary structure resembling the otocyst, thus suggesting an early arrest in the developmental progression of ventral inner ear structures in Shh−/− embryos (Fig. 1a,b).
Figure 1.

Morphological assessment of Shh−/− inner ears. The membranous labyrinth of wild-type (a,c,e) and Shh−/− (b,d,f) inner ears is shown after injection with latex paint. (a,b) Lateral views of inner ears from 15.5 dpc embryos. Note that the inner ear from the Shh−/− embryo appears to lack all six sensory chambers including the cochlear duct, utricule, saccule, and three ampullae. The lateral semicircular canal and endolymphatic duct are also absent in Shh mutants. Lateral (c,d) and dorsal (e,f) views of inner ears from 12.5 dpc embryos. The primordial structures for the semicircular canals, the vertical (vp) and horizontal (hp) outpockets are present in Shh−/− embryos, but resorption is not yet evident in these structures. aa, anterior ampulla; asc, anterior semicircular canal; cc, common crus; cd, cochlear duct; ed, endolymphatic duct; la, lateral ampulla; lsc, lateral semicircular canal; pa, posterior ampulla; psc, posterior semicircular canal; s, saccule; u, utricle. A, anterior; D, dorsal; M, medial. Bar, 100 μm.
The lack of both medial (endolymphatic duct) and lateral (lateral semicircular canal and ampulla) structures in Shh mutants at E15.5 suggested that the specification of the medial/lateral axis might be perturbed in these mice. This prompted us to examine inner ears at E12.5, to determine whether earlier phases of semicircular canal formation were altered in Shh−/− embryos. Each semicircular canal develops from an epithelial outpocket of the otocyst. Over time, the opposing epithelia in the central region of the outpocket come together, fuse, and resorb, leaving behind a tube-shaped canal (Martin and Swanson 1993). In the wild-type inner ear, this process is almost complete by 12.5 dpc. A delay in resorption was observed in the prospective anterior and posterior canals from age-matched Shh−/− embryos (Fig. 1c,d). Interestingly, despite being absent at later stages, the horizontal outpocket, which forms the lateral canal, was present in Shh−/− embryos at E12.5 (Fig. 1c–f). No evidence of endolymphatic duct outgrowth was detected at E12.5 in Shh−/− embryos; however, initial outgrowth of this structure was observed at E10.5 (Fig. 3k,l, see below). These results suggest that specification of the medial/lateral axis of the inner ear is normal in Shh−/− embryos, whereas morphogenesis of the endolymphatic duct and semicircular canals is dependent on Shh.
Figure 3.
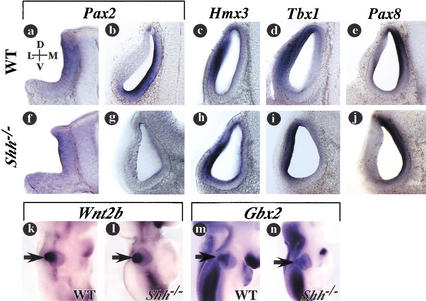
Shh regulates the expression of markers along the mediolateral axis of the otic vesicle. Assessment of otic markers in wild-type (a–e,k,m) and Shh−/− (f–j,l,n) embryos by in situ hybridization. (a,b,f,g) Transverse sections through the otic vesicles probed for Pax2 expression at otic cup (a,f; 8.5 dpc) and otic vesicle (b,g; 9.5 dpc), stages of development. Despite being expressed at otic cup stages, Pax2 is not maintained in the medial side of the otic vesicle at 9.5 dpc in Shh−/− embryos. (c,h) Hmx3 expression along the lateral wall of the otic vesicle is unaffected in Shh−/− embryos. (d,i) Tbx1; neither the lateral epithelial expression of Tbx1 nor the medial epithelial expression of Pax8 (e,j) were altered in Shh−/− embryos at 9.5 dpc. (k–n) Lateral views of embryos stained by whole-mount in situ hybridization for Wnt2b (k,l) and Gbx2 (m,n) at 10.5 dpc. Arrows mark the initial outgrowth of the endolymphatic duct.
Shh is required to pattern the otic vesicle along the dorsal-ventral axis
With the complete lack of a coiling cochlea in late-gestational-stage Shh mutant embryos, we decided to address the molecular nature of these defects at otic vesicle stages of development. The ventralmost cells of the otic vesicle are fated to give rise to the cochlear duct and are marked by the overlapping expression of the homeobox genes Otx1 and Otx2 (Morsli et al. 1999). Otx1 expression also extends dorsally along the lateral wall of the otic vesicle where it contributes to the formation of the lateral semicircular canal and ampulla (Morsli et al. 1999). To address whether Shh signaling is required in the ventralmost cells of the otic vesicle, we surveyed the expression of Otx genes in Shh−/− embryos. Interestingly, the expression of Otx2 was not detected in the otic vesicles of Shh−/− embryos either at the time of its initiation at 10.5 dpc or any time thereafter (Fig. 2a,e; data not shown). Moreover, the domain of Otx1 expression was reduced to a small ventral patch (Fig. 2b,c,f,g).
Figure 2.
Shh regulates the expression of markers along the dorsoventral axis of the otic vesicle. Transverse section and whole-mount views of otic vesicles from wild-type (a–d,i–l,q,s,u,w) and Shh−/− (e–h,m–p,r,t,v,x) embryos analyzed for gene expression by RNA in situ hybridization. (a,e) Otx2 is absent from the ventral otic epithelium of Shh−/− embryos; (b,c,f,g) Otx1 expression is reduced in Shh−/− embryos. Red arrowhead in g points to the reduced domain of Otx1 expression. (d,h) Dlx5 is expanded ventrally in the absence of Shh. Brackets mark the distance between the ventral limit of Dlx5 expression and the ventral extent of the otic vesicle. (i,m) Fgf3. (j,k,n,o) Lfng, the ventral shift in Lfng expression in Shh−/− embryos (o) is marked by a red arrowhead. (l,p) Bmp4; red arrowhead points to the Bmp4 expression domain that is shifted ventrally in Shh−/− embryos. (q,r) Gli1and (s,t) Ptc are detected in the otic epithelium and periotic mesenchyme of wild-type but not Shh−/− embryos. Arrowheads in q and s point to faint expression of Gli1 and Ptc in the periotic mesenchyme and medial wall of the otic vesicle. The semblance of expression of Gli1 in r is the result of trapping rather than specific staining. (u,v) Tbx1; expression is detected in the lateral wall of the otic vesicle in both wild-type and Shh−/− embryos. However, the periotic mesenchyme expression of Tbx1 (bracket) is absent in Shh−/− mutants. (w,x) Brn4; expression in the condensing mesenchyme (bracket) is absent from Shh−/− embryos at 10.5 dpc. Asterisk in x marks the branchial arch expression of Brn4, which is unaffected in Shh−/−embryos. All sections are from embryos at 10.5 dpc except d and h, which are from embryos at 9.5 dpc. M, medial; L, lateral; D, dorsal; V, ventral.
