Abstract
Aims
To identify the cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) isoforms responsible for the formation of the primary metabolite(s) of zaltoprofen, and to predict possible drug interactions by investigating the inhibition of CYP isoforms in vitro.
Methods
The metabolism of zaltoprofen was studied in vitro using recombinant CYP and UGT isoform cDNA-expression systems. The effects of selective isoform inhibitors on zaltoprofen metabolism were studied using human liver microsomes. The inhibitory effects of zaltoprofen on the metabolism of selective probe substrates for CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4 were also determined in human liver microsomes.
Results
Zaltoprofen was extensively metabolized by CYP2C9 and UGT2B7. CYP2C9 catalysed sulphoxidation but not hydroxylation of zaltoprofen. In the human liver microsomal metabolism study, zaltoprofen metabolism was markedly inhibited by sulphaphenazole, a selective inhibitor of CYP2C9. In the drug interaction study, negligible inhibition (< 15%) of the activities of CYP1A2, CYP2C19, CYP2D6, CYP2E1 and CYP3A4 was apparent at 5 µg ml−1, the maximum plasma concentration observed in humans after oral administration of an 80 mg zaltoprofen tablet. However, zaltoprofen inhibited CYP2C9 by 26% at 5 µg ml−1. At higher concentrations, zaltoprofen produced some inhibition of CYP2C9 (IC50 = 19.2 µg ml−1; 64.4 µm) and CYP3A4 (IC50 = 53.9 µg ml−1; 181 µm). The free drug concentrations in plasma (0.02 µg ml−1, 67.0 nm) at the Cmax of the clinically effective doses are much lower than the IC50 values corrected for the nonspecific binding ratio of zaltoprofen to microsomal protein (15.5 µg ml−1 for CYP3A4, 49.5 µg ml−1 for CYP3A4). Furthermore, the maximum free drug concentrations in the hepatic intracellular was calculated to be 0.068 µg ml−1 and the increase in the AUC in the presence of zaltoprofen was estimated to be only 0.4% for CYP2C9 substrates and 0.1% for CYP3A4 substrates, respectively.
Conclusions
Zaltoprofen is predominantly metabolized by CYP2C9 and UGT2B7, and is considered unlikely to cause significant drug interactions in vivo when coadministered with CYP substrates at clinically effective doses.
Keywords: CYP2C9, human liver microsomes, in vitro metabolism, UGT2B7, zaltoprofen
Introduction
Zaltoprofen, (±)-2-(10,11-dihydro-10-oxodibenzo [b, f] thiepin-2-yl)propionic acid (Figure 1), belongs to the therapeutic class of nonsteroidal anti-inflammatory drugs (NSAIDs), which are used in the treatment of rheumatoid arthritis and osteoarthritis as well as to relieve inflammation and pain after surgery, injury and tooth extraction [1].
Figure 1.
Chemical structures of zaltoprofen and its metabolites.
After oral administration of zaltoprofen to humans, 62% of the dose is excreted as conjugates in the urine and only 3% is excreted as unchanged compound by this route [2]. Zaltoprofen is also biotransformed to S-oxide-zaltoprofen (M-2), 10-hydroxy-zaltoprofen (M-3) and S-oxide-10-hydroxy-zaltoprofen (M-5) in humans, and conjugates of M-2 and M-3 are excreted in the urine, although urinary levels of each of these metabolites account for less than 10% of the dose [2].
The existence and functions of cytochrome P450 (CYP) isoforms in human liver microsomes are well known [3–7]. Numerous CYP isoforms have now been cloned and expressed in various cells using cDNA technology, and these systems have become available for the evaluation of drug metabolism [8, 9]. Furthermore, many reports have described serious adverse effects caused by drug–drug interactions resulting from the inhibition of CYP isoforms [5, 6, 9]. The selective substrates of many individual CYP isoforms have now been identified, and potential drug–drug interactions can be predicted by studying the inhibition of their metabolismin vitro[6, 9–12]. Conjugation with glucuronide is another major metabolic pathway for drugs, and is catalysed by UDP-glucuronosyltransferase (UGT) phase II enzymes [13, 14]. Recombinant human UGTs have also been used to study metabolism in vitro[13, 14]. However, the CYP and UGT enzymes involved in the metabolism of zaltoprofen have not previously been identified.
In the present study, the principal CYP and UGT isoforms involved in the metabolism of zaltoprofen were investigated using human liver microsomes and cDNA-expression systems. Potential interactions between zaltoprofenand other drugs that act as selective substrates for CYPs were also investigated by determining any inhibition of their metabolism in human liver microsomes.
Methods
Chemicals
Zaltoprofen (Lot Nos. P390 and 3007), [14C]-zaltoprofen, S-oxide-zaltoprofen (M-2; 2-(10,11-dihydro-5,10-dioxodibenzo[b, f] thiepin-2-yl) propionic acid), 10-hydroxy-zaltoprofen (M-3; sodium 2-(10,11-dihydro-10-hydroxydibenzo[b, f] thiepin-2-yl) propionate) and S-oxide-10-hydroxy-zaltoprofen (M-5; 2-(10,11-dihydro-10-hydroxy-5-oxodibenzo [b, f] thiepin-2-yl) propionic acid) were supplied from Nippon Chemiphar Co., Ltd. (Tokyo, Japan); (Figure 1).
