Abstract
Aims
Albendazole (ABZ) is effective in the treatment of neurocysticercosis. ABZ undergoes extensive metabolism to (+) and (−)-albendazole sulphoxide (ASOX), which are further metabolized to albendazole sulphone (ASON). We have investigated the distribution of (+)-ASOX (−)-ASOX, and ASON in cerebrospinal fluid (CSF) of patients with neurocysticercosis.
Methods
Twelve patients with a diagnosis of active brain parenchymal neurocysticercosis treated with albendazole for 8 days (15 mg kg−1 day−1) were investigated. On day 8, serial blood samples were collected during the dose interval (0–12 h) and one CSF sample was taken from each patient by lumbar puncture at different time points up to 12 h after the last albendazole dose. Albendazole metabolites were determined in CSF and plasma samples by h.p.l.c. using a Chiralpak AD column and fluorescence detection. Population curves for CSF albendazole metabolite concentration vs time were constructed.
Results
The mean plasma/CSF ratios were 2.6 (95% CI: 1.9, 3.3) for (+)-ASOX and 2.7 (95% CI: 1.8, 3.7) for (−)-ASOX, with the two-tailed P value of 0.9873 being non-significant. These data indicate that the transport of ASOX through the blood–brain barrier is not enantioselective, but rather depends on passive diffusion. The present results suggest the accumulation of the (+)-ASOX metabolite in the CSF of patients with neurocysticercosis. The CSF AUC(+)/AUC(−) ratio was 3.4 for patients receiving albendazole every 12 h. The elimination half-life of both ASOX enantiomers in CSF was 2.5 h. ASOX was the predominant metabolite in the CSF compared with ASON; the CSF AUCASOX/AUCASON ratio was approximately 20 and the elimination half-life of ASON in CSF was 2.6 h.
Conclusions
We have demonstrated accumulation of the (+)-ASOX metabolite in CSF, which was about three times greater than the (−) antipode. ASOX concentrations were approximately 20 times higher than those observed for the ASON metabolite.
Keywords: albendazole, cerebrospinal fluid, cysticercosis, enantiomers, metabolism, pharmacokinetics
Introduction
Albendazole (methyl 5-propylthio-1H-benzimidazol-2-yl carbamate) is a broad spectrum antihelminthic drug whose activity resides both in the parent compound and its sulphoxide metabolite. Albendazole was introduced for the treatment of hydatidosis in 1983 and was later shown to be effective for the treatment of neurocysticercosis [1, 2]. The pharmacokinetic properties of albendazole have been determined in different animal species [3–6] and in man, including healthy volunteers [4, 7, 8] and patients with hydatidosis [7, 9–11] and intraparenchymatous neurocysticercosis [12–16]. The pharmacokinetic parameters are almost exclusively derived from measurements of plasma concentrations.
Clinical studies have reported poor absorption of albendazole, with wide interindividual variability as a consequence of its low solubility in aqueous solutions (<1 µg ml−1 water, pH 7.4). A fatty meal improves albendazole absorption. Apparently, the presence of neutral fat in the duodenum may increase bile flow and improve the absorption of albendazole by the detergent action of the bile acids. High presystemic elimination of albendazole impairs the detection of the drug in its unaltered form in plasma, thus preventing the determination of its bioavailability [1, 7, 10].
In human liver microsomes, albendazole is oxidized to the active albendazole sulphoxide (ASOX) metabolite by flavin monooxygenases (FMO) and the cytochrome P450 system (CYP), mainly CYP3A4, with the CYP component being the major contributor [17]. Albendazole sulphoxide is oxidized by CYP to the inactive albendazole sulphone (ASON) metabolite [18]. The sulphoxidation reaction seems to be a rapid process, with part of the sulphoxide undergoing a slow and irreversible oxidation to albendazole sulphone [19].
The steady-state plasma concentration of ASOX shows high intra- and interpatient variability. Plasma concentrations of the (+)-ASOX enantiomer are approximately nine times higher (7.6–10.9) than those observed for the (−)-ASOX antipode. The (+)/(−) plasma concentration ratio changes with time after albendazole administration. The (+)/(−) ratio decreases up to tmax (2–3 h) and increases during the elimination phase, with evidence of preferential (−)-ASOX formation and (+)-ASOX accumulation [14].
ASOX is eliminated in the urine with a renal clearance rate of the order of 0.01–0.04 l kg−1 h−1 [15]. Marriner et al. (1986) [7] observed an extremely low ASOX concentration in bile, indicating that bile does not represent a quantitatively important route for the elimination of the metabolite.