The altered expression of Otx1 and Otx2 in Shh−/− embryos could indicate that Shh signaling regulates the transcription of Otx genes, or that Shh is required for the specification of ventral otic vesicle cells that express Otx genes, or both. To begin to distinguish between these possibilities we assessed the expression of additional markers localized along the dorsal-ventral axis of the otic vesicle. If the ventralmost cells of the otic vesicle are not specified in Shh−/− embryos, then a possible outcome could be the ventral expansion of dorsal markers, reminiscent of the neural tube patterning defects observed in Shh−/− embryos (Chiang et al. 1996). The homeobox gene Dlx5, which marks the dorsal half of the otic vesicle, is expanded ventrally in Shh−/− embryos, suggesting that Shh signaling normally antagonizes Dlx5 expression (Fig. 2d,h). This is not a general feature for all dorsal otic markers, as Wnt2b and Lmx1b did not expand ventrally in embryos lacking Shh (Fig. 3k,l; data not shown).
The ventral expansion of Dlx5 expression in the otic vesicles of Shh−/− embryos did not include the ventralmost cells. To determine the identity of these cells, we surveyed the expression of other markers expressed in intermediate regions of the otic vesicle. In the anteroventrolateral compartment of the otic vesicle, Fibroblast growth factor-3 (Fgf3) and high levels of Lunatic Fringe (Lfng) are coexpressed in a sensory competent zone from where a portion of the cvg and possibly the macula of the utricle will form (Fig. 2i,j,k). Low levels of Lfng can also be found in the ventralmost portion of the otic epithelium (Fig. 2j). Cells immediately dorsal to the high Lfng and Fgf3 domains in the lateral otic vesicle express Bone morphogenetic protein-4 (Bmp4; Fig. 2l). In the otic vesicles of Shh−/− embryos, the expression patterns of Fgf3, Lfng, and Bmp4 were each found to shift ventrally, with Fgf3 and high levels of Lfng occupying the ventralmost portion of the otocyst (Fig. 2m–p). The low level of Lfng that normally occupies the ventralmost region of the otic vesicle was absent in Shh−/− embryos (Fig. 2n). Despite the lack of Shh, Bmp4-expressing cells preserved their spatial relationship with Fgf3 and Lfng, lying immediately dorsal in position. From these results we conclude that Shh signaling is required for the specification of the ventralmost cells of the otic vesicle from where the cochlear duct emerges. In the absence of Shh there is an expansion of some dorsal inner ear markers and a shift in the positioning of some markers of lateral otic cell fates.
Shh signaling acts directly on the otic epithelium and periotic mesenchyme
The effects of Hedgehog (Hh) signaling can be detected over short- or long-range distances. Long-range Hh signaling can act directly, by an unresolved mechanism that transports Hh via its lipid linkages over several cell diameters, or indirectly, through a secondary-signal relay (Ingham and McMahon 2001). This raises the question as to whether Shh acts directly or indirectly to pattern the otic epithelium. In this regard we assessed the expression of two transcriptional targets of Shh signaling, the zinc-finger containing transcription factor Gli1 and the Shh receptor Patched (Ptc), both of which serve as molecular readouts for pathway activation (Goodrich et al. 1996). Between 9.5 and 10.5 dpc, Gli1 and Ptc transcripts are expressed broadly in the otic epithelium and ventral periotic mesenchyme (Fig. 2q,s; data not shown). The absence of Gli1 and Ptc expression from these tissues in Shh−/−embryos (Fig. 2r,t) excludes the possibility that other members of the hedgehog family might be signaling to the otic vesicle at these stages, lending support for a direct role of Shh in inner ear development.
With the observation that Shh signaling is active in the periotic mesenchyme, we next determined whether this tissue was also compromised in Shh−/− embryos. The transcription factors Tbx1 and Brn4 are markers of the periotic mesenchyme and condensing mesenchyme, respectively (Fig. 2u,w). Condensation of the mesenchyme initiates within a subpopulation of periotic cells immediately adjacent to the ventral otic epithelium and, subsequently, the mesenchyme will encompass the inner ear to form the bony labyrinth. In the absence of Shh, neither Tbx1 nor Brn4 are detected in the mesenchyme at 10.5 dpc (Fig. 2v,x). In contrast, Tbx1 expression along the lateral wall of the otic vesicle is maintained in the absence of Shh, suggesting that the two inner ear domains of Tbx1 expression are regulated independently (Fig. 2u,v). By 15.5 dpc, Brn4 transcripts are detected in Shh−/−embryos, indicating the existence of an alternate pathway for Brn4 activation (data not shown). Our findings suggest that Shh signaling impacts directly on both the otic epithelium and periotic mesenchyme and identify Tbx1 and Brn4 as two potential targets of the Shh signaling pathway in the development of the inner ear.
Maintenance of Pax2 expression is dependent on Shh
The paired-box gene Pax2 is an early marker of otic fate, expressed initially throughout the otic placode and later within cells along the medial side of the otic vesicle (Nornes et al. 1990; Puschel et al. 1992; Groves and Bronner-Fraser 2000). As it was reported that Pax2−/− embryos lack the cochlear duct, we reasoned that Pax2 expression might be altered in the inner ears of mice lacking Shh (Favor et al. 1996; Torres et al. 1996). Pax2 was expressed at the otic cup stage in Shh−/−embryos but was lost from the medial wall of the otic vesicle by 9.5 dpc (Fig. 3a,b,f,g). The failure to maintain Pax2 expression in the absence of Shh is unlikely to be the only cause for the lack of ventral cells in the otic vesicle of Shh−/− embryos, because Otx2, which is also essential for cochlear development, was absent in Shh−/− mutants (Fig. 2a,e; Morsli et al. 1999). We therefore conclude that the lack of cochlear duct outgrowth in Shh−/− embryos is likely explained by a combination of defects, that is, failure to maintain Pax2 expression in conjunction with the lack of ventral otic cell specification.