α-Naphthoflavone, sulphaphenazole, tranylcypromine, quinidine and troleandomycin were purchased from Sigma (MO, USA), furafylline was purchased from Ultrafine Chemicals Ltd (Salford, UK), and ketoconazole was purchased from ICN Biomedicals Inc (CA, USA). Mefenamic acid and omeprazole were purchased from Wako Pure Chemical Industry (Osaka, Japan), diethyldithiocarbamate was purchased from Sigma and Wako.
cDNA-expressed microsomes from baculovirus-infected insect cells for human CYP1A1, CYP1A2, CYP1B1, CYP2B6, CYP2C9*1(Arg144), CYP2C19, CYP2D6*1(Val374), CYP2E1, CYP3A4, flavin-containing monooxygenase (FMO) 3, UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B7 and UGT2B15, and CYP and UGT insect cell control SUPERSOMES® were obtained from Gentest (MA, USA).
Pooled human liver microsomes were obtained from Gentest (Lot No. 16) and Tissue Transformation Technologies (Lot No. HHM-0281, NJ, USA) with the approval of the local ethics committee.
All other reagents used were of at least analytical grade.
Metabolism of zaltoprofen in CYP and UGT cDNA-expression systems
For the metabolic study using CYP isoforms, the reaction mixture (total volume, 0.5 ml) consisted of 40 mm Tris-HCl buffer (pH 7.4) containing 10 mmβ-NADPH, 1 mm EDTA and 6 mm MgCl2, 5 µg ml−1 zaltoprofen, and 2 mg ml−1 microsomal protein from the enzyme cDNA-expression system (160 pmol CYP ml−1). The mixture was incubated at 37 °C for 2 h. For the metabolic study using UGT isoforms, the reaction mixture (total volume of 0.25 ml) consisted of 125 mm Tris-HCl buffer (pH 7.4) containing 0.004% Triton X, 4 mm MgCl2, 0.8 mm UDP-glucuronic acid (UDPGA), 1 µg ml−1 zaltoprofen, and 1 mg ml−1 microsomal protein from the enzyme cDNA-expression system. The mixtures were activated by preincubation at 37 °C for 20 min without zaltoprofen. The reaction was then initiated by adding zaltoprofen, and incubation was continued for 4 h.
The reaction was terminated by adding acetonitrile containing mefenamic acid as an internal standard, and the mixture was centrifuged at 9000g for 10 min. The supernatant was injected onto a high-performance liquid chromatography (h.p.l.c.) column, and the disappearance of zaltoprofen was determined.
Reaction mixtures containing UGT2B7 were also hydrolysed by overnight incubation at 37 °C with β-glucuronidase (type H1, 1000 unit ml−1) in 0.1 m acetate buffer (pH 5.0). After incubation, the samples were treated as described above. The experiments for each isoform were performed in triplicate.
To identify the metabolites of zaltoprofen, the above procedure was carried out using a zaltoprofen concentration of 20 µg ml−1 and 500 pmol ml−1 CYP2C9 at 37 °C. The reaction was allowed to proceed for 20 min, and was terminated by adding 1 m acetate buffer (pH 4.5) and dichloromethane. After adding methyl p-hydroxybenzoate as an internal standard, the mixture was centrifuged at 2000g for 10 min. The organic phase was evaporated to dryness under a stream of nitrogen gas, and the residue was dissolved in the mobile phase used for h.p.l.c.
Effects of chemical inhibitors on the metabolism of zaltoprofen by CYP in human liver microsomes
Chemical enzyme inhibitors were added to the reaction medium in order to determine the quantitative contribution of individual CYP isoforms to zaltoprofen metabolism. The reaction mixture (total volume, 0.5 ml) consisted of 0.1 m Tris-0.1 m HCl buffer (pH 7.4) containing 10 mmβ-NADPH, 1 mm EDTA and 6 mm MgCl2. In the experiment to determine the effect of diethyldithiocarbamate, preincubation was carried out at 37 °C for 15 min before initiating the reaction by adding zaltoprofen. The final concentrations of inhibitors were based on theKi values published in the literature: α-naphthoflavone (0.04, 0.2, 1 µm; CYP1A1/2) [8], tranylcypromine (0.08, 0.4, 2 µm; CYP2A6) [7], sulphaphenazole (0.2, 1, 5 µm; CYP2C9) [3–5,8], omeprazole (0.4, 2, 10 µm; CYP2C19) [5, 6], quinidine (0.04, 0.2, 1 µm; CYP2D6) [3, 5, 8], diethyldithiocarbamate (1, 5, 25 µm; CYP2E1) [3], ketoconazole (0.04, 0.2, 1 µm; CYP3A4) [3, 4].