Albendazole sulphoxide is also found in the brain and a high proportion of the drug reaches the cerebrospinal fluid (CSF) compared with its concentration in plasma (2:1 serum to CSF ratio) [1]. Jung et al. (1992) [13] observed high concentrations of the metabolite (0.1–0.5 µg ml−1) in the CSF of eight patients with a diagnosis of neurocysticercosis who were treated with albendazole (15 mg kg−1 day−1). The ASOX concentration in CSF varies as a function of the pharmacokinetics of the drug. Other factors such as age, sex and inflammation of the subarachnoid space do not seem to be related to this variability. The high concentrations of ASOX attained in CSF probably explain the high efficacy of albendazole in the treatment of intraparenchymatous neurocysticercosis [1].
There are no data in the literature about the biological activity of the isolated isomers (+)-ASOX and (−)-ASOX. However, CSF albendazole sulphoxide enantiomers concentrations are important in order to evaluate the therapeutic role of albendazole in the neurocysticercosis treatment. In view of the lack of data about enantioselectivity in ASOX concentration in CSF, the aim of the present study was to determine the (+)/(−) enantiomeric ratios of ASOX in CSF and to construct population curves for the distribution of the albendazole metabolites in CSF of patients with neurocysticercosis who were treated with albendazole.
Methods
Patients
The study was conducted in 12 adult patients of both sexes (6 men and 6 women), aged 23–46 years and weighing 51–142 kg, with clinical and laboratory examinations (computed tomography and/or nuclear magnetic resonance, and ELISA for cysticercosis in CSF) being compatible with a diagnosis of the active form of intraparenchymatous neurocysticercosis and whose hepatic and renal functions were normal. All patients received detailed information about the study and signed the Free and Informed Consent form. The clinical protocol was approved by the Research Ethics Committee of Hospital das Clı´nicas, Faculdade de Medicina de Ribeira˜o Preto, Universidade de Sa˜o Paulo, Brazil.
The patients included in the study were admitted to the hospital and received albendazole (Zentel®, tablets, SmithKline Beecham Laboratórios Ltda, Rio de Janeiro, Brazil) as a multiple dose regimen (7.5 mg kg−1 12 h−1) for 8 days. Nine of the 12 patients were treated with antiepileptic drugs as phenobarbitone, carbamazepine or phenytoin in order to avoid epileptic convulsive seizures, which represent the main neurological manifestation of neurocysticercosis. Blood samples of approximately 5 ml were collected with heparinized syringes (Liquemine® 5000 IU, Roche) on the 8th day of treatment after administration of the last albendazole dose at time 0, and at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 h. Plasma was obtained by centrifugation for 20 min at 1800 g and stored at −20° C. On day 8 only one cerebrospinal fluid sample (lumbar puncture) was taken from each patient during a 12 h dose interval. The construction of albendazole metabolite concentration curves in CSF (0–12 h) was based on 12 samples collected from 12 patients at different time points up to 12 h after drug administration. The CSF samples were stored at −20° C until the time for analysis.
Albendazole metabolites in CSF
The CSF samples (500 µl) were added to 200 µl of a sodium metabisulfite solution and 500 µl of 0.75 n acetate buffer, pH 7.0, and extracted with 5.0 ml ethyl acetate for 20 min in a mechanical shaker (220±10 cycles min−1). After freezing of the aqueous phases for 15 h at −20° C, the organic phases (4.0 ml) were separated and the extracts evaporated to dryness under an air flow. The residues were dissolved in 100 µl of the mobile phase and submitted to chromatographic analysis (50 µl). The h.p.l.c. system consisted of a Shimadzu chromatograph (Kyoto, Japan) equipped with an LC-10 AS pump, a RF 551 fluorescence detector (λexc=280 nm, λem=320 nm), a CR-6 integrator and a 7125 Rheodyne injector with a 50 µl loop. The albendazole metabolites were separated on a 250×4 mm Chiralpak® AD chiral phase column (Chiral Technologies, Inc., Exton, PA) containing 10 µm particles, equipped with a 4×4 mm CN Lichrospher® 100 (Merck) precolumn containing 10-µm particles, using n-hexane/isopropanol/ethanol (81:14.75:4.25, v/v/v) as the mobile phase at a flow rate of 1.1 ml min−1.
Validation of the analytical method was carried out with drug-free CSF samples after excluding the possibility of interfering endogenous components. For evaluation of the selectivity of the analytical method, drugs commonly used in combination with albendazole such as dexamethasone, phenobarbitone, phenytoin, carbamazepine, cimetidine, and ranitidine, among others, were analysed.
CSF samples were analysed using calibration curves constructed by duplicate analysis of 500 µl aliquots of water (blank CSF was replaced with water considering that similar recoveries of the albendazole metabolites were proved) enriched with standard albendazole metabolite solutions (5–500 ng of each ASOX enantiomer ml−1 water and 1–100 ng of ASON ml−1 water). The samples were submitted to the extraction procedures and chromatographic analysis as described above. The linear regression equations and the correlation coefficients were calculated from the height of the peaks plotted against the respective concentrations.
Albendazole metabolites in plasma
Plasma albendazole metabolite concentrations were determined as described by Lanchote et al. (1998) [20]. The drugs were extracted from 500 µl plasma with ethyl acetate and the resolution of ASOX enantiomers and ASON was obtained on a ChiralpakTM AD column using hexane-isopropanol-ethanol (81:14:25:4.75, v/v/v) as the mobile phase. The drugs were detected by fluorescence (λexc=280 nm, λem=320 nm). Linear standard curves were obtained in the concentration range of 5–2500 ng ml−1 for ASOX enantiomers and 1–500 ng ml−1 for ASON. The method is precise and accurate for the three compounds, as judged by the coefficients of variation and relative errors lower than 10%.
Pharmacokinetic and statistical analysis
The CSF concentration vs time areas under the curve (AUC) during the interval from 0 to 12 h were determined using the linear trapezoidal method. Distribution and CSF elimination half-lives and rate constants were determined as previously described [14]. Differences between the data obtained for (+)- and (−)-ASOX were analysed by the paired Student t-test, accepting P≤0.05 as significant.
Results
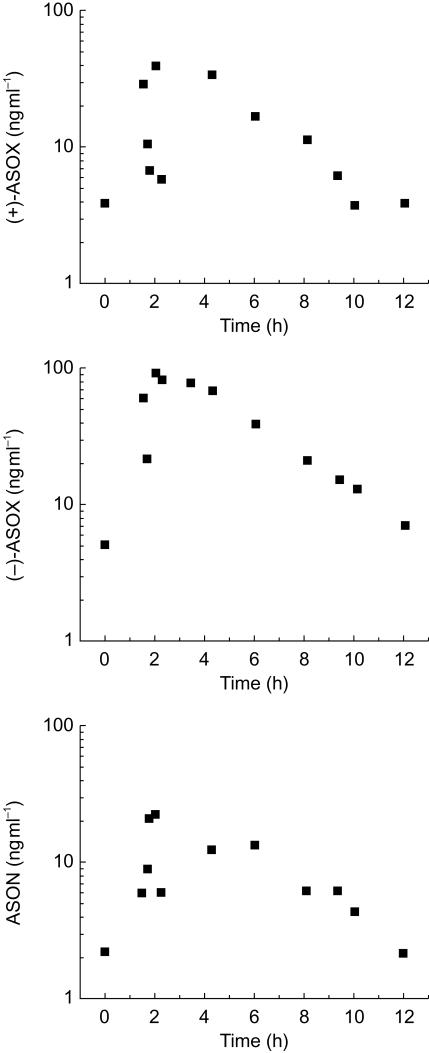
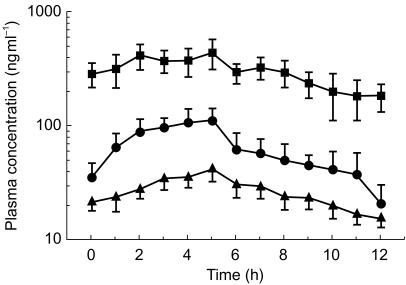
Figure 1 shows the population curves of steady-state albendazole metabolite concentrations in CSF as a function of a 12 h dose interval for 12 patients with neurocysticercosis after treatment for 8 days with albendazole. Only one CSF sample was collected from each patient at different time points during the 12 h dose interval. Table 1 shows the population distribution of albendazole metabolites in CSF samples of the patients with neurocysticercosis included in the study; data are presented as mean±s.e. mean (n=12). The plasma concentration vs time curves for (+)-ASOX, (−)-ASOX and ASON are presented in Figure 2 as means±s.e. mean (n=12).
Figure 1.
CSF concentrations vs time population curves for (+)-ASOX (−)-ASOX and ASON for 12 patients with neurocysticercosis after treatment for 8 days with albendazole at the dose of 7.5 mg/12 h (only one CSF sample was collected from each patient at different time points during the dose interval).
Table 1.
Population distribution of albendazole metabolites in CSF of patients with neurocysticercosis (n=12; one CSF sample was collected from each patient at different time points during the dose interval).
| AUC(0,12 h) (ng ml−1 h) | Cmax (h) | tmax (h) | t1/2d (h) | Kd (h−1) | t1/2 (h) | Kel (h−1) | |
|---|---|---|---|---|---|---|---|
| (+)-ASOX | 1836.13 | 385.5 | 2.0 | 2.0 | 0.347 | 2.5 | 0.277 |
| (−)-ASOX | 536.55 | 88.2 | 2.0 | >1.9 | 0.365 | 2.5 | 0.277 |
| ASON | 116.11 | 22.2 | 2.0 | 1.6 | 0.433 | 2.6 | 0.267 |
AUC, area under the CSF concentration-time curve; Cmax, maximum CSF concentration; tmax, time to reach Cmax; t1/2d, CSF distribution half-life; Kd, CSF distribution rate constant; t1/2, CSF elimination half-life; Kel CSF elimination rate constant.
Figure 2.
Plasma concentrations vs time curves for (+)-ASOX (▪), (−)-ASOX (•) and ASON (▴). Data presented as mean±s.e. mean for 12 patients with neurocysticercosis after treatment for 8 days with albendazole (7.5 mg/12 h).
Discussion
The ability of ASOX to cross the blood–brain barrier may explain the efficacy of albendazole in the treatment of human intraparenchymatous neurocysticercosis [1]. Jung et al. (1992) [13] observed high concentration of ASOX in the CSF of patients with neurocysticercosis treated with albendazole. Sotelo & Jung (1998) [1] reported serum/CSF concentration ratios close to 2 for the enantiomeric mixture of the active ASOX metabolite. No data are available about the (+)/(−) ASOX ratio in CSF or about the ability of the inactive ASON metabolite to reach the CSF.
The study on the enantioselectivity of ASOX in CSF requires the development and validation of an analytical method capable of chiral discrimination. The method was developed as previously described by Lanchote et al. [20] for the sequential analysis of albendazole metabolites in plasma. The most significant modification concerns the need of buffer addition to keep the pH of the CSF at 7.0 so that ASOX and ASON recovery rates close to 80% can be obtained. Using the Chiralpak AD column it was possible to achieve complete resolution of the albendazole metabolites as symmetrical peaks eluted within 30 min. The quantification limit of 5 ng ml−1 for each ASOX enantiomer (within day precision CV% 7.7 for (+) and 9.8 for (−) and within day accuracy bias% 9.0) permitted the analysis of the albendazole metabolites in the CSF of the 12 patients studied during the 12 h dose interval. The method proved to be highly reproducible and accurate, with relative standard deviations and systematic errors in the range of 10%. Drugs commonly administered in combination with albendazole during neurocysticercosis treatment (dexamethasone, ranitidine, cimetidine, antiepileptic drugs) did no interfere with the analytical method. Carbamazepine and phenobarbitone were not detected by fluorescence.
Figure 1 shows the population curves of steady-state albendazole metabolite concentrations in CSF as a function of the 12 h dose interval. (+)-ASOX concentrations in CSF ranged from 37 to 386 ng ml−1 after albendazole administration (7.5 mg kg−1 12 h−1), whereas (−)-ASOX concentrations reached 88 ng ml−1 at most, with some patients showing concentrations close to the quantification limit of 5 ng ml−1. The plasma concentration vs time curves for (+)-ASOX (−)-ASOX and ASON are presented in Figure 2 as means±s.e. mean (n=12). The plasma concentrations of (+)-ASOX were approximately five times higher than those of (−)-ASOX (AUC(0,12)(+)/AUC(0,12)(−)=4.78 as mean, n=12). The mean plasma/CSF ratios were 2.6 (95% CI: 1.9, 3.3) for (+)-ASOX and 2.7 (95% CI: 1.8, 3.7) for (−)-ASOX. These data indicate that the transport of ASOX through the blood–brain barrier is not enantioselective, but rather depends on passive diffusion of the drug. Marriner et al. [7] demonstrated approximately 70% binding of albendazole to plasma proteins. However, no data are available on ASOX and ASON binding to plasma proteins. The quantification limit of 1 ng ml−1 for ASON permitted the analysis of all patient samples, with concentrations ranging from 2 to 22 ng ml−1. The mean plasma/CSF ASON concentration ratio of 3.0 (95% CI: 2.3, 3.8) indicates an approximately three times higher ASON concentration in plasma than in CSF.
The present results demonstrate an accumulation of the (+)-ASOX metabolite in CSF of patients with neurocysticercosis. The CSF AUC(+)/AUC(−) ratio for patients treated with albendazole every 12 h was 3.4. The elimination half-life of both ASOX enantiomers in CSF was 2.5 h (Table 1).
ASOX was the predominant metabolite in the CSF compared to ASON; the CSF AUCASOX/AUCASON ratio was approximately 20 for the population studied. The CSF AUCASON/AUCASOX+AUCASON ratio was only 4.7%, indicating the rapid formation and slow oxidation of ASOX to ASON. The elimination half-life of ASON in CSF was 2.6 h.
In conclusion, the present data demonstrate an accumulation of the (+)-ASOX metabolite in CSF, which was about three times higher than that of the (−) antipode, and ASOX concentrations approximately 20 times higher than those observed for the ASON metabolite. The clinical significance of these findings remains to be tested. Although ALB is useful for therapy for cysticercosis in the subarachnoid space [21] where CSF (+)-ASOX and (−)-ASOX concentration would be of paramount importance, ALB is mostly indicated for therapy of brain intraparenchymatous cysticercosis [1, 2]. However, because of the difficulty in measuring sulphoxide enantiomers within the brain parenchyma, CSF sampling is the best substitute for CNS drug penetration [22, 23]. Secondly, there are no data in the literature about which ALB metabolite (+)-ASOX, (−)-ASOX or both, is actually effective or the respective concentrations required for proper treatment of neurocysticercosis. In the future, studies concerning CSF enantiomer concentration and clinical outcome of neurocysticercosis would clarify the question about the therapeutic role of each one of the ALB metabolites against the cysticerci.
Acknowledgments
The authors are grateful to FAPESP (Fundaça˜o de Amparo a Pesquisa do Estado de Sa˜o Paulo) for financial support and to CNPq (Conselho Nacional de Desenvolvimento Cientı´fico e Tecnológico) for granting research fellowships. The authors thanks Mrs Andréia M. Pontoglio for her technical assistance.
References
- 1.Sotelo J, Jung H. Pharmacokinetics optimisation of the treatment of neurocysticercosis. Clin Pharmacokin. 1998;34:503–515. doi: 10.2165/00003088-199834060-00006. [DOI] [PubMed] [Google Scholar]
- 2.Takayanagui OM, Jardim E. Therapy for neurocysticercosis. Arch Neurol. 1992;49:290–294. doi: 10.1001/archneur.1992.00530270106026. [DOI] [PubMed] [Google Scholar]
- 3.Benoit E, Besse S, Delatour P. Effect of repeated doses of albendazole on enantiomerism of its sulfoxide metabolite in goats. Am J Vet Res. 1992;53:1663–1665. [PubMed] [Google Scholar]
- 4.Delatour P, Benoit E, Besse S, Boukraa A. Comparative enantioselectivity in the sulphoxidation of albendazole in man, dogs and rats. Xenobiotica. 1991;21:217–221. doi: 10.3109/00498259109039463. [DOI] [PubMed] [Google Scholar]
- 5.Delatour P, Benoit E, Caude M, Tambute A. Species differences in the generation of the chiral sulfoxide metabolite of albendazole in sheep and rats. Chirality. 1990;2:156–160. doi: 10.1002/chir.530020306. [DOI] [PubMed] [Google Scholar]
- 6.Delatour P, Garnier F, Benoit E, Caude I. Chiral behaviour of the metabolite albendazole sulphoxide in sheep, goats and cattle. Res Vet Sci. 1991;50:134–138. doi: 10.1016/0034-5288(91)90095-6. [DOI] [PubMed] [Google Scholar]
- 7.Marriner SE, Morris DL, Dickson B, Bogan JA. Pharmacokinetics of albendazole in man. Eur J Clin Pharmacol. 1986;30:705–708. doi: 10.1007/BF00608219. [DOI] [PubMed] [Google Scholar]
- 8.Homeida M, Leahy W, Copeland S, Ali MMM, Harron DWG. Pharmacokinetic interaction between praziquantel and albendazole in Sudanese men. Ann Trop Med Parasitol. 1994;88:551–559. doi: 10.1080/00034983.1994.11812903. [DOI] [PubMed] [Google Scholar]
- 9.Cotting J, Zeugin T, Steiger U, Reichen J. Albendazole kinetics in patients with echinococcosis; delayed absorption and impaired elimination in cholestasis. Eur J Clin Pharmacol. 1990;38:605–608. doi: 10.1007/BF00278590. [DOI] [PubMed] [Google Scholar]
- 10.Lange H, Eggers R, Bircher J. Increased systemic availability of albendazole when taken with a fatty meal. Eur J Clin Pharmacol. 1988;34:315–317. doi: 10.1007/BF00540964. [DOI] [PubMed] [Google Scholar]
- 11.Steiger U, Cotting J, Reichen J. Albendazole treatment of echinococcosis in humans: effects on microsomal metabolism and drug tolerance. Clin Pharmacol Ther. 1990;47:347–353. doi: 10.1038/clpt.1990.38. [DOI] [PubMed] [Google Scholar]
- 12.Jung H, Hurtado M, Medina MT, Sanchez M, Sotelo J. Dexamethasone increases plasma levels of albendazole. J Neurol. 1990;231:279–280. doi: 10.1007/BF00314741. [DOI] [PubMed] [Google Scholar]
- 13.Jung H, Hurtado M, Sanchez M, Medina MT, Sotelo J. Clinical pharmacokinetics of albendazole in patients with brain cysticercosis. J Clin Pharmacol. 1992;32:28–31. doi: 10.1002/j.1552-4604.1992.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 14.Marques MP, Takayanagui OM, Bonato PS, Santos SRCJ, Lanchote VL. Enantioselective kinetic disposition of albendazole sulfoxide in patients with neurocysticercosis. Chirality. 1999;11:218–223. doi: 10.1002/(SICI)1520-636X(1999)11:3<218::AID-CHIR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Sánches M, Suástegui R, Gonzáles-Esquivel D, Sotelo J, Jung H. Pharmacokinetic comparison of two albendazole dosage regimens in patients with neurocysticercosis. Clin Neuropharmacol. 1993;16:77–82. doi: 10.1097/00002826-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Takayanagui OM, Lanchote VL, Marques MPC, Bonato PS. Therapy for neurocysticercosis. pharmacokinetic interaction of albendazole sulfoxide with dexamethasone. Ther Drug Monit. 1997;19:51–55. doi: 10.1097/00007691-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Rawden HC, Kokwaro GO, Ward SA, Edwards G. Relative contribution of cytochromes P-450 and flavin-containing monoxygenases to the metabolism of albendazole by human liver microsomes. Br J Clin Pharmacol. 2000;49:313–322. doi: 10.1046/j.1365-2125.2000.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souhaili-El Amri H, Mothe O, Totis M, et al. Albendazole sulfonation by rat liver cytochrome P-450c. J Pharmacol Exp Ther. 1988;46:758–764. [PubMed] [Google Scholar]
- 19.Moroni P, Buronfosse T, Longin-Sauvageon C, Delatour P, Benoit E. Chiral sulfoxidation of albendazole by the flavin adenine dinucleotide-containing and cytochrome P450-dependent monoxygenases from rat liver microsomes. Drug Metab Dispos. 1995;23:160–164. [PubMed] [Google Scholar]
- 20.Lanchote VL, Marques MPC, Takayanagui OM, Carvalho R, Bonato PS. Simultaneous determination of albendazole sulfoxide enantiomers and albendazole sulfone in plasma. J Chromatogr B. 1998;709:273–279. doi: 10.1016/s0378-4347(98)00082-6. [DOI] [PubMed] [Google Scholar]
- 21.Proano JV, Madrazo I, Avelar F, Lopez-Felix B, Diaz G, Grijalva I. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med. 2001;345:879–885. doi: 10.1056/NEJMoa010212. [DOI] [PubMed] [Google Scholar]
- 22.Kearney BP, Aweeka FT. The penetration of anti-infectives into the central nervous system. Neurol Clin. 1999;17:883–900. doi: 10.1016/s0733-8619(05)70171-7. [DOI] [PubMed] [Google Scholar]
- 23.Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System. 2. Saunders, Philadelphia: 1992. [Google Scholar]