In addition to promoting cochlear duct outgrowth, Pax2 has been postulated to participate in the establishment of a compartmental boundary within the otic vesicle, at the dorsal juncture of the medial-lateral axis (Brigande et al. 2000). The homeobox- and T-box-containing transcription factors Hmx3 and Tbx1, respectively, mark the complementary side of the proposed boundary within cells along the lateral wall of the otic vesicle (Fig. 3c,d). A suggested purpose for the creation of such a boundary is to establish a site from where the endolymphatic duct will emerge, at the dorsal intersection between the medial-lateral and anterior-posterior axes (Brigande et al. 2000). If Pax2 is required to maintain the medial-lateral boundary within the otic vesicle, then in its absence Hmx3 and Tbx1 expression should be altered and endolymphatic duct outgrowth inhibited. Interestingly, in the otic vesicles of Shh−/− embryos, the lateral expression of Hmx3 and Tbx1 remained fixed (Fig. 3c,d,h,i). Moreover, initial phases of endolymphatic duct outgrowth occurred normally in Shh−/− embryos as assessed by the dorsal expression of Wnt2b (Fig. 3k,l). The failure to maintain endolymphatic duct outgrowth in Shh−/− embryos may have more to do with the downregulation of Gbx2 at 10.5dpc, than with possible alterations to the compartmental boundary (Fig. 3m,n). The persistence of a medial-lateral boundary within the otic vesicle of Shh−/− embryos may be explained in part by the presence of Pax8, which maintains its expression along the dorsomedial wall of the otic vesicle in embryos lacking Shh (Fig. 3e,j). Pax8 does not compensate for Pax2 in all regions of the otocyst, given its inability to rescue the cochlear duct defects in either Shh−/− or Pax2−/− embryos. These findings suggest that Pax2 function is dispensable for medial-lateral boundary formation in the otocyst.
The cvg fail to form in Shh−/− embryos
The cvg derive from anteroventral regions of the otic vesicle. Once specified these neuronal precursors delaminate from the otic epithelium, migrate ventrally, and aggregate to form the cvg at the base of the otocyst. The cvg later segregate into the cochlear and vestibular nerves, which function to innervate the thousands of sensory hair cells of their respective structures. The requirement of Shh in specifying ventral otic cell fates makes the neuronal precursors of the cvg a potential target of Shh signaling.
Prior to their delamination from the otic epithelium, neuronal precursors express the bHLH transcription factors Ngn1 and NeuroD (Ma et al. 1998). Ngn1 is the earliest of the neuronal determinants to be expressed in cvg progenitors, at E9.0. followed by NeuroD at E9.5. Both the levels and domains of Ngn1 and NeuroD transcripts were markedly reduced in the otic vesicles from Shh−/− embryos compared to age-matched wild-type littermates (Fig. 4a–d), inferring that Shh is involved in specifying the number of cvg progenitors by regulating Ngn1 transcription.
Figure 4.
Shh is required for the specification and delamination of cvg precursors. Transverse sections through the anterior portion of the otic vesicles of wild-type (a,c,e,g) and Shh−/− (b,d,f,h) embryos. NeuroD (a,b) and Ngn1 (c,d) expression is downregulated within cvg precursors of Shh−/− embryos at 9.5 dpc. NeuroD (e,f) and Fgf10 (g,h) expression is reduced within the cvg and geniculate ganglia of Shh−/− embryos at 10.5 dpc. Red lines in e and f highlight the protracted expression of NeuroD in the mutant vs. wild-type otic epithelium. Adjacent sections probed with Lfng (i,l), Bmp4 (j,m), and Myo XV (k,n) expression from wild-type and Shh−/− inner ears at 15.5 dpc. Arrows point to the positive Lfng and Myo XV domains that are negative for Bmp4. The level of the sections is shown in each representative diagram. ca, crista ampullaris; gg, geniculate ganglia; lc, lateral crista; mu, macula utriculi; pc, posterior crista. Bar, 100 μm.
To further investigate the dependence on Shh for neural fate specification in the inner ear, we looked to the ganglion proper at slightly later stages. By 10.5 dpc, many of the wild-type neuroblasts expressing NeuroD had delaminated from the otic epithelium, migrated ventrally, and aggregated to form a compact ganglion (Fig. 4e). This contrasts with the situation in Shh−/− embryos, where a smaller proportion of NeuroD-expressing precursors are found scattered below the otic vesicle, with the majority remaining confined to the otic epithelium (Fig. 4f). Assessment of a second cvg marker, Fgf10, is consistent with this result (Fig. 4g,h). Moreover, few neurons contributing to the cochlear ganglia and no neurons contributing to the vestibular ganglia were identified at 14.5 dpc (data not shown). The reduction in delaminating neuroblasts from the otic epithelium of Shh−/− embryos bears remarkable similarity to the phenotype displayed by NeuroD−/−embryos and suggests that NeuroD activity may be modulated by Shh (Liu et al. 2000; Kim et al. 2001).
Because the neurogenic domain is thought to partially overlap with the sensory competent region in the inner ear, we investigated the development of the sensory patches in Shh−/− embryos at 15.5 dpc using established sensory organ markers. In the wild-type ear, all sensory patches express Lfng by 13 dpc and only the three cristae express Bmp4 (Fig. 4i,j; Morsli et al. 1998). In Shh−/− inner ears, a broad Lfng-positive domain was observed on the medial side of the membranous chamber (Fig. 4l). This Lfng expression is thought to give rise to the maculae of the utricle and saccule and the organ of corti, but none of these sensory structures was distinguishable in Shh mutants. Nevertheless, Bmp4-positive crista-like structures were detected (Fig. 4m) and sensory hair cells did form in each sensory patch, as indicated by the expression of an early hair cell marker, Myosin XV (Fig. 4k,n). Thus, Shh is required to specify the majority of the neurogenic but not sensory lineages in the inner ear.
Otic targets of the Shh signaling pathway are upregulated in ShhP1 embryos
Two questions arise from the results described thus far; first, what is the source of Shh responsible for patterning the otic vesicle, and second, is Shh sufficient to induce ventral otic fates. To address the first question we assessed Shh expression in and around the developing inner ear. Between 8.5 dpc and 10.5 dpc, no discernible expression of Shh mRNA or protein could be detected in the otic epithelium or surrounding mesenchyme, suggesting that the likely source of Shh responsible for patterning the inner ear emanates from the notochord and/or floor plate (Figs. 5b, 6Aa).
Figure 5.
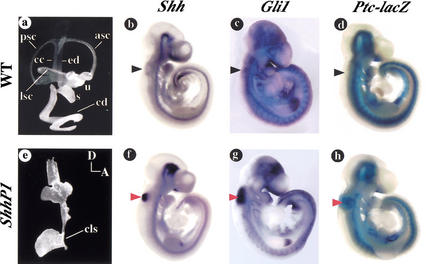
Embryos carrying the ShhP1 transgene show impaired inner ear morphogenesis as a result of ectopic Shh expression. Analysis of paint-fill injections of inner ears from wild-type (a) and ShhP1 (e) embryos at 15.5 dpc. Note the absence of dorsal structures in ShhP1 compared to wild-type inner ears. Lateral views of embryos stained by whole-mount in situ hybridization for Shh (b,f) and Gli1 (c,g) in wild-type (b,c) and ShhP1 (f,g) embryos at 9.5 dpc. X-gal staining of PtclacZ/+ (d) and PtclacZ/+; ShhP1 (h) embryos at 9.5 dpc. The absence of Shh, Gli1, and Ptc expression in the dorsal otic vesicle is marked with a black arrowhead in b–d, whereas ectopic expression of these markers in ShhP1 embryos is highlighted with red arrowheads in f–h. cls, cochlear-like structure.
With regard to the second question, we took advantage of a transgenic mouse line that ectopically expresses Shh in the otic vesicle to determine whether Shh signaling is sufficient for establishing ventral otic fates. Independent mouse lines carrying a 100-kb P1 clone (ShhP1) overlapping the Shh open reading frame exhibit behavioral abnormalities related to vestibular apparatus dysfunction including circling, hyperactivity, and head bobbing. Paint-fill analyses of inner ears from ShhP1 transgenic mice reveal an absence of identifiable vestibular structures including semicircular canals, utricle, and saccule (Fig. 5a,e). The appearance of the cochlear duct was also irregular, with limited outgrowth and coiling (5/6 embryos).
To determine the molecular nature underlying the phenotype exhibited by embryos carrying the ShhP1 transgene, we first assessed the expression of Shh at early stages of otic development. Whereas wild-type embryos show no evidence of Shh expression at any stage of inner ear development between 8.5 dpc and 10.5 dpc, embryos carrying the ShhP1 transgene show ectopic expression of Shh in the otic epithelium at 9.5 dpc (Fig. 5b,f). The ectopic expression of Shh likely results from the absence of a negative regulatory element in the ShhP1 transgene that normally functions to repress Shh transcription from the otic vesicle (D. Epstein, unpubl.). Indication that Shh signaling is active from this ectopic source was confirmed by the significant upregulation of Gli1 and Ptc transcription (Fig. 5c,d,g,h). Although X-gal staining of embryos carrying the PtclacZ allele recapitulates most aspects of Ptc expression, lacZ activity was only detected in the otic epithelium of ShhP1 transgenic and not wild-type embryos. The failure to observe lacZ expression in the otic vesicles of wild-type embryos presumably results from altered transcription from the targeted allele, because Ptc mRNA is normally present in wild-type otic vesicles (Fig. 2s).
Localization of ectopic Shh expression in ShhP1 embryos is confined to the dorsal portion of the otic epithelium (Fig. 6Aa,e). To address the consequence of this new source of Shh on otic vesicle patterning, we surveyed a number of genes that were shown previously to depend on Shh for their expression. Remarkably, the domain of Pax2, which normally is maintained by Shh on the medial side of the otic epithelium, was found to encompass the entire otic vesicle in ShhP1 embryos (Fig. 6Ab,f). In contrast, the expression of Dlx5 and Hmx3 were reduced in the otic vesicles of ShhP1 embryos by 9.5 dpc and almost completely absent by 10.5 dpc (Fig. 6Ac,d,g,h; data not shown). Given that both of these genes are essential for establishing the vestibular apparatus, their downregulation in ShhP1 embryos likely explains the absence of semicircular canals, utricle, and saccule in these transgenic mice (Hadrys et al. 1998; Wang et al. 1998; Acampora et al. 1999; Depew et al. 1999). These results offer further support of the mutual antagonism between Dlx5 and Shh in establishing dorsal and ventral otic fates.
Since the ventralmost cells of the otic vesicle are dependent on Shh for their specification and the ventral limit of Pax2 may partially overlap these cells, we sought to address whether ventralmost cells are represented in the expanded domain of Pax2 expression in ShhP1 embryos. Otx2 expression remained restricted to the ventral portion of the otic vesicle in ShhP1 embryos, confirming that dorsal Shh expression is insufficient to activate either Otx2 transcription or ventralmost cell fates (data not shown). However, within the periotic mesenchyme, ectopic Shh is sufficient to expand the expression domain of Brn4 ventrally and activate ectopic Tbx1 expression dorsally (Fig. 6Ba,b,f,g). These results suggest that in some contexts Shh signaling is sufficient to activate target gene expression—Pax2 in the otic epithelium and Brn4 and Tbx1 in the periotic mesenchyme—whereas in other contexts, Shh is likely acting in conjunction with cooperating signals, for instance in the specification of Otx2-expressing ventral cell fates.
As Shh is required for cvg formation, we next investigated whether ectopic Shh affects the neuronal lineage. Interestingly, the number of NeuroD-expressing neurons contributing to the cvg is expanded more than twofold in ShhP1 embryos compared to age-matched wild-type littermates (Fig. 6Bc,h). Moreover, NeuroD-expressing precursors migrate from ectopic locations within the otic epithelium of ShhP1 embryos (Fig. 6Bd,i). In comparison to wild-type littermates, a notable expansion of Ngn1-expressing neuronal precursors was detected in the otic epithelium of ShhP1 embryos prior to their delamination (Fig. 6Be,j). No enhancement in cell survival or proliferation was detected in the vicinity of the expanded neuroblast lineage in ShhP1 embryos compared to wild-type littermates (data not shown). It is possible that the expansion of neuronal progenitors comes at the expense of sensory cells, because only one or two sensory patches were evident in ShhP1 ears at 15.5 dpc (data not shown). These findings reinforce the view that Shh is both necessary and sufficient for the specification of neuronal cell fates within the otic vesicle.
Discussion
Shh patterns the otic vesicle by specifying distinct ventral cell fates
The morphogenetic programs that shape the inner ear into auditory (ventral) and vestibular (dorsal) components are established early during otic development and are heavily influenced by extrinsic cues from surrounding tissues (Torres and Giraldez 1998; Baker and Bronner-Fraser 2001). In the present work we demonstrate that Shh secreted from the notochord and/or floor plate acts as a long-range signal to promote ventral cell identity within the adjacent otic vesicle. In mouse embryos lacking Shh function, ventral otic derivatives including the cochlear duct and cvg failed to develop. A direct action of Shh signaling on ventral otic cells is suggested by the expression of Gli1 and Ptc in the otic epithelium and periotic mesenchyme, two tissues that show altered expression of these and other target genes in Shh−/− embryos.
As the cochlear duct and cvg emerge from different locations within the otic epithelium, we propose that ventral otic progenitor cells respond differentially to Shh signaling. Consistent with this hypothesis, in the otic vesicles of Shh−/− embryos we detected specific alterations in the expression of ventral otic genes localized along the anteroposterior axis (Fig. 7). For instance, in posterior regions of the otic vesicle, ventral cells contributing to the cochlear duct marked by the overlapping expression of Otx1, Otx2, and possibly Pax2 were absent in Shh−/− embryos, whereas the medial expression of Pax2 was not maintained (Fig. 7). In anterior regions of the otic vesicle where neuronal precursors are derived, the expression of Ngn1 and NeuroD was significantly downregulated in Shh−/− embryos, resulting in a failure to generate the cvg (Fig. 7).
Figure 7.
Schematic representation of the Shh loss and gain-of-function phenotypes revealed in the inner ear. In posterior regions of the otic vesicle, Shh is required for the specification of the ventralmost cells of the otic epithelium marked by the overlapping expression of Otx1, Otx2, and possibly Pax2 (royal blue) that contribute to the outgrowth of the cochlear duct. Shh is also required for the maintenance of Pax2 expression along the medial wall of the otic vesicle (light blue). In the absence of Shh function, there is a ventral expansion in the expression of some dorsal genes including Dlx5 (yellow) and a shift in the expression of markers of lateral fates (red, green) to the ventralmost portion of the otic vesicle. Within the condensing mesenchyme (small white circles underlying the otic vesicle), there is a delay in Brn4 and an absence of Tbx1 expression. In comparison, ShhP1 embryos, which exhibit ectopic expression of Shh in the dorsal otocyst (dark blue), depict a phenotype reciprocal to that of Shh−/− embryos in that dorsal markers are downregulated (loss of yellow) and Pax2 expression (light blue) expands throughout the circumference of the otic vesicle. In addition, there is an increase in the population of condensing mesenchyme expressing Brn4 and ectopic Tbx1, as well as ectopic ganglia formation (purple). Hatched portions of the otic vesicle represent coexpression with Pax2. In the anterior portion of the otic vesicle, Shh is required for the specification of the majority of cvg precursors through the regulation of Ngn1 expression (pink). The domain and intensity of Ngn1 transcription are substantially reduced in Shh−/− embryos, and consequently the size of the cvg (purple) is diminished. In ShhP1 embryos, the ectopic expression of Shh in the inner ear (dark blue) causes an expansion of the Ngn1-positive domain (pink) and an increase in the size of the cvg. For the purpose of illustration, not all genes expressed in the anterior otic vesicle are shown.
That the misexpression of Shh in the dorsal otocyst of ShhP1 transgenic embryos was sufficient to induce the ectopic expression of some of these markers including Pax2 and Ngn1 is further indication that Shh acts in an instructive rather than permissive manner to pattern the otic vesicle (Fig. 7). How Shh differentially regulates the expression of ventral otic genes along the anteroposterior axis is unclear, but given that cooperative interactions between Shh and other signaling pathways specify distinct neuronal progenitor cells along the anteroposterior axis of the neural tube, it is conceivable that a similar strategy is employed to pattern the otic vesicle (Jessell 2000). Interestingly, Fgf family members are differentially expressed within the otic vesicle (Pickles 2001).
Although Shh is required for the specification of ventral otic cells expressing Otx2, ectopic Otx2 expression was not detected in the otic vesicles of ShhP1 embryos. Any of several interpretations may explain these results. First, Otx2 may be a marker of ventral otic cells that is not regulated by Shh signaling. Second, Shh may require cooperating signals to activate Otx2 expression. Third, Shh may be sufficient to activate Otx2 expression but the level of Shh emanating from the dorsal otocyst of ShhP1 embryos is below a critical threshold. A final possibility is that the loss of Otx2 in Shh−/− embryos is an indirect consequence of the altered neural patterning in these mutants and hence ectopic Otx2 expression would not be an expected outcome in ShhP1 embryos. We feel this final possibility to be the least likely given that (1) known Shh target genes including Gli and Ptc are expressed ventrally in the otic vesicle, (2) Shh is sufficient to activate other otic markers in ShhP1 embryos, and (3) Shh is known to signal to other paraxial tissues.
In addition to the loss of ventral otic cell fates, the absence of Shh impacts the positioning of dorsal and lateral markers. Dlx5, a gene required for dorsal (vestibular) cell fates, was expanded ventrally in Shh−/− embryos (Fig. 7). Further indication that Shh antagonizes the expression of Dlx5 stems from the downregulation of Dlx5 in response to ectopic Shh in ShhP1 embryos. The role of Shh in patterning the otic vesicle along the dorsal/ventral axis thus bears some similarity to the manner by which Shh establishes ventral fates in the neural tube (Ericson et al. 1996). Both processes involve the initial repression of dorsal markers in order to promote ventral cell fates. It will be interesting to determine whether other similarities exist between inner ear and neural patterning, for instance whether BMPs from the surface ectoderm and/or dorsal neural tube function as dorsalizing signals in the otic vesicle acting in opposition to the ventralizing effects of Shh.
Pax2 is a mediator of Shh signaling responsible for cochlear duct outgrowth
The failure in cochlear duct outgrowth in Shh−/− embryos is most likely mediated by the lack of Pax2, Otx1, and Otx2, genes previously ascribed with required roles in this process (Favor et al. 1996; Torres et al. 1996; Morsli et al. 1999). Furthermore, our observations that Shh is both necessary and sufficient for the expression of Pax2 along the medial wall of the otic vesicle implicates Pax2 as a downstream effector of Shh signaling in the otocyst (Fig. 7). The regulation of Pax2 by Shh in inner ear development resembles the relationship between Pax2 and Shh in the formation of another placode-derived sensory organ, the eye. In generating the proximal-distal axis of the optic cup, Shh signaling from the ventral forebrain promotes Pax2-expressing proximal fates (optic fissure, optic stalk) at the expense of Pax6-expressing distal fates (prospective retina, pigmented epithelium, and lens; Ekker et al. 1995; MacDonald et al. 1995; Chiang et al. 1996; Zhang and Yang 2001). To maintain the border between proximal and distal lineages, Pax2 and Pax6 antagonize each other by mutual transcriptional repression (Schwarz et al. 2000). The commonality in response by Pax genes to Hh signaling can be broadened to include Pax1 in the ventral somite and Pax6 in the ventral neural tube (Fan and Tessier-Lavigne 1994; Johnson et al. 1994; Ericson et al. 1997; Zhang et al. 2001). In both of these cases, Pax family members with opposing functions are expressed adjacent to sites of Pax1 and Pax6 activity (Fan and Tessier-Lavigne 1994; Johnson et al. 1994; Ericson et al. 1996). This is not a general rule, as Pax genes are not expressed complementary to Pax2 in the inner ear, although other transcription factors may be fulfilling an antagonistic role in this tissue (Brigande et al. 2000). Our observations thus add to the growing list of functions for Pax transcription factors in mediating cellular responses to Shh signaling (Mansouri et al. 1996).
Pax2-independent functions of the Shh signaling pathway in otic development
The downregulation of Pax2 expression may contribute to the failure of cochlear duct outgrowth in embryos lacking Shh function. However, the absence of the lateral semicircular canal, endolymphatic duct, and cvg in Shh−/− embryos, structures that are present in Pax2−/− embryos, reveals a more complex role for Shh than simply regulating Pax2 expression (Favor et al. 1996; Torres et al. 1996). The lack of lateral semicircular canals in Shh−/− embryos may be explained in part by the initial reduction and ventralward shift in expression of Otx1, a gene required for the formation of this structure (Acampora et al. 1996; Morsli et al. 1999). However, since the primordium of the lateral canal, the horizontal outpocket, is still present in Shh−/− embryos, it is unlikely that Otx1 function is completely compromised in the absence of Shh. Alternatively, the disruption in lateral canal formation may stem from the disregulated growth of ventral otic cells in Shh−/− embryos. The ventral overgrowth that appears after 12.5 dpc in Shh−/− mutants could interfere with the development of intermediate structures such as the lateral canal. Similar reasoning is unlikely to explain the failure to maintain endolymphatic duct outgrowth in Shh−/− embryos, however, because this dorsally derived structure arrests at 10.5 dpc, prior to the appearance of the ventral cyst. Interestingly, the arrest in endolymphatic duct development is concomitant with the downregulation of Gbx2 expression in this tissue. Whether maintenance of Gbx2 expression is a direct response to Shh signaling and whether Gbx2 is required for endolymphatic duct outgrowth are two questions that remain to be addressed.
The downregulated expression of Tbx1 and Brn4 in the periotic mesenchyme of Shh−/− embryos at 10.5 dpc indicates that this tissue is also responsive to Shh signaling. Moreover, since Tbx1 and Brn4 are upregulated in ShhP1 embryos, it is likely that these two genes are transcriptional targets of the Shh pathway. Interestingly, Tbx1 has also been shown to be dependent on Shh in the pharyngeal arches (Garg et al. 2001). Although the contribution of signals from the periotic mesenchyme in patterning the otic epithelium is not well understood, Brn4 is involved in forming the mature bony labyrinth surrounding the inner ear (Phippard et al. 1999). The expression of Brn4 in Shh−/− embryos at later stages in conjunction with the presence of a cartilage capsule suggests that a compensatory pathway functions to promote chondrogenesis in the absence of Shh (data not shown).
Neuroblast precursors of the cvg are dependent on Shh signaling
The mechanism by which neuronal precursors of the cranial sensory ganglia are specified is dependent on a genetic cascade mediated by basic helix-loop-helix (bHLH) transcription factors. With regard to the cvg, Ngn1 is at the top of the genetic hierarchy required to instruct a population of naïve otic epithelial cells to adopt a neuronal precursor fate (Ma et al. 1998). NeuroD, which is activated half-a-day later than Ngn1, is required for the delamination of neuroblasts from the otic epithelium as well as for their survival during the differentiation process (Ma et al. 1998; Liu et al. 2000; Kim et al. 2001). Although in many contexts activation of proneural genes in the neuronal determination pathway is regulated by Notch-Delta signaling, a growing body of evidence indicates that the initial selection of cvg precursors is controlled by alternative mechanisms (Ma et al. 1998).
Our studies suggest that the expression of Ngn1 in cvg precursors is established in part by the Shh signaling pathway. Ngn1 is downregulated in cvg precursors from Shh−/− embryos and upregulated in an expanded pool of neuroblasts in ShhP1 transgenic mice that ectopically express Shh (Fig. 7). Additional secreted factors acting independently of Shh are likely responsible for the specification of some cvg precursors, because a small number of Ngn1-expressing neuroblasts persist in Shh−/− embryos. Of the cvg precursors that are specified in Shh−/− embryos, it is unclear as to why the majority fail to emerge from the otic epithelium to form what would be perhaps a smaller ganglion. The answer may point once again to a function of Ngn1. A consequence of Ngn1 downregulation in Shh−/− mutants is a reduction in the level of NeuroD expression. Given the requirement for NeuroD in promoting neuroblast delamination from the otic epithelium, it is possible that the reduction in NeuroD expression in Shh−/− mutants prevents the emergence of cvg precursors from the otic epithelium. We favor this explanation over a cell survival role for Shh because at the stages of neuroblast delamination examined (between 9.5 and 10.5 dpc), there was no detectable increase in the number of dying cells in a comparison of wild-type and Shh−/− embryos (data not shown). Similar conclusions were drawn in the analysis of NeuroD−/−embryos (Liu et al. 2000).
Overlapping roles for Shh and retinoids in otic vesicle patterning
Retinoic acid (RA) signaling has been shown to play an active role in patterning the otic vesicle (Dupe et al. 1999; Niederreither et al. 2000; Pasqualetti et al. 2001). Interestingly, the inner ears from Shh−/− embryos bear some resemblance to the phenotype displayed by mice lacking Raldh2, a gene that catalyzes RA formation (Niederreither et al. 2000). In otic vesicles from both mutants, Pax2 expression is not maintained and Hmx3 expression is expanded ventrally (the present study; Niederreither et al. 2000). Unfortunately, further comparisons are not possible due to the early lethality of Raldh2−/− embryos. RA can also rescue the Hoxa1−/− inner ear phenotype, which mimics features of Shh−/− embryos including a lack of cochlear duct outgrowth (Pasqualetti et al. 2001). Numerous examples exist supporting the actions of Shh and RA in the same or parallel pathways in embryonic development (Riddle et al. 1993; Ogura et al. 1996; Pierani et al. 1999; Schneider et al. 2001). Thus, the possibility exists that Shh and RA signaling may converge in the patterning of the otic vesicle.
Since the otic phenotype in Raldh2 and Hoxa1 mutants is attributed to a hindbrain patterning defect rather than a primary effect on the otic vesicle per se, it raises the question as to whether aspects of the Shh−/− otic phenotype can be explained by alterations in the expression of genes that pattern the hindbrain (Niederreither et al. 2000; Pasqualetti et al. 2001). The hindbrain expression of kreisler and Fgf3 in rhombomeres 5 and 6 is altered in a number of mouse mutants with inner ear phenotypes, including Raldh2−/−, Hoxa1−/−, kreisler−/−, and Fgf3−/− embryos (Mansour et al. 1993; McKay et al. 1996; Niederreither et al. 2000; Pasqualetti et al. 2001). Nevertheless, the spatial and temporal domains of Kreisler and Fgf3 expression were unaffected in the hindbrain of Shh−/− embryos, suggesting that the Shh−/− otic phenotype does not result from misregulation of these hindbrain genes (data not shown). It will be interesting to determine whether the similarities in inner ear phenotypes of Shh−/−, Raldh2−/−, and Hoxa1−/− embryos stem from crosstalk between the Shh and RA pathways at other junctures or result from mutually independent mechanisms.
Similar usage of the Shh signaling pathway to pattern dissimilar paraxial structures
The otic vesicles and somites originate from two different tissue sources, the surface ectoderm and paraxial mesoderm, respectively, yet they share several features common to their development. For instance, both are transient structures that form adjacent to the neural tube. As a consequence, the two tissues are subjected to the same inductive signals expressed broadly along the anteroposterior axis as well as distinct signals expressed from localized sources.
Previous studies, in addition to our present work, support a general role for Shh in patterning paraxial structures (for review, see Bailey et al. 2001). What is particularly intriguing about the common use of this signaling pathway is the similarities in the types of genes used to affect disparate processes of differentiation. For instance, within the dorsal somite, Shh signaling specifies the epaxial musculature lineage through the direct activation of Myf5 expression in the dorsomedial lip of the dermamyotome (Gustafsson et al. 2002). In the ventral somite, both Shh and Indian hedgehog cooperatively regulate sclerotome differentiation by controlling Pax1 expression (Fan and Tessier-Lavigne 1994; Johnson et al. 1994; Zhang et al. 2001). Myf5, like Ngn1 in the otic vesicle, is a bHLH transcription factor that functions as a primary determinant of cell fate (for review, see Bailey et al. 2001). Similarly, Pax1 and Pax2 are paired box transcription factors responsible for mediating differentiation programs in a variety of contexts (Mansouri et al. 1996). Greater insight into the mechanisms by which genes utilized by Shh to pattern paraxial structures are recruited will require further unraveling of the regulatory networks underlying otic vesicle and somite gene expression.
Materials and methods
Production and genotyping of transgenic mice
A P1 bacteriophage library (Genome Systems) was screened with primers directed against exons 1 and 3 of the Shh locus to isolate P1 5527, a 100-kb clone overlapping the Shh open reading frame and extending approximately 10 kb upstream and 90 kb downstream of the Shh translational start site. The P1 clone was linearized with SalI and purified for pronuclear injection by standard protocol at a concentration of 1ng/μL (Hogan et al. 1994). Genotyping of founder animals using primers against the sacB gene contained in the P1 vector (J50, 5′-GGTCGGCGA CAACTCAATCG-3′; J51, 5′-GTGAGGGTCTCTCAGCGTAT G-3′) as well as Southern blot hybridization with Shh-specific probes identified two independent mouse lines, each carrying 4–6 copies of an intact P1 5527 transgene (Shh P1). The two lines of mice exhibited similar behavioral abnormalities including hyperlocomotor activity, circling, and head bobbing. The transgenic lines were maintained on a CD-1 background (Charles River). The Shh+/− animals were kindly provided by H. Westphal (NIH; Chiang et al. 1996) and maintained on a CD-1 background (Charles River). PtclacZ/+ mice were obtained from Jackson Laboratories.
Paint-fill studies
Mouse embryos for paint-fill analysis were harvested in PBS and placed overnight in Bodian fixative at room temperature. Specimens were subsequently dehydrated in ethanol and cleared in methyl salicylate. A 0.1% latex paint solution in methyl salicylate was injected into the lumen of the membranous labyrinth using a micromanipulator (Martin and Swanson 1993; Morsli et al. 1998).
In situ hybridization and whole-mount β-galactosidase staining
Whole-mount RNA in situ hybridization was performed essentially as described (Matise et al. 1998) using digoxigenin-UTP-labeled riboprobes. Three to five embryos of each genotype were analyzed for every probe. After whole-mount staining, representative embryos were postfixed in 4% paraformaldehyde, rinsed in PBS, embedded in 4% agarose, and sectioned on a vibratome at 50–75 μm. The assessment of β-galactosidase activity in PtclacZ/+ embryos was performed by histochemical staining using X-gal (GIBCO-BRL) as substrate (Epstein et al. 2000).
Immunohistochemistry
Embryos of various genotypes were fixed for 1 h in 4% PFA, sunk in 30% sucrose overnight, embedded and frozen in OCT, and sectioned at 16–20 μm on a cryostat. Primary antibodies used and dilutions were as follows: Pax2 (Zymed) 1:250; Shh (5E1, DSHB) 1:100. Detection of primary antibodies was achieved using Cy3-conjugated goat anti-rabbit (Pax2) and goat anti-mouse (Shh) secondary antibodies (Jackson ImmunoResearch Laboratories).
Figure 6.
Ectopic expression of Shh-responsive genes in ShhP1 embryos. (A) Upregulation of cochlear and downregulation of vestibular markers in ShhP1 embryos. Antibody staining for Shh (a,e) and Pax2 (b,f) on transverse sections through the otic vesicles of wild-type (a,b) and ShhP1 (e,f) embryos at 9.5 dpc. Shh protein is ectopically expressed in the dorsal region of the otic vesicle of ShhP1 embryos (e). White arrowheads in f mark the ectopic expression of Pax2 protein on the lateral side of the otic vesicle. Dlx5 (c,g) and Hmx3 (d,h) expression is downregulated in ShhP1 embryos compared to wild-type littermates. (B) Markers of the periotic mesenchyme and cvg precursors are upregulated in ShhP1 embryos. Brn4 (a,f) and Tbx1 (b,g) expression in lateral views of whole-mount stained wild-type and ShhP1 embryos at 10.5 dpc. Dashed circles mark the position of the otic vesicle. Expression of Brn4 in the condensing mesenchyme of ShhP1 embryos is expanded (red arrowheads in f) compared to wild-type embryos (white arrowheads in a). The asterisks in a and f mark the branchial arch expression of Brn4. (g) Red arrowheads point to the ectopic expression of Tbx1 in ShhP1 embryos. NeuroD (c,d,h,i) and Ngn1 (e,j) expression on transverse sections through the otic vesicles of wild-type (c–e) and ShhP1 (h–j) embryos. Note the expansion of cvg precursors in anterior and posterior locations of the otic vesicle in ShhP1 embryos (red arrowheads in i,j). The ectopic ganglia depicted in i correlate with the ectopic Ngn1 expression (red arrowheads in j). All sections are from embryos at 10.5 dpc except e and j, which are from embryos at 9.5 dpc.
Acknowledgments
We thank the following individuals for probes: D. Anderson, B. Crenshaw, C-M. Fan, M. Frohman, P. Gruss, B. Hogan, R. Johnson, A. Joyner, T. Lufkin, A. McMahon, B. Morrow, J. Rubenstein, P-X. Xu, and T. Yamaguchi. D.J.E. acknowledges Alex Joyner for her support in generating the ShhP1 mouse line. Kenneth Campbell and Maja Bucan provided helpful comments on the manuscript. Funding for this project in D.J.E.'s laboratory was supported by NIH grant R01 NS39421 from NINDS, a Basil O'Conner Starter Scholar Research Award from the March of Dimes, and a Pew Scholar Award in the Biomedical Sciences.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL epsteind@mail.med.upenn.edu; FAX (215) 573-5892.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1013302.
References
- Acampora D, Mazan S, Avantaggiato V, Barone P, Tuorto F, Lallemand Y, Brulet P, Simeone A. Epilepsy and brain abnormalities in mice lacking the Otx1 gene. Nat Genet. 1996;14:218–222. doi: 10.1038/ng1096-218. [DOI] [PubMed] [Google Scholar]
- Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, Bober E, Barbieri O, Simeone A, Levi G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–3809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- Bailey P, Holowacz T, Lassar AB. The origin of skeletal muscle stem cells in the embryo and the adult. Curr Opin Cell Biol. 2001;13:679–689. doi: 10.1016/s0955-0674(00)00271-4. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Brigande JV, Kiernan AE, Gao X, Iten LE, Fekete DM. Molecular genetics of pattern formation in the inner ear: Do compartment boundaries play a role? Proc Natl Acad Sci. 2000;97:11700–11706. doi: 10.1073/pnas.97.22.11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Liu JK, Long JE, Presley R, Meneses JJ, Pedersen RA, Rubenstein JL. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development. 1999;126:3831–3846. doi: 10.1242/dev.126.17.3831. [DOI] [PubMed] [Google Scholar]
- Dupe V, Ghyselinck NB, Wendling O, Chambon P, Mark M. Key roles of retinoic acid receptors alpha and beta in the patterning of the caudal hindbrain, pharyngeal arches and otocyst in the mouse. Development. 1999;126:5051–5059. doi: 10.1242/dev.126.22.5051. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Martinu L, Michaud JL, Losos KM, Fan C, Joyner AL. Members of the bHLH-PAS family regulate Shh transcription in forebrain regions of the mouse CNS. Development. 2000;127:4701–4709. doi: 10.1242/dev.127.21.4701. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: Evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle, et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci. 1996;93:13870–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Garg V, Yamagishi C, Hu T, Kathiriya IS, Yamagishi H, Srivastava D. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev Biol. 2001;235:62–73. doi: 10.1006/dbio.2001.0283. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Studer M, Lumsden A, Rijli FM, Krumlauf R, Chambon P. Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development. 1998;125:1123–1136. doi: 10.1242/dev.125.6.1123. [DOI] [PubMed] [Google Scholar]
- Giraldez F. Regionalized organizing activity of the neural tube revealed by the regulation of lmx1 in the otic vesicle. Dev Biol. 1998;203:189–200. doi: 10.1006/dbio.1998.9023. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by Hedgehog. Genes & Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Groves AK, Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein DJ, Emerson CP., Jr Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes & Dev. 2002;16:114–126. doi: 10.1101/gad.940702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrys T, Braun T, Rinkwitz-Brandt S, Arnold HH, Bober E. Nkx5–1 controls semicircular canal formation in the mouse inner ear. Development. 1998;125:33–39. doi: 10.1242/dev.125.1.33. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. New York: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes & Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–1173. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1967. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes & Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:4069–4082. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Goddard JM, Capecchi MR. Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development. 1993;117:13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Hallonet M, Gruss P. Pax genes and their roles in cell differentiation and development. Curr Opin Cell Biol. 1996;8:851–857. doi: 10.1016/s0955-0674(96)80087-1. [DOI] [PubMed] [Google Scholar]
- Mark M, Lufkin T, Vonesch JL, Ruberte E, Olivo JC, Dolle P, Gorry P, Lumsden A, Chambon P. Two rhombomeres are altered in Hoxa-1 mutant mice. Development. 1993;119:319–338. doi: 10.1242/dev.119.2.319. [DOI] [PubMed] [Google Scholar]
- Martin P, Swanson GJ. Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev Biol. 1993;159:549–558. doi: 10.1006/dbio.1993.1263. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- McKay IJ, Lewis J, Lumsden A. The role of FGF-3 in early inner ear development: An analysis in normal and kreisler mutant mice. Dev Biol. 1996;174:370–378. doi: 10.1006/dbio.1996.0081. [DOI] [PubMed] [Google Scholar]
- McKay IJ, Muchamore I, Krumlauf R, Maden M, Lumsden A, Lewis J. The kreisler mouse: A hindbrain segmentation mutant that lacks two rhombomeres. Development. 1994;120:2199–2211. doi: 10.1242/dev.120.8.2199. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126:2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- Nornes HO, Dressler GR, Knapik EW, Deutsch U, Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development. 1990;109:797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- Ogura T, Alvarez IS, Vogel A, Rodriguez C, Evans RM, Izpisua Belmonte JC. Evidence that Shh cooperates with a retinoic acid inducible co-factor to establish ZPA-like activity. Development. 1996;122:537–542. doi: 10.1242/dev.122.2.537. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Neun R, Davenne M, Rijli FM. Retinoic acid rescues inner ear defects in Hoxa1 deficient mice. Nat Genet. 2001;29:34–39. doi: 10.1038/ng702. [DOI] [PubMed] [Google Scholar]
- Phippard D, Lu L, Lee D, Saunders JC, Crenshaw EB., III Targeted mutagenesis of the POU-domain gene Brn4/Pou3f4 causes developmental defects in the inner ear. J Neurosci. 1999;19:5980–5989. doi: 10.1523/JNEUROSCI.19-14-05980.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO. The expression of fibroblast growth factors and their receptors in the embryonic and neonatal mouse inner ear. Hear Res. 2001;155:54–62. doi: 10.1016/s0378-5955(01)00247-7. [DOI] [PubMed] [Google Scholar]
- Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Westerfield M, Dressler GR. Comparative analysis of Pax-2 protein distributions during neurulation in mice and zebrafish. Mech Dev. 1992;38:197–208. doi: 10.1016/0925-4773(92)90053-m. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Hu D, Rubenstein JL, Maden M, Helms JA. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–2767. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Torres M, Giraldez F. The development of the vertebrate inner ear. Mech Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Wang W, Van De Water T, Lufkin T. Inner ear and maternal reproductive defects in mice lacking the Hmx3 homeobox gene. Development. 1998;125:621–634. doi: 10.1242/dev.125.4.621. [DOI] [PubMed] [Google Scholar]
- Wang W, Chan EK, Baron S, Van de Water T, Lufkin T. Hmx2 homeobox gene control of murine vestibular morphogenesis. Development. 2001;128:5017–5029. doi: 10.1242/dev.128.24.5017. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001;233:271–290. doi: 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–792. [PubMed] [Google Scholar]