Abstract
The established place of regular long-acting β2-adrenoceptor agonists at step 3 in asthma management guidelines has evolved as a consequence of evidence showing additive effects of salmeterol and formoterol on exacerbation rates, resulting in a putative inhaled corticosteroid sparing effect. There is however, evidence to show that although long-acting β2-adrenoceptor agonists facilitate using a lower dose of inhaled corticosteroid, this may occur at the expense of suboptimal anti-inflammatory control. This is likely to be the case especially with fixed dose combination inhalers where it is not possible to properly titrate anti-inflammatory therapy with inhaled corticosteroids without also inadvertently overtreating with unnecessarily high doses of long-acting β2-adrenoceptor agonists. Most patients with mild to moderate persistent asthma can be adequately controlled on monotherapy with inhaled corticosteroid in low or medium dosage, which is considerably cheaper than concomitant use of a long-acting β2-adrenoceptor agonist. Subsensitivity to long-acting β2-adrenoceptor agonists is a predictable pharmacological phenomenon which occurs despite concomitant inhaled corticosteroid therapy and occurs more readily for bronchoprotective than bronchodilator effects. Subsensitivity of salbutamol protection against bronchoconstrictor stimuli occurs in patients receiving concomitant long-acting β2-adrenoceptor agonists, which may be due to β2-adrenoceptor down-regulation or prolonged receptor occupancy. Prospective large scale long-term studies are required to further define the clinical relevance of β2-adrenoceptor polymorphisms, to look at clinical control outcomes as well as propensity for subsensitivity. It would therefore make more sense to first of all optimize the dose of anti-inflammatory therapy with inhaled corticosteroid and to then consider adding a long-acting β2-adrenoceptor agonist for patients who are poorly controlled.
Keywords: asthma, β2-adrenoceptor agonists, formoterol, salmeterol, subsensitivity
Introduction
The asthma triad is characterized by airway inflammation with associated airway hyperresponsiveness and reversible airflow obstruction. The pivotal role of the inflammatory cascade in the asthmatic disease process is well established. This in turn has driven asthma management guidelines in terms of emphasizing the use of inhaled corticosteroids as the cornerstone of anti-inflammatory therapy for persistent asthma [1, 2]. At the same time there has been increasing awareness of the potential for dose-related systemic adverse effects of inhaled corticosteroids, especially with the more potent lipophilic drugs such as fluticasone propionate [3].
The use of inhaled β2-adrenoceptor agonists has turned full circle over the past two decades. During the 1970s and 1980s, evidence emerged to suggest that regular use of short-acting β2-adrenoceptor agonists may have adverse effects on asthmatic disease control, although it was unclear whether this was due to a rebound increase in airway hyperresponsiveness or subsensitivity to protection against bronchoconstrictor stimuli [4, 5]. Other studies have subsequently failed to substantiate the deleterious effects of regular salbutamol [6, 7]. This led to the recommendation in guidelines for only using short-acting β2-adrenoceptor agonists on demand for relief of acute episodes of bronchoconstriction. Indeed, the dose of inhaled corticosteroid is conventionally titrated against short-acting β2-adrenoceptor agonist reliever use, as a surrogate marker of asthmatic disease activity [2].
During the 1990s, the availability of long-acting β2-adrenoceptor agonists such as salmeterol and formoterol led to a change in perception with regard to the use of regular β2-adrenoceptor agonists in asthma therapy. This came from studies which showed that diurnal asthma control was better with regular twice daily long-acting β2-adrenoceptor agonist as compared with a regular four times daily short-acting β2-adrenoceptor agonist [8, 9]. Moreover, a number of studies showed that the addition of regular twice daily long-acting β2-adrenoceptor agonist to an inhaled corticosteroid conferred improvements in terms of reducing exacerbations of asthma [10–13]. These data were taken on board into management guidelines for the treatment of moderate to severe persistent asthma, whereby regular long-acting β2-adrenoceptor agonists were advocated as add-on controller therapy to low or medium dose inhaled corticosteroid, in order to obviate corticosteroid related systemic adverse effects. This recommendation has been reinforced by the recent availability of new combination inhalers, which contain a fixed dose of inhaled corticosteroid in conjunction with a long-acting β2-adrenoceptor agonist – i.e. fluticasone/salmeterol or budesonide/formoterol. The arguments for improved disease control and better compliance with combination inhalers has resulted in increased use of regular long-acting β2-adrenoceptor agonists in the management of persistent asthma, both in Europe and the USA.
The purpose of this review article is therefore to critically appraise the use of long-acting β2-adrenoceptor agonists, focusing on their effects on asthmatic inflammation and exacerbations, the occurrence of subsensitivity with their regular use and the effects of β2-adrenoceptor genotype. It is not within the scope of this brief review to provide an in depth appraisal of the use of regular short-acting β2-adrenoceptor agonists, although certain studies will be mentioned where appropriate to illustrate important points.
Effects on airway inflammation
A number of studies have evaluated the effects of long-acting β2-adrenoceptor agonists on airway inflammatory cells as assessed in induced sputum, bronchoalveolar lavage and bronchial biopsy, which have revealed conflicting results. Two studies comparing regular salmeterol with placebo in corticosteroid naïve or corticosteroid treated asthmatics showed no effects on inflammatory cells from bronchoalveolar lavage or bronchial biopsy [14, 15]. Li and coworkers evaluated the effects of supplementary treatment over 12 weeks with regular salmeterol 50 µg twice daily, fluticasone propionate 100 µg twice daily or placebo, on submucosal inflammatory cells in symptomatic asthmatics taking up to 500 µg per day of inhaled corticosteroid [16]. Salmeterol produced a significant but variable within group reduction in the number of EG1 positive eosinophils but not mast cells, lymphocytes or macrophages, but this effect was not significant compared to placebo. Interestingly the positive control with inhaled fluticasone was found to have no significant reduction in inflammatory cells, further questioning the relevance of the small salmeterol response. Additionally, neither salmeterol nor fluticasone significantly affected the cell profile on bronchoalveolar lavage. Wallin et al. showed that regular formoterol 24 µg twice daily significantly decreased the number of submucosal eosinophils and mast cells, but not T-cells comparing pre and post treatment, although this was not significant compared to placebo [17]. In contrast inhaled budesonide 400 µg twice daily produced a significant reduction in mast cells, eosinophils and T-cells as compared to the placebo group. Other data have shown that treatment with either regular twice daily formoterol 24 µg or budesonide 400 µg produces a significant fall in number of submucosal activated EG2 positive eosinophils and epithethial expression of activated nuclear factor kappa B compared with placebo, although for formoterol these changes were not accompanied by reduced immunoreactivity for adhesion molecules or cytokines [18].
Kips and coworkers evaluated induced sputum after an initial nonrandomized run-in period with budesonide 1600 µg daily. Over the subsequent 12 month randomized treatment period, step-down therapy to budesonide 800 µg daily on its own resulted in a nonsignificant increase in sputum eosinophils from 0.88% to 1.74%, as compared with an increase from 0.60% to 3.41% during step-down with budesonide 200 µg daily plus formoterol 9 µg twice daily [19]. The proportion of patients with a median sputum eosinophil count less than 2.5% was not different between the groups −42% with budesonide 200 µg plus formoterol vs 65% with budesonide 800 µg alone. There were no significant differences in the proportion of EG2 + cells in the budesonide plus formoterol group (from 1.5% to 3.09%) compared with budesonide alone (from 0.75 to 2.63). However, the clinical relevance of these data are uncertain due to the inherent variability in sputum eosinophilia and emphasizes the need for larger studies looking at this endpoint. Not surprisingly, the patients taking formoterol in the study had better domiciliary peak flow rates as compared with the group taking the higher dose of budesonide as monotherapy [20].
In another study of severe asthmatics, rapid tapering of inhaled corticosteroid was evaluated in patients randomized to receive add-on therapy with placebo or salmeterol, until an exacerbation occurred [21]. The addition of salmeterol facilitated a greater degree of steroid tapering, although this occurred at the expense of a progressive increase in airway eosinophilia. The mean inhaled corticosteroid dose at exacerbation was 227 µg per day in the salmeterol group vs 612 µg per day in the placebo group, whilst at the week before exacerbation sputum eosinophils were 20% in the salmeterol group vs 9% in the placebo group. At the same time point prior to exacerbation, patients taking salmeterol had apparently better asthma control in terms of improved lung function and symptoms as well as reduced reliever medication, suggesting that salmeterol may mask the underlying airway inflammation in patients who are receiving suboptimal doses of inhaled corticosteroid. It is unclear whether such effects would be seen during more gradual tapering as occurs in real-life clinical practise during step-down therapy with inhaled corticosteroid in addition to salmeterol. Calhoun et al. showed no effect of salmeterol on the bronchoalveolar lavage cell profile after segmental allergen challenge despite improvements in lung function, concluding that salmeterol exhibited no clinically meaningful anti-inflammatory activity in vivo[22].
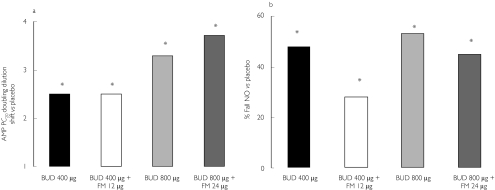
In a study of mild to moderate asthmatics, after an initial steroid free run-in period, patients were randomized in crossover fashion to receive once daily budesonide with or without formoterol over a 4 week period [23]. The results showed that formoterol 9 µg or 18 µg daily conferred no significant additive anti-inflammatory effects to budesonide 400 µg or 800 µg daily, in terms of improvements in bronchial hyperresponsiveness to adenosine monophosphate challenge (the primary endpoint) or exhaled breath nitric oxide, whilst formoterol produced significantly better domiciliary peak flows and associated better patient rated preference (Figure 1). This also illustrates the importance of distinguishing between the effects of treatment on β2-adrenoceptor agonist responsive endpoints such as lung function and reliever requirements as opposed to inflammatory surrogates.
Figure 1.
Effects of low or medium dose inhaled budesonide given alone or in combination with formoterol, for effects on (a) bronchial hyperresponsiveness to adenosine monophosphate challenge and (b) suppression of exhaled nitric oxide. Asterisk denotes a significant (P < 0.05) difference from placebo. Formoterol (FM) did not potentiate the response to budesonide (BUD) at either dose. Modified after [23].
Effects on asthma exacerbations
Several studies have been performed comparing adding in salmeterol with a given dose of inhaled corticosteroid as compared with doubling the dose of inhaled corticosteroid, looking at exacerbations over a period of up to 6 months [24]. These types of studies were based on the assumption that any significant additive effect of salmeterol on exacerbations would be due to the presence of putative anti-inflammatory activity on top of that seen with inhaled corticosteroid. However as salmeterol exhibits little or no clinically meaningful anti-inflammatory activity in vivo, such additive effects on exacerbations are more likely to be due to its prolonged duration of action on airway smooth muscle, resulting in an airway stabilizing effect, which is complimentary to the anti-inflammatory effects of an inhaled corticosteroid. An alternative explanation might be that the sustained bronchodilatation with salmeterol increases peripheral airway calibre and consequently improves aerosol delivery for the inhaled corticosteroid. Although there are some preliminary in vitro data to suggest the possibility of potentiated nuclear translocation of the cytosolic glucocorticoid receptor complex by salmeterol, this putative synergistic process has never actually been demonstrated in vivo[25]. Indeed any clinical effects of combined therapy with inhaled corticosteroid and long-acting β2-adrenoceptor agonist on asthma control can be explained solely on the grounds of their additive effects on the separate components of airway inflammation and smooth muscle.
In a meta-analysis of nine parallel group trials comparing adding in salmeterol with increasing the dose of inhaled corticosteroid, none of the individual studies showed any difference for effects on exacerbation rates [24]. However the pooled estimate for moderate or severe exacerbations from the meta-analysis of 3685 patients showed a significant difference in favour of adding in salmeterol amounting to 2.42% (95% CI 0.24%, 4.6%). This equated to needing to treat 40 patients with salmeterol in order to prevent a moderate or severe exacerbation in one additional patient.
In a randomized controlled trial over 4 months, patients who were suboptimally controlled on inhaled steroid (triamcinolone 800 µg daily) had the dose reduced by half for 2 months followed by subsequent elimination for 2 months, with the addition of salmeterol 50 µg twice daily or placebo in two groups; with the third group receiving salmeterol in the presence of an unchanged inhaled corticosteroid dose throughout the 4 months [26]. For the primary outcome variable of percentage treatment failures during inhaled corticosteroid reduction and elimination, there was no overall difference between the placebo or salmeterol groups (47.4%vs 43.2%, respectively), whilst the control group who received salmeterol with an unchanged inhaled corticosteroid dose had significantly fewer treatment failures (12.2%). Unfortunately, in this trial there was no comparator limb where patients had their inhaled corticosteroid dose left unchanged, but without the addition of salmeterol. In a study of persistent asthmatics controlled on inhaled corticosteroid (triamcinolone 800 µg daily), switching to placebo or salmeterol 50 µg twice daily alone over 4 months resulted in a comparable deterioration of asthma control which was not seen in patients who continued with inhaled corticosteroid [27]. Moreover the deterioration with salmeterol was accompanied by worsening of surrogate inflammatory markers including sputum eosinophils, eosinophilic cationic protein and tryptase, exhaled nitric oxide and methacholine hyperresponsiveness. Both of these studies [26, 27] point to a lack of any clinical meaningful anti-inflammatory activity exhibited by salmeterol.
Three key studies have evaluated the effects of inhaled formoterol on asthma exacerbations. In patients with moderate persistent asthma over a 12 month period, in comparison with budesonide 100 µg twice daily, there was a 26% reduction in severe exacerbations with budesonide 100 µg twice daily plus formoterol 9 µg twice daily, a 49% reduction with budesonide 400 µg twice daily and a 63% reduction with budesonide 400 µg twice daily plus formoterol 9 µg twice daily [20]. The addition of formoterol to either low or medium dose budesonide conferred a significant further reduction in exacerbations, although the effect of increasing the dose of budesonide alone was significantly superior than adding in formoterol to the lower dose of budesonide. This clearly shows that the dose of inhaled corticosteroid should first be optimized before considering adding in a regular long-acting β2-adrenoceptor agonist. It is also interesting to note that the peak flow rate was significantly higher in the group taking low dose budesonide plus formoterol as compared with the group taking medium dose budesonide alone, despite the latter exhibiting fewer exacerbations and a relatively lower degree of airway eosinophilia [19]. This reinforces the potential pitfalls of following β2-adrenoceptor agonist responsive endpoints such as lung function in patients taking long-acting bronchodilators.
In another study of moderate persistent asthmatics taking inhaled corticosteroids, patients over 12 weeks were randomized to receive add-on therapy with formoterol or terbutaline for on demand reliever use, in order to evaluate both safety and efficacy [28]. The time to the first severe exacerbation was significantly (P = 0.013) longer in the formoterol group than in the terbutaline group. The relative risk of a first severe exacerbation was significantly reduced by formoterol (relative risk = 0.55, 95% CI 0.34, 0.89), while the relative risk was not significant for having a first exacerbation defined by the need for oral corticosteroids (relative risk = 0.61, 95% CI 0.35, 1.06). In a third study, corticosteroid naïve and corticosteroid treated mild persistent asthmatics were evaluated over 12 months. In corticosteroid naïve patients budesonide 200 µg daily alone significantly reduced exacerbations compared with placebo, whilst adding in formoterol 4.5 µg twice daily had no further significant effect [29]. In corticosteroid treated patients there was no difference between 200 µg and 400 µg daily of inhaled budesonide on its own, although adding in formoterol produced a significant further small reduction in exacerbations. In the corticosteroid treated group, patients were presumably already near the plateau in the dose–response curve for budesonide response prior to enrolment in the study, which would have biased the result towards showing a significant additive response to a long-acting β2-adrenoceptor agonist. Indeed there was no placebo control group with which to compare the response to the budesonide alone. For the majority of patients with mild persistent asthma a low dose of inhaled corticosteroid as monotherapy would be sufficient to achieve adequate asthma control.
To summarize this section, optimizing the dose of inhaled corticosteroid alone will have the single greatest impact on the underlying asthmatic inflammatory process and associated exacerbations. This should always be the first logical step before considering adding in a long-acting β2-adrenoceptor agonists in patients who are symptomatic and requiring regular reliever therapy. Fixed dose combination inhalers do not allow for inhaled corticosteroid titration or flexibility, especially during initiation of asthma therapy or during exacerbations when patients may require a higher dose of inhaled corticosteroid. This is analogous to putting salt and pepper in the same shaker and trying to adjust to achieve the correct taste on each occasion. There may be a period when certain trigger factors are present which aggravate asthma control, when patients may need to use a long-acting β2-adrenoceptor agonist, but by the same token there may also be periods when the disease is more quiescent and patients may manage on an inhaled corticosteroid on its own, with the dose titrated according to the patients needs.
In some respects it would be logical to directly substitute a short-acting with a long-acting β2-adrenoceptor agonist to use on demand and allow the patient to decide how frequently to use it. The likelihood is that patients probably end up adopting this dosing strategy in real life. In the UK formoterol, which is a fast onset long-acting β2-adrenoceptor agonist, is indicated for use in this way on an on demand basis up to a maximum daily dose of 36 µg, whilst salmeterol, which has a slower onset of action, is only licensed for regular twice daily use and is therefore inherently less flexible. Moreover, the regular use of a long-acting β2-adrenoceptor agonist will mask the requirement for reliever therapy, which in itself is an important marker of disease activity. By taking a long-acting β2-adrenoceptor agonist on demand, its frequency of use can be used as a marker of whether or not the underlying inflammatory process has been adequately suppressed.
There is however, another argument to advocate using combination inhalers, in that the patients' perception of improved airway calibre may in theory help to reinforce long-term compliance with the inhaled corticosteroid moiety, although this remains unproven. It is however, difficult for the clinician to know which outcome measure should be used to optimize the inhaled corticosteroid dose, especially as the dose–response against lung function and symptoms is relatively flat and the exacerbation frequency may vary from one every 6–12 months to one every 12–24 months. The main problem is that there are no readily available reliable surrogate inflammatory markers which can be used in everyday clinical practice.
β2-adrenoceptor subsensitivity
Subsensitivity to the effects of regular β2-adrenoceptor ag-onist therapy occurs as a consequence of β2-adrenoceptor down-regulation and associated subsensitivity of response. In vitro studies in human lung β2-adrenoceptors have shown that acute exposure to salbutamol, salmeterol and formoterol at a concentration of 1 µm leads to a significant reduction in β2-adrenoceptor density within 24 h and a significant reduction in expression of β2-adrenoceptor mRNA within 4 h [30]. The degree of clinical subsensitivity which is evident in patients taking regular β2-adrenoceptor agonist therapy will depend on the degree of functional antagonism exhibited by the prevailing bronchomotor tone. For the bronchodilator effect of a β2-adrenoceptor agonist, there is a weak degree of functional antagonism conferred by a low level of basal bronchomotor tone, which results in a minimal degree of clinical subsensitivity. The opposite applies to the bronchoprotective effect of a β2-adrenoceptor agonist, where there is a strong degree of functional antagonism in the presence of high bronchomotor tone, which results in a maximal degree of clinical subsensitivity. For studies which have evaluated subsensitivity with long-acting β2-adrenoceptor agonists it is also important to consider whether they were administered in corticosteroid naïve or corticosteroid treated patients, as the latter would be more clinically relevant in terms of current asthma management guidelines, which advocate using long-acting β2-adrenocpetor agonists only as add-on therapy at step 3 to inhaled corticosteroids. This may also be relevant as corticosteroids may have facilitatory effects on β2-adrenoceptor regulation and responsiveness in vitro.
Bronchodilator subsensitivity
In a double-blind crossover study in corticosteroid naïve mild persistent asthmatics, treatment was given for 4 weeks with regular low dose (200 µg four times daily) or high dose (1000 µg four times daily) salbutamol or placebo, followed by a dose–response curve to salbutamol administered as repeated cumulative doubling doses, to mimic what might happen during an acute asthma attack [31]. For the bronchodilator dose–response curve as change in FEV1 from baseline, there was no effect after regular treatment with low or high dose salbutamol as compared with placebo. In contrast for the systemic dose–response curve as the chronotropic or hypokalaemic delta response, there was a progressive right-ward shift, which was greater with high dose vs low dose salbutamol, or in turn vs placebo. This suggests that β2-adrenoceptors are more prone to subsensitivity in systemic compared with airway tissue, at least in corticosteroid naïve patients taking regular short-acting β2-adrenoceptor agonist therapy. However one can question the clinical relevance of these data as patients would not normally be taking regular short-acting β2-adrenoceptor agonists in the absence of background inhaled corticosteroid therapy.
Two similar but separate studies were performed with regular formoterol in moderate persistent asthmatics receiving inhaled corticosteroid therapy [32, 33]. In both of these studies after an initial β2-adrenoceptor agonist free run-in period, patients were randomized to receive in crossover fashion treatment for 4 week with either regular formoterol 24 µg twice daily or placebo, with a dose–response curve to cumulative repeated doubling doses of formoterol being performed 2–24 h after the last dose of each randomized treatment. The results showed a persistent degree of down-regulation and desensitization of peripheral blood lymphocyte β2-adrenoceptors, which was accompanied by a significant rightward shift in the bronchodilator and systemic dose–response curves. Moreover, the degree of subsensitivity for the bronchodilator response was greater at 6 h after the last cumulative dose of formoterol as compared to the peak response. In a multicentre study by Pauwels and coworkers, the addition of regular formoterol to budesonide 200 µg or 800 µg daily exhibited a 50% loss of bronchodilator effect on domiciliary peak flow rate within the first 2 weeks of treatment, but thereafter remained at a predictable level throughout the rest of the 12 month study period, with values being higher than treatment with inhaled budesonide alone [20].
Bronchoprotective subsensitivity
There are two broad types of bronchial challenge testing which can be performed in the laboratory, namely those using direct bronchoconstrictor stimuli acting on airway smooth muscle (e.g. methacholine or histamine), and those stimuli acting indirectly to produce bronchoconstriction via airway inflammatory cells (e.g. allergen or adenosine monophosphate challenge) or via neural mechanisms (e.g. exercise or cold air challenge). Everyday bronchoconstrictor stimuli are mediated via indirect mechanisms and so these types of challenges may be considered to be more clinically relevant. The protective effect of a β2-adrenoceptor agonist against a bronchoconstrictor stimuli may be considered kindred to what might happen in acute asthma where there is an increase in bronchomotor tone. Regular treatment with short-acting β2-adrenoceptor agonists in corticosteroid naïve mild asthmatics results in subsensitivity to its bronchoprotective effects, which is more evident with an indirect than a direct bronchoconstrictor stimulus [34, 35].
It is therefore not surprising that bronchoprotective subsensitivity also occurs to a marked degree with regular long-acting β2-adrenoceptor agonists, where there is persistent β2-adrenoceptor occupancy over the 12 h dosing interval with a twice daily regimen. Several studies with regular twice daily salmeterol have shown reproducible subsensitivity developing between first and last dose protection against methacholine challenge, in either corticosteroid naïve or corticosteroid treated patients [26, 36–41]. Indeed subsensitivity to salmeterol protection against methacholine can be demonstrated within 24 h after initiating treatment [42]. Similar findings have been reported in studies with regular salmeterol showing loss of bronchoprotection against exercise or allergen challenge [43–45]. In a parallel group study of corticosteroid treated adult asthmatics, the residual degree of bronchoprotection against methacholine as add-on therapy was not significant after 2 weeks and was the same for formoterol 18 µg twice daily, 4.5 µg twice daily or 9 µg once daily [46]. This indicates that there is no appreciable recovery of β2-adrenoceptors due to persistent diurnal occupancy, even in association with a 24 h dosing interval. In the same study, a comparison was made as a control with terbutaline 500 µg four times daily which showed 3.9 fold vs 1.9 fold shift in methacholine hyperresponsiveness after the first and last dose, respectively, as compared to a 6.4 fold and 1.5 fold respective shift with formoterol 9 µg once daily, with a 5.5 fold and 1.6 fold response shift with formoterol 4.5 µg twice daily. Similar results were observed in a different study of corticosteroid treated asthmatics using adenosine monophosphate challenge, where formoterol 24 µg twice daily produced a 6.7 fold and 1.7 fold shift after the first and last doses, respectively, as compared to 5.2 fold and 1.7 fold shift after formoterol 24 µg once daily, with the residual protection being nonsignificant compared to placebo with both dosing regimens [47]. Simons et al. have also shown subsensitivity to exercise protection occurring with once daily salmeterol in asthmatic children [48].
It is also pertinent to consider whether other second-line nonsteroidal agents, such as leukotriene-receptor antagonists, show the same degree of bronchoprotective subsensitivity during chronic dosing. Two studies have been performed comparing montelukast 10 mg once daily vs salmeterol 50 µg twice daily over 8 weeks, showing sustained protection with the leukotriene-antagonist and loss of protection with the long-acting β2-adrenoceptor agonist [49, 50]. In a study using adenosine monophosphate challenge in corticosteroid treated asthmatics, add-on therapy for 2 weeks with montelukast 10 mg once daily resulted in sustained bronchoprotection compared with placebo at 24 h after the first dose and 2 weeks, whilst for salmeterol 50 µg twice daily significant protection was seen after the first but not the last dose [51].
Interaction between long and short-acting β2-adrenoceptor agonists
As well as evaluating subsensitivity to the long-acting β2-adrenoceptor agonist itself, it is also important to consider whether there is cross subsensitivity to a short-acting β2-adrenoceptor agonist such as salbutamol. Current asthma management guidelines recommend using a long-acting β2-adrenoceptor agonist as add-on therapy in patients not controlled on an inhaled corticosteroid, but in addition to have a short-acting β2-adrenoceptor agonist available for on demand relief.
The first study to properly evaluate this potential interaction was in moderate persistent adult asthmatics receiving inhaled corticosteroids, who after a 2 week run-in period without β2-adrenoceptor agonists, were randomized in crossover fashion to receive 4 weeks of double-blind treatment with either salmeterol 50 µg twice daily or placebo [52]. A dose–response curve to inhaled salbutamol was performed at 36 h after the last dose of each randomized treatment as well as measurement of peripheral blood lymphocyte β2-adrenoceptor parameters. Treatment with salmeterol was associated with a significant decrease in β2-adrenoceptor numbers at 36 h after the last dose as compared to placebo, while at the same time point the baseline lung function was not significantly different. The bronchodilator dose–response curve to salbutamol showed a rightward shift with salmeterol as compared to placebo for FEV1 (P = 0.06) and peak flow (P = 0.04). Regression analysis from the salbutamol dose–response curves showed that to produce a 0.5 l change in FEV1 response, a 2.5 fold higher dose of salbutamol was required after salmeterol vs placebo, while to produce an 80 l min−1 change in peak flow response, a 4 fold higher dose of salbutamol was required after salmeterol. However, there was no significant difference in the final value for peak absolute FEV1 response to cumulative doses of salbutamol, expressed as a percentage of the predicted normal value. In a study of asthmatic children, the bronchodilator dose–response to terbutaline was completely blunted by salmeterol, whereas the response was preserved after treatment with placebo [53].
Other studies evaluating the salbutamol bronchodilator response after salmeterol have shown no evidence of cross-subsensitivity, although these studies did not have an adequate washout period without salmeterol prior to the salbutamol dose–response curve [54, 55]. This carryover effect of salmeterol resulted in there being relatively little room for improvement in response to salbutamol. For example, in the study of Nelson et al. [55] after 4 weeks of treatment with salmeterol the mean increase in FEV1 between 180 µg and 2880 µg of salbutamol amounted to 0.2 l, whilst in the study of Wilding et al. [54], there was only a 0.1 l improvement between 90 µg and 720 µg of salbutamol. It is therefore meaningless to draw any valid conclusions regarding the lack of bronchodilator cross-subsensitivity to salbutamol, in the presence of such a flat dose–response curve [56].
It is probably more clinically relevant to look at studies which have evaluated cross-subsensitivity to salbutamol where bronchoprotective effects were measured in the presence on increase bronchomotor tone. Yates et al. evaluated methacholine protection at 15 min after salbutamol, before and after 1 week of treatment with salmeterol 50 µg twice daily, during concomitant treatment with placebo or inhaled budesonide. Salmeterol induced cross-subsensitivity to salbutamol bronchoprotection, was seen in the presence of concomitant budesonide or placebo, with the only difference being that the budesonide group started from and fell to a higher value [57]. A similar degree of blunting of the acute bronchoprotective effects of salbutamol against methacholine challenge has been reported in other studies in patients treated with salmeterol compared to placebo [40, 41].