H.p.l.c. analysis of zaltoprofen and its metabolites
The zaltoprofen content of the mixtures was determined by h.p.l.c. system using an L-Column ODS (4.6 mm i.d. × 250 mm, Chemicals Evaluation and Research Institute, Tokyo, Japan) with a guard column, CAPCELL PAK C18 UG120 (4.0 mm i.d. × 10 mm; Shiseido, Tokyo, Japan) at 35 °C. The mobile phase was acetonitrile: purified water: acetic acid (60 : 40 : 1, v/v/v) and was pumped at a flow-rate of 0.7 ml min−1. The eluate was monitored by a u.v. detector set at 330 nm. In the experiment to identify zaltoprofen metabolites, zaltoprofen and M-2 were simultaneously determined on an analytical column, CAPCELL PAK C18 UG120, 4.6 mm i.d. × 250 mm, with a guard column, CAPCELL PAK C18 UG120, 4.0 mm i.d. × 10 mm at 35 °C. The mobile phase was 65 mm NaH2PO4 containing 5 mm tetrabutylammonium chloride (adjusted to pH 6.0 with triethylamine): acetonitrile (35 : 15, v/v) and was pumped at a flow-rate of 1 ml min−1. The eluate was monitored by a u.v. detector set at 270 nm. The within-run and between-run accuracy and precision of these assays did not deviate by more than 15% from the nominal concentration (20% at the limit of quantification), and the analytes remained stable for at least 48 h in the final sample solution at room temperature.
Inhibition of the metabolism of isoform-selective substrates by zaltoprofen
The following marker substrate activities were used to evaluate the potential inhibitory effects of zaltoprofen on CYP isoforms in human liver microsomes: 7-ethoxyresorufin O-deethylase (CYP1A2) [15], tolbutamide methyl hydroxylase (CYP2C9)[16],S-mephenytoin 4′-hydroxylase (CYP2C19) [17], debrisoquine 4-hydroxylase (CYP2D6) [18], lauric acid 11-hydroxylase (CYP2E1) [19] and testosterone 6β-hydroxylase (CYP3A4) [20]. The experiments were conducted at zaltoprofen concentrations of 5, 15 and 50 µg ml−1 for CYP1A2, CYP2C19, CYP2D6 and CYP2E1, and 5, 10, 20, 35, 50 and 75 µg ml−1 for CYP2C9 and CYP3A4. Inhibition by the selective chemical inhibitors furafylline (30 µm; CYP1A2), sulpha-phenazole (20 µm; CYP2C9), tranylcypromine (100 µm; CYP2C19), quinidine (5 µm; CYP2D6), diethyldithiocarbamate (300 µm; CYP2E1) and troleandomycin (101 µm; CYP3A4) were also investigated. To determine the IC50 values (50% inhibition of the enzymatic activity observed in the absence of the inhibitor) for CYP2C9 and CYP3A4, activity data obtained from the following equation were analysed by nonlinear regression analysis (WinNonlin, Pharsight Corp., USA):
 |
where v0 is the velocity without the inhibitor, v is the observed velocity and [I] is the inhibitor concentration.
Prediction of the inflow concentration (Iin) of the inhibitor into the liver after oral administration can be expressed as follows [10]:
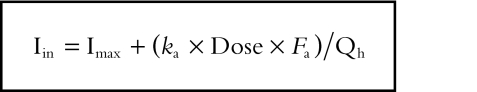 |
where Imax is the maximum concentration of the inhibitor in the blood, ka is the first-order rate constant for gastrointestinal absorption, Fa is the fraction absorbed from the gastrointestinal tract into the portal vein, and Qh is the hepatic blood flow.
Imax is calculated as follows:
 |
where Cmax is the maximum concentration of the inhibitor in the plasma and RBS is the ratio of the concentration in the blood to that in the serum concentration.
The free concentration of zaltoprofen in the liver (Iu,liver) can be calculated as follows:
 |
where fplasma is the unbound fraction in the plasma and RLB is the ratio of the zaltoprofen concentration in the liver to that in the blood.
The data on the zaltoprofen used in the above calculations were obtained from the following sources. Cmax (= 5 µg ml−1) and ka (= 1.84 h−1) values were determined after an oral dose of 80 mg zaltoprofen [2], fplasma (= 0.004) was obtained from a report by Sano et al.[21], RBS (= 0.5) and RLB (= 2.1) from rat radioisotope tracer experiments [22], and Qh (= 1610 ml min−1) from Ito et al.[10]. The Fa value for zaltoprofen was taken to be 1.
Determination of nonspecific binding of zaltoprofen to liver microsomes
The nonspecific binding of zaltoprofen to the human liver microsomal fraction was determined by an ultra-centrifugation method. [14C]-Zaltoprofen (at concentrations of 5, 20 and 75 µg ml−1) was mixed with human liver microsomes in 50 mm Tris-HCl buffer (pH 7.4). The concentrations of microsomal protein were 0.5 and 2 mg ml−1, which were those used for the determination of IC50 values of CYP3A4 and CYP2C9, respectively.
The mixtures were incubated at 37 °C for 5 min, then centrifuged at 105 000g at 10 °C for 1 h. The radioactivity of zaltoprofen was determined by liquid scintillation counting (2500TR, Packard, Meriden, CT, USA). Binding to the microsomal protein was calculated using the equation:
 |
where B is the binding (%), Sup is the d min−1 in the supernatant after centrifugation, and Mix is the d min−1 in the mixture before centrifugation.
Furthermore, the IC50 value corrected by unbound microsomal fraction (ICu,50) was calculated using the equation:
 |
Results
Incubation with human CYP isoforms in a cDNA-expression system
During incubation for 2 h with microsomes from baculovirus-infected insect cells containing human CYPs, the most obvious disappearance of zaltoprofen, a 53.6% reduction compared with initial values, was observed with microsomes expressing CYP2C9 (Figure 2). The percentage decreases produced by CYP3A4 and CYP1A1 were 13.1% and 11.8%, respectively. The degree of metabolism produced by other CYP isoforms was equivalent to that observed with control microsomes (< 10% reduction from initial zaltoprofen). Metabolism by FMO3 enzyme was also negligible.
Figure 2.
Metabolism of zaltoprofen by CYP and FMO3 isoforms in a cDNA-expression system. R; CYP reductase: b5; Cytochrome b5. The values show the percentage reduction from initial values (mean ± s.d., n = 3). The microsomal protein concentration was 2 mg ml−1 (160 pmol CYP ml−1).
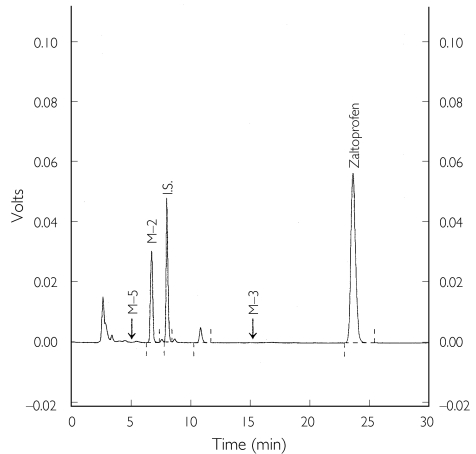
In the experiment to characterize zaltoprofen metabolism by CYP2C9, M-2 was identified as the predominant metabolite, and neither M-3 nor M-5 was observed on the chromatogram (Figure 3). The velocity of M-2 formation was 2.34 ± 0.08 pmol pmol −1 of CYP min−1 (mean ± s.d., n = 3), which was equivalent to the disappearance rate of zaltoprofen (2.23 ± 0.13 pmol pmol −1 of CYP min−1).
Figure 3.
H.p.l.c. profile of zaltoprofen metabolism by human CYP2C9*1 in a cDNA-expression system. Analysis of the sample obtained after incubation for 20 min with CYP2C9*1 (500 pmol CYP ml−1).
Incubation with human UGT isoforms in a cDNA-expression system
During incubation for 4 h with microsomes expressing UGT, the most obvious disappearance of zaltoprofen, a 53.0% reduction from the initial values, was observed with microsomes expressing UGT2B7 (Figure 4). The degree of metabolism observed with other UGT isoforms was equivalent to that produced control microsomes (< 10% reduction from initial zaltoprofen). After enzymatic hydrolysis of the UGT2B7 incubation mixture, the proportion of zaltoprofen metabolized was drastically decreased to 14.3%.
Figure 4.
Metabolism of zaltoprofen by human UGT isoforms in a cDNA-expression system. The values show the percentage reduction from initial values (mean ± s.d., n = 3). The microsomal protein concentration was 1 mg ml−1.
Inhibitory effects of isoform-selective chemical inhibitors on the metabolism of zaltoprofen by human liver microsomes
The effects of selective CYP inhibitors on zaltoprofen metabolism in human liver microsomes are shown in Table 1. After incubation for 30 min in the absence of chemical inhibitors, the rate of disappearance of zaltoprofen from the reaction mixture was between 0.279 and 0.292 nmol min−1 mg−1 of protein, and about 50% of the initial zaltoprofen content was metabolized.
Table 1.
Effect of selective CYP inhibitors on the metabolism of zaltoprofen in human liver microsomes.
| Inhibitor | µmol l-1 | % of control |
|---|---|---|
| a-Naphthoflavone (CYP1A2) | 0.04 | 97.9 ± 3.9 |
| 0.2 | 98.3 ± 2.2 | |
| 1 | 96.1 ± 4.0 | |
| Tranylcypromine (CYP2A6) | 0.08 | 99.9 ± 1.7 |
| 0.4 | 98.2 ± 2.9 | |
| 2 | 97.9 ± 1.3 | |
| Sulfaphenazole (CYP2C9) | 0.2 | 72.7 ± 1.6 |
| 1 | 33.1 ± 1.1 | |
| 5 | 14.6 ± 2.7 | |
| Oumeprazole (CYP2C19) | 0.4 | 96.3 ± 3.5 |
| 2 | 94.3 ± 1.5 | |
| 10 | 86.5 ± 2.5 | |
| Quinidine (CYP2D6) | 0.04 | 95.7 ± 4.4 |
| 0.2 | 94.5 ± 0.4 | |
| 1 | 95.5 ± 2.4 | |
| Diethyldithiocarbamate (CYP2E1) | 1 | 94.6 ± 2.1 |
| 5 | 94.0 ± 1.9 | |
| 25 | 83.5 ± 1.7 | |
| Ketoconazole (CYP3A4) | 0.04 | 92.5 ± 3.9 |
| 0.2 | 92.7 ± 0.3 | |
| 1 | 91.1 ± 1.4 |
The values are mean ± s.d. (n = 3).
This metabolism of zaltoprofen was inhibited by sulphaphenazole in a concentration-dependent manner, decreasing to 14.6% of the control level with 5 µm sulphaphenazole. Zaltoprofen metabolism was also slightly decreased with 25 µm diethyldithiocarbamate (to 83.5% of control), 10 µm omeprazole (to 86.5%) and 1 µm ketoconazole (to 91.1%). The other inhibitors tested did not inhibit zaltoprofen metabolism, which remained at over 95% of the control level even with high concentrations of inhibitors.
Effect of zaltoprofen on CYP isoform activities in human liver microsomes
The potential of zaltoprofen to inhibit the catalytic activities of CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4 was assessed by measuring the metabolism of marker substrates (Table 2). CYP1A2, CYP2C19, CYP2D6 and CYP2E1 were inhibited by less than 15% with zaltoprofen 5 µg ml−1, and by less than 21.1% with 50 µg ml−1. On the other hand, zaltoprofen inhibited the activitiy of CYP2C9, by 25.6% at 5 µg ml−1 and 75.3% at 50 µg ml−1. Furthermore, although the activity of CYP3A4 was decreased by only 3.6% with 5 µg ml−1 of zaltoprofen, the reduction was 48.8% with 51 µg ml−1. IC50 values for these two isoforms were determined as 19.2 µg ml−1 (64.4 µm) for CYP2C9 and 53.9 µg ml−1 (181 µm) for CYP3A4.
Table 2.
Effect of zaltoprofen on CYP activites in human liver microsomes.
| % of inhibition | ||
|---|---|---|
| CYP1A2 (7-Ethoxyresorufin O-deethylase activity) | ||
| Zaltoprofen | 5 mg ml−1 | 0.0 ± 0.0 |
| 15 mg ml-1 | 1.3 ± 2.3 | |
| 50 mg ml-1 | 5.8 ± 5.2 | |
| Furafylline | 30 mmol l-1 | 100.0 ± 0.0 |
| CYP2C9 (Tolbutamide methyl hydroxylase activity) | ||
| Zaltoprofen | 5 mg ml−1 | 25.6 ± 3.6 |
| 20 mg ml-1 | 51.0 ± 0.5 | |
| 50 mg ml−1 | 75.3 ± 6.3 | |
| Sulphaphenazole | 20 mmol l−1 | 85.1 ± 4.8 |
| CYP2C19 (S-Mephenytoin 4’-hydroxylase activity) | ||
| Zaltoprofen | 5 mg ml−1 | 14.8 ± 5.8 |
| 15 mg ml-1 | 8.2 ± 5.2 | |
| 50 mg ml−1 | 16.6 ± 4.7 | |
| Tranylcypromine | 100 mmol l−1 | 74.5 ± 7.9 |
| CYP2D6 (Debrisoquine 4-hydroxylase activity) | ||
| Zaltoprofen | 5 mg ml-1 | 1.4 ± 1.5 |
| 15 mg ml-1 | 2.9 ± 2.5 | |
| 50 mg ml−1 | 4.6 ± 2.2 | |
| Quinidine | 5 mmol l−1 | 60.6 ± 2.6 |
| CYP2E1 (Lauric 11-hydroxylase activity) | ||
| Zaltoprofen | 5 mg ml−1 | 6.9 ± 3.2 |
| 15 mg ml−1 | 12.9 ± 6.8 | |
| 50 mg ml−1 | 21.1 ± 0.2 | |
| Diethyldithiocarbamate | 300 mmol l−1 | 56.6 ± 1.4 |
| CYP3A4 (Testosterone 6b-hydroxylase activity) | ||
| Zaltoprofen | 5 mg ml−1 | 3.6 ± 4.2 |
| 20 mg ml−1 | 26.7 ± 1.6 | |
| 51 mg ml−1 | 48.8 ± 2.4 | |
| Troleandomycin | 101 mmol l-1 | 68.9 ± 2.9 |
The values are mean ± s.d. (n = 3).
Nonspecific binding of zaltoprofen to human liver microsomal protein
The nonspecific binding of zaltoprofen was determined as 4.0–8.2% at 0.5 mg protein ml−1, and 16.7–19.5% at 2 mg protein ml−1, respectively (Table 3). A slight decrease in binding to microsomal subcellular fractions with increase in total zaltoprofen concentration was observed. The minimum free fraction of zaltoprofen was 0.918 at 0.5 mg protein ml−1 and 0.805 at 2 mg protein ml−1, respectively. Therefore, the IC50 values corrected for the nonspecific binding ratio were calculated to be 15.5 µg ml−1 (52.0 µm) for CYP2C9 and 49.5 µg ml−1 (166 µm) for CYP3A4.
Table 3.
Nonspecific binding of zaltoprofen to human liver microsomal protein (%)
| Microsomal protein (mg ml-1) | ||
|---|---|---|
| Zaltoprofen (mg ml-1) | 0.5 | 2 |
| 5 | 8.2 ± 1.8 | 19.5 ± 0.7 |
| 20 | 5.2 ± 0.9 | 16.8 ± 1.3 |
| 75 | 4.0 ± 1.5 | 16.7 ± 1.1 |
The values are mean ± s.d. (n = 3).
Prediction of the drug interaction in clinical setting
Zaltoprofen is 99.6% bound to human plasma proteins; the free fraction in the plasma is therefore only 0.4%[21]. At Cmax (5 µg ml−1), the concentration of free zaltoprofen in the plasma is only 0.02 µg ml−1 (67.0 nm). Thus, the IC50 values corrected by nonspecific binding ratio for CYP2C9 and CYP3A4 are approximately 800 times and 2500 times higher than the free plasma concentration at Cmax, respectively. The maximum inflow concentration of zaltoprofen into the liver (Iin) and the unbound concentration in the hepatic intracellular (Iu,liver) were calculated to be 4.02 and 0.068 µg ml−1, respectively. Comparing the Iu,liver to the IC50 corrected by nonspecific binding ratio, the increase in the AUC in the presence of zaltoprofen was estimated to be only 0.4% for CYP2C9 substrates and 0.1% for CYP3A4 substrates, respectively.
Discussion
To identify the CYP and UGT isoforms involved in the metabolism of zaltoprofen, studies were conducted using human liver microsomes and recombinant human enzymes. Zaltoprofen was extensively metabolized by CYP2C9 expressed in microsomes derived from baculovirus-infected insect cells. In the human liver microsomal metabolism study, zaltoprofen metabolism was markedly inhibited by sulphaphenazole, a selective inhibitor of CYP2C9. CYP2C9 is responsible for the metabolism of a number of drugs in clinical use, such as tolbutamide, S-warfarin, phenytoin and hexobarbitone [11, 23, 24]. A number of other NSAIDs, such as diclofenac, ibuprofen, mefenamic acid, piroxicam and tenoxicam have also been reported to be metabolized by CYP2C9 [23–26]; however, these metabolic changes involved hydroxylation, whereas sulphoxidation was generally catalysed by CYP3A4 or the FMO [27, 28]. It has been reported that CYP2C9 and CYP3A4 catalysed the oxidation of sulphinpyrazone, a uricosuric agent, although CYP3A4 was approximately 10 times more effective than CYP2C9 [28]. The present study of the catalytic activities of isoforms produced by cDNA-expression systems also indicated the involvement of CYP3A4, but not FMO enzymes, in the metabolism of zaltoprofen. The catalytic activitiy of CYP3A4 on zaltoprofen was much smaller than that of CYP2C9, although the CYP3A4 content of the human liver is usually greater than the CYP2C9 content (CYP3A4/CYP2C9 ratio is between 1 and 8) [29]. With this in mind, it was initially speculated that zaltoprofen would be catalysed not only by CYP2C9 but also by CYP3A4, and that M-2 (the sulphoxide-metabolite) might be produced by CYP3A4. However, the involvement of CYP3A4 in zaltoprofen metabolism under physiological conditions may actually be negligible, since ketoconazole, a selective inhibitor of CYP3A4, did not inhibit the metabolism of zaltoprofen by human liver microsomes. In addition, recombinant CYP2C9*1 was found to catalyse the sulphoxidation, but not the hydroxylation, of zaltoprofen. It is therefore interesting that the sulphoxidation of zaltoprofen involved CYP2C9, but not CYP3A4 or FMO. Furthermore, the present results highlight the need to verify data obtained with cDNA-expression systems with those from incubations with human liver microsomes before the identification of CYP isoforms involved in the metabolism of a drug can be confirmed.
A further main metabolite of zaltoprofen, the glucuronide, was produced by UGT2B7 and hydrolysed by β-glucuronidase. UGTs are encoded by multiple genes of at least two families, and the UGT2 family is divided into two subfamilies [13, 14]. Enzymes of the UGT2B subfamily catalyse the glucuronidation of several endogenous compounds (including bile acids, steroids, fatty acids and carboxylic acids) and exogenous substrates such as phenolic compounds and drugs [13, 14]. UGT enzymes are abundantly expressed in the liver and other organs, and their substrate specificity is generally considered to be low. UGT1A3 and UGT2B7 isoforms have been reported to be involved in the glucuronidation of NSAIDs in human liver. The present results showed that the ability of UGT2B7 to glucuronidate zaltoprofen was about seven times greater than that of UGT1A3. Therefore, zaltoprofen glucuronidation is mainly catalysed by UGT2B7, and other UGT isoforms contribute little to its metabolism under physiological conditions.
Serious adverse effects caused by drug–drug interactions are a great cause for concern, and any significant information that can be obtained from in vitro studies using human liver microsomes may be valuable in avoiding such incidents in the clinical setting [6, 9, 10]. Indeed, several methods of predicting clinical drug interactions have been established [9, 10]. In the present drug interaction study, the activities of CYP1A2, CYP2C19, CYP2D6, CYP2E1 and CYP3A4 were inhibited to a negligible extent (< 15%) by zaltoprofen 5 µg ml−1, the maximum plasma concentration observed in humans after oral administration of an 80 mg zaltoprofen tablet. However, at this concentration, zaltoprofen inhibited CYP2C9 activity by 25.6%, whilst at 10 times the maximum plasma concentration it inhibited CYP2C9 activity by 75.3% and CYP3A4 activity by 48.8%. It was therefore considered appropriate to determine IC50 values for the effect of zaltoprofen on the activities of both these enzymes so that the likelihood of drug interactions could be predicted. The IC50 values were determined as 19.2 µg ml−1 (64.4 µm) for CYP2C9 and 53.9 µg ml−1 (181 µm) for CYP3A4. It is generally accepted that comparing the free concentration of a drug in the plasma with its IC50 or Ki value will give an estimation of the likelihood of drug interactions [10]. The nonspecific binding of an enzyme inhibitor to microsomal protein must also be taken into account to avoid underestimation of the degree of inhibition. However, the IC50 values corrected for the nonspecific binding ratio were only slightly changed to be 15.5 µg ml−1 (52.0 µm) for CYP2C9 and 49.5 µg ml−1 (166 µm) for CYP3A4, since the binding of zaltoprofen to microsomal protein was very low. The free fraction of zaltoprofen in the plasma is only 0.4%[21], this is consistent with zaltoprofen's acidity, since acidic drugs are highly bound to plasma proteins but show low binding to microsomes [30]. At Cmax, the concentration of free zaltoprofen in the plasma is only 0.02 µg ml−1 (67.0 nm). This is much lower than the total plasma concentration. Thus, the IC50 values corrected for nonspecific binding ratio for CYP2C9 and CYP3A4 are approximately 800 times and 2000 times higher than the amount of free zaltoprofen presented in the plasma at Cmax, respectively, even allowing for the slight change in IC50 resulting from the correction for nonspecific binding. Similar results were obtained when the ratio of the areas under the plasma level vs time curves (AUCs) for CYP2C9 and CYP3A4 substrates in the presence and absence of zaltoprofen were calculated from the prediction method[10]. The unbound concentration of zaltoprofen in the liver was calculated to be 0.068 µg ml−1 and the increase in the AUC in the presence of zaltoprofen was estimated to be only 0.4% for CYP2C9 substrates and 0.1% for CYP3A4 substrates, respectively. Taken together, these findings suggest that zaltoprofen is unlikely to cause significant drug–drug interactions involving these isoforms. Indeed, there have as yet been no reports of clinically serious drug interactions with zaltoprofen; this may result from the fact that zaltoprofen is metabolized mainly by UGT2B7 rather than by CYP isoforms.
References
- 1.Ito A, Mori Y. Effect of a novel anti-inflammatory drug, 2-(10, 11-dihydro-10-oxo-dibenzo [b, f] -thiepin-2-yl) propionic acid (CN-100), on the proteoglycan biosynthesis in articular chondrocytes and prostaglandin E2 production in synovial fibroblasts. Res Commun Chem Pathol Pharmacol. 1990;70:131–142. [PubMed] [Google Scholar]
- 2.Sasaki K, Iizuka K, Sano H, Miwa M, Haruki S. Pharmacokinetics of CN-100 for 80 mg tablet of final preparation in healthy volunteers. Jpn Pharmacol Ther. 1992;20:2167–2174. [Google Scholar]
- 3.Newton DJ, Wang RW, Lu AYH. Cytochrome P450 inhibitors: evaluation of specificities in the in vitro metabolism of therapeutic agents by human liver microsomes. Drug Metab Dispos. 1995;23:154–158. [PubMed] [Google Scholar]
- 4.Baldwin SJ, Bloomer JC, Smith GJ, et al. Ketoconazole and sulphaphenazole as the respective selective inhibitors of P4503A and 2C9. Xenobiotica. 1995;25:261–270. doi: 10.3109/00498259509061850. [DOI] [PubMed] [Google Scholar]
- 5.Moody GC, Griffin SJ, Mather AN, McGinnity DF, Riley RJ. Fully automated analysis of activities catalysed by the major human liver cytochrome P450 (CYP) enzymes: assessment of human CYP inhibition potential. Xenobiotica. 1999;29:53–75. doi: 10.1080/004982599238812. [DOI] [PubMed] [Google Scholar]
- 6.Furuta S, Kamada E, Suzuki T, et al. Inhibition of drug metabolism in human liver microsomes by nizatidine, cimetidine and omeprazole. Xenobiotica. 2001;31:1–10. doi: 10.1080/00498250110035615. [DOI] [PubMed] [Google Scholar]
- 7.Taavitsainen P, Juvonen R, Pelkonen O. In vitro inhibition of cytochrome P450 enzymes in human liver microsomes by a potent CYP2A6 inhibitor, trans-2-phenylcyclopropylamine (tranylcypromine), and its nonamine analog, cyclopropylbenzene. Drug Metab Dispos. 2001;29:217–222. [PubMed] [Google Scholar]
- 8.Ono S, Hatanaka T, Hotta H, et al. Specificity of substrate and inhibitor probes for cytochrome P450s: evaluation of in vitro metabolism using cDNA-expressed human P450s and human liver microsomes. Xenobiotica. 1996;26:681–693. doi: 10.3109/00498259609046742. [DOI] [PubMed] [Google Scholar]
- 9.Ohyama K, Nakajima M, Suzuki M, et al. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49:244–253. doi: 10.1046/j.1365-2125.2000.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K, Iwatsubo T, Kanamitsu S, et al. Prediction of pharmacokinetic alterations caused by drug–drug interactions: metabolic interaction in the liver. Pharmacol Rev. 1998;50:387–411. [PubMed] [Google Scholar]
- 11.Kohl C, Steinkellner M. Prediction of pharmacokinetic drug/drug interactions from in vitro data: interactions of the nonsteroidal anti-inflammatory drug lornoxicam with oral anticoagulants. Drug Metab Dispos. 2000;28:161–168. [PubMed] [Google Scholar]
- 12.Prakash C, Kamel A, Cui D, et al. Identification of the major human liver cytochrome P450 isoform (s) responsible for the formation of the primary metabolites of ziprasidone and prediction of possible drug interactions. Br J Clin Pharmacol. 2000;49(Suppl 1):35S–42S. doi: 10.1046/j.1365-2125.2000.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin C, Miners JO, Lillywhite KJ, Mackenzie PI. Complementary deoxyribonucleic acid cloning and expression of a human liver uridine diphosphate-glucuronosyltransferase glucuronidating carboxylic acid-containing drugs. J Pharmacol Exp Ther. 1993;264:475–479. [PubMed] [Google Scholar]
- 14.Coffman BL, King CD, Rios GR, Tephly TR. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y (268) and UGT2B7H (268) Drug Metab Dispos. 1998;26:73–77. [PubMed] [Google Scholar]
- 15.Burke MD, Prough RA, Mayer RT. Characteristics of a microsomal cytochrome P-448-mediated reaction. Ethoxyresorufin O-de-ethylation. Drug Metab Dispos. 1977;5:1–8. [PubMed] [Google Scholar]
- 16.Miners JO, Smith KJ, Robson RA, et al. Tolbutamide hydroxylation by human liver microsomes. Kinetic characterisation and relationship to other cytochrome P-450 dependent xenobiotic oxidations. Biochem Pharmacol. 1988;37:1137–1144. doi: 10.1016/0006-2952(88)90522-9. [DOI] [PubMed] [Google Scholar]
- 17.Chauret N, Gauthier A, Martin J, Nicoll-Griffith DA. In vitro comparison of cytochrome P450-mediated metabolic activities in human, dog, cat, and horse. Drug Metab Dispos. 1997;25:1130–1136. [PubMed] [Google Scholar]
- 18.Kronbach T, Mathys D, Gut J, Catin T, Meyer UA. High-performance liquid chromatographic assays for bufuralol 1′-hydroxylase, debrisoquine 4-hydroxylase, and dextromethorphan O-demethylase in microsomes and purified cytochrome P-450 isozymes of human liver. Anal Biochem. 1987;162:24–32. doi: 10.1016/0003-2697(87)90006-6. [DOI] [PubMed] [Google Scholar]
- 19.Parker GL, Orton TC. Induction by oxyisobutyrates of hepatic and kidney microsomal cytochrome P-450 with specificity towards hydroxylation of fatty acids. In: Gustafsson JÅ, Carlstedt-Duke J, Mode A, Rafter J, editors. Biochemistry, Biophysics and Regulation of Cytochrome P-450. Amsterdam: Elsevier/North Holland Biomedical Press; pp. 373–377. [Google Scholar]
- 20.Sonderfan AJ, Arlotto MP, Dutton DR, McMillen SK, Parkinson A. Regulation of testosterone hydroxylation by rat liver microsomal cytochrome P-450. Arch Biochem Biophys. 1987;255:27–41. doi: 10.1016/0003-9861(87)90291-8. [DOI] [PubMed] [Google Scholar]
- 21.Sano H, Segawa Y, Furuta S, et al. Drug interaction of zaltoprofen, a novel anti-inflammatory drug, and its effect on hepatic drug metabolizing enzyme. Jpn Pharmacol Ther. 1995;23:1477–1487. [Google Scholar]
- 22.Haruki S, Yamada A. Absorption, distribution, metabolism and excretion of CN-100, (±) -2-(10, 11-dihydro-10-oxodibenzo[b, f]thiepin-2-yl) propionic acid in rats. Jpn Pharmacol Ther. 1990;18:3843–3863. [Google Scholar]
- 23.Carlile DJ, Hakooz N, Bayliss MK, Houston JB. Microsomal prediction of in vivo clearance of CYP2C9 substrates in humans. Br J Clin Pharmacol. 1999;47:625–635. doi: 10.1046/j.1365-2125.1999.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miners JO, Birkett DJ. Cytochrome P4502C9 is an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–538. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JH, Begg EJ, Kennedy MA, Roberts R, Barclay ML. Is cytochrome P450 2C9 genotype associated with NSAID gastric ulceration? Br J Clin Pharmacol. 2001;51:627–630. doi: 10.1046/j.0306-5251.2001.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takanashi K, Tainaka H, Kobayashi K, et al. CYP2C9 Ile359 and Leu359 variants: enzyme kinetic study with seven substrates. Pharmacogenetics. 2000;10:95–104. doi: 10.1097/00008571-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Hodgson E, Levi PE. Species, organ and cellular variation in the flavin-containing monooxygenase. Drug Metabol Drug Interact. 1988;6:219–233. doi: 10.1515/dmdi.1988.6.3-4.219. [DOI] [PubMed] [Google Scholar]
- 28.He M, Rettie AE, Neal J, Trager WF. Metabolism of sulfinpyrazone sulfide and sulfinpyrazone by human liver microsomes and cDNA-expressed cytochrome P450s. Drug Metab Dispos. 2001;29:701–711. [PubMed] [Google Scholar]
- 29.Tang C, Shou M, Rushmore TH, et al. In-vitro metabolism of celecoxib, a cyclooxygenase-2 inhibitor, by allelic variant forms of human liver microsomal cytochrome P450 2C9: correlation with CYP2C9 genotype and in-vivo pharmacokinetics. Pharmacogenetics. 2001;11:223–235. doi: 10.1097/00008571-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27:1350–1359. [PubMed] [Google Scholar]