Abstract
Aims
We determined whether the start of selective serotonin reuptake inhibitors (SSRI) in levodopa users was followed by a faster increase of antiparkinsonian drug treatment.
Methods
Selected were all levodopa users of 55 years and older from the PHARMO prescription database. The rate of increase of antiparkinsonian drug treatment was compared using Cox's proportional hazard model for starters of SSRI (n = 15) with starters of tricyclic antidepressants (TCA) (n = 31) and with patients not using antidepressants (n = 304), and was adjusted for age, gender, and duration of levodopa use.
Results
The hazard ratio for the SSRI group compared with the TCA group was 4.2 (95% confidence interval 1.4, 12.6) and compared with the second control group was 2.7 (1.2, 5.2).
Conclusions
The start of SSRI therapy in levodopa users is followed by a faster increase of antiparkinsonian drug treatment.
Keywords: antidepressant drugs, levodopa, Parkinson's disease, pharmacoepidemiology, selective serotonin reuptake inhibitors, tricyclic antidepressants
Introduction
Depression occurs in approximately 40% of patients with Parkinson's disease (PD) [1]. Few studies have been performed with antidepressants in these patients. There are no controlled studies with selective serotonin reuptake inhibitors (SSRI) [2]. There are conflicting reports whether SSRI worsen symptoms of PD [3–8]. Because none of these studies included a control group, it was not possible to determine whether a worsening of disease was due to SSRI or to normal disease progression.
We determined from pharmacy records whether in levodopa users start of SSRI treatment is followed by a faster increase of antiparkinsonian drug treatment compared with starters of tricyclic antidepressants (TCA) and compared with patients who did not use antidepressants.
Methods
Data were obtained from the PHARMO system, which includes drug dispensing information for all 300 000 residents of six Dutch cities [9]. All available data from 1985 until 1998 were used.
Selected were all persons aged 55 years and older who used levodopa for at least 180 days. In a previous study we found that 95% of all levodopa users had PD [10]. Levodopa is therefore a reliable marker for PD in pharmacy records. From the levodopa users we selected a group of starters of SSRI, a control group of starters of TCA and a second control group of patients not using any antidepressants. The second control group was included because TCA have anticholinergic properties which may be beneficial in PD [11]. To be included in the SSRI or TCA group, patients had to start such an antidepressant at least 90 days after the first dispensing date of levodopa in the file. Patients, who had used another antidepressant within 180 days before start of SSRI or TCA, were omitted. Patients, who met the criteria for both antidepressant groups, were included in the SSRI group. Start of follow-up in the SSRI and TCA group, was the first dispensing date of the antidepressant. Patients started using SSRI at average 1068 days later than the first dispensing date of levodopa in the file. Start of follow-up in the second control group (no antidepressants) was the first dispensing date of levodopa at least 1068 days later than the start of levodopa. Patients who consecutively used levodopa for less than 90 days were not included. Users of antipsychotics, metoclopramide, cinnarizine and flunarizine were omitted because these drugs can induce parkinsonism.
The endpoint of follow-up was reached if an increase of antiparkinsonian drug treatment occurred within 180 days after start of follow-up. This increase occurred when the dosage of any antiparkinsonian drug was increased, or a new antiparkinsonian drug was started.
A higher rate of increase of antiparkinsonian drug treatment, can be explained by closer monitoring by a physician. We therefore compared the number of days a patient filled a prescription within 90 days after start of follow-up with the unpaired t-test.
The rates of increase of antiparkinsonian drug treatment were compared with use of Cox's proportional hazard model, which enabled adjustment for age, gender, and the time a patient had received levodopa in the file before start of follow-up.
Results
Fifteen patients fulfilled the inclusion criteria for the SSRI group. They used fluoxetine (6 patients), fluvoxamine (3), and paroxetine (6). The 31 persons included in the TCA group used amitriptyline (23), doxepine (1), maprotiline (2), imipramine (1), nortriptyline (2) and clomipramine (2). The second control group included 304 persons. Table 1 lists the characteristics of the three groups at the start of follow-up.
Table 1.
Number, sex, mean age, mean levodopa dosages, mean time on levodopa, number of users of antiparkinsonian drugs other than levodopa, at start of follow-up.
| Characteristic | SSRI | TCA | No antidepressant |
|---|---|---|---|
| Number | 15 | 31 | 304 |
| Male patients | 3 (20%) | 11 (36%) | 146 (48%) |
| Mean age (years) | 71 | 77 | 75 |
| Mean time on levodopa (days) ± s.d. | 1068 ± 816 | 1301 ± 850 | 1140 ± 221 |
| Number of visits to pharmacy* | 9.0 ± 5.0 | 9.7 ± 4.9 | 5.5 ± 3.7 |
| Mean levodopa dosage (mg) ± s.d. | 322 ± 160 | 385 ± 220 | 359 ± 211 |
| Dopamine agonist | 3 (20%) | 9 (29%) | 69 (23%) |
| Selegiline | 5 (33%) | 10 (32%) | 73 (24%) |
| Amantadine | 4 (27%) | 1 (3%) | 31 (10%) |
| Anticholinergics | 3 (20%) | 4 (13%) | 54 (18%) |
SSRI vs TCA P = 0.63, SSRI vs no antidepressant P = 0.001.
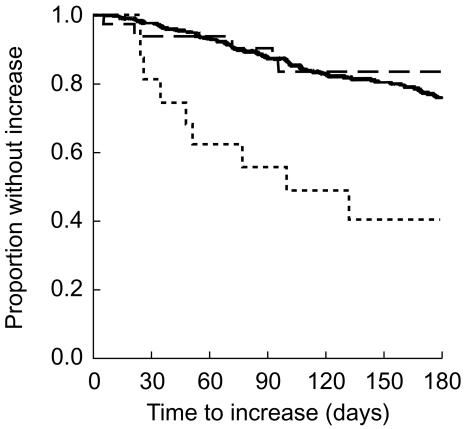
Antiparkinsonian drug treatment increased faster among starters of SSRI compared with both control groups (Figure 1). The crude hazard ratio comparing the SSRI with the TCA group was 5.0 (95% confidence interval 1.7, 14.9); the adjusted hazard ratio was 4.6 (1.4, 15.2). For the comparison of the SSRI group with the second control the results were 4.2 (2.1, 8.4) and 3.8 (1.8, 7.9), respectively.
Figure 1.
Proportion of patients without increase of antiparkinsonian drug treatment (Kaplan Meier estimate) within 180 days after start of follow-up in three groups of users of levodopa: patients who started a selective serotonin reuptake inhibitors (SSRI) (…), patients who started a tricyclic antidepressant (TCA) (--), and patients who did not use an antidepressant (—).
Discussion
This study shows that in levodopa users start of SSRI was accompanied by faster increase of antiparkinsonian drug treatment during the first 180 days of treatment compared with starters of TCA and patients not receiving antidepressants.
In a previous study we found that 95% of the levodopa users had PD. The clinical diagnosis of PD is difficult, because of similarities with other parkinsonian syndromes [12]. However, these syndromes are less common, do usually not respond to levodopa, and can often only be distinguished from PD at a postmortem examination [12]. The vast majority of the patients will therefore have a clinical diagnosis of PD.
There is no relation between disease stage (Hoehn Yahr) and depression [1, 11, 13]. It is therefore not likely that a possible difference in the severity of depression between the groups explains our results.
More than 60% of the prescriptions for either TCA or SSRI are for treatment of depression [14, 15]. The antidepressant groups will therefore for the most part be comparable with respect to the underlying indication. Other indications include anxiety, which will be found a little more in the SSRI group, and insomnia, found more often in the TCA group [14, 15]. Anxiety and insomnia are associated with the ‘off’ state and should be treated by increasing antiparkinsonian drug treatment. SSRI or TCA are only indicated when this approach fails [11]. Our analysis showed that our findings probably cannot be explained by differences in attention of health care providers as measured by visits to community pharmacies. The anticholinergic properties of TCA may be beneficial in PD [11]. However, the increase of antiparkinsonian drug treatment in the TCA group was comparable in patients not using antidepressants. Therefore, the difference between both antidepressant groups is probably explained by worsening of PD in patients starting SSRI.
Although we cannot completely rule out that differences between our groups are partly explained by differences in prescribing practices among physicians, there is further evidence for worsening of PD by SSRI. First, fluoxetine stimulates serotonergic projections from the dorsal raphe that project directly to the substantia nigra and inhibit the firing of the dopaminergic neurones [16]. Second, extrapyramidal symptoms have been reported as side-effect of SSRI [17].
There are conflicting results whether SSRI can be used safely in PD [3–5] or not [6–8]. All of these studies included patients that experienced a worsening of parkinsonian signs. Because the studies did not include a control group, it was not possible to determine whether a worsening occurred more often than normally during the course of disease.
This study suggests that SSRI worsen PD. The findings of this study should be confirmed in a double-blind placebo controlled clinical trial. However, based on our results we feel that physicians should use SSRI with caution in these patients.
Acknowledgments
This study was supported financially by the royal Dutch organization for the advancement of pharmacy (KNMP) and Utrecht University.
References
- 1.Cummings JL. Depression and Parkinson's disease: a review. Am J Psychiatry. 1992;149:443–454. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- 2.Klaassen T, Verhey FRJ, Sneijders GHJM, et al. Treatment of depression in Parkinson's disease: a meta-analysis. J Neuropsychiatry Clin Neurosci. 1995;7:281–286. doi: 10.1176/jnp.7.3.281. [DOI] [PubMed] [Google Scholar]
- 3.Caley CF, Friedman JH. Does fluoxetine exacerbate Parkinson's disease? J Clin Psychiatry. 1992;53:278–282. [PubMed] [Google Scholar]
- 4.Ceravolo R, Nuti A, Piccinni A, et al. Paroxetine in Parkinson's disease: effects on motor and depressive symptoms. Neurology. 2000;55:1216–1218. doi: 10.1212/wnl.55.8.1216. [DOI] [PubMed] [Google Scholar]
- 5.Tesei S, Antonini A, Canesi M, et al. Tolerability of paroxetine in Parkinson's disease: a prospective study. Mov Disord. 2000;15:986–989. doi: 10.1002/1531-8257(200009)15:5<986::aid-mds1034>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Jansen Steur ENH. Increase of Parkinson disability after fluoxetine medication. Neurology. 1993;43:211–213. doi: 10.1212/wnl.43.1_part_1.211. [DOI] [PubMed] [Google Scholar]
- 7.Richard IH, Maughn A, Kurlan R. Do serotonin reuptake inhibitor antidepressants worsen Parkinson's disease? A retrospective case series. Mov Disorder. 1999;14:155–157. doi: 10.1002/1531-8257(199901)14:1<155::aid-mds1026>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Simons JA. Fluoxetine in Parkinson's disease. Mov Disorder. 1996;11:581–582. doi: 10.1002/mds.870110517. [DOI] [PubMed] [Google Scholar]
- 9.Herings RM, Bakker A, Stricker BH, Nap G. Pharmaco-morbidity linkage: a feasibility study comparing morbidity in two pharmacy based exposure cohorts. J Epidemiol Community Health. 1992;46:136–140. doi: 10.1136/jech.46.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Vijver DAMC, Stricker BHC, Breteler MMB, et al. Evaluation of antiparkinsonian drugs as a marker for Parkinson's disease in pharmacy records. Pharm World Sci. 2001;23:148–152. doi: 10.1023/a:1011807919632. [DOI] [PubMed] [Google Scholar]
- 11.Olanow CW, Koller WC. An algorithm (decision tree) for the management of Parkinson's disease: treatment guidelines. American Academy of Neurology. Neurology. 1998;50:S1–S57. doi: 10.1212/wnl.50.3_suppl_3.s1. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Waal MW, Stolk J, van Marwijk HW, Springer MP. Prescription of antidepressants in family practice. Ned Tijdschr Geneeskd. 1996;140:2131–2134. [PubMed] [Google Scholar]
- 15.Isacsson G, Redfors I, Wasserman D, Bergman U. Choice of antidepressants: questionnaire survey of psychiatrists and general practitioners in two areas of Sweden. Br Med J. 1994;309:1546–1549. doi: 10.1136/bmj.309.6968.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldessarini RJ, Marsh ER, Kula NS. Interactions of fluoxetine with metabolism of dopamine and serotonin in rat brain regions. Brain Res. 1992;579:152–156. doi: 10.1016/0006-8993(92)90754-w. [DOI] [PubMed] [Google Scholar]
- 17.Leo RJ. Movement disorders associated with the serotonin selective reuptake inhibitors. J Clin Psychiatry. 1996;57:449–454. doi: 10.4088/jcp.v57n1002. [DOI] [PubMed] [Google Scholar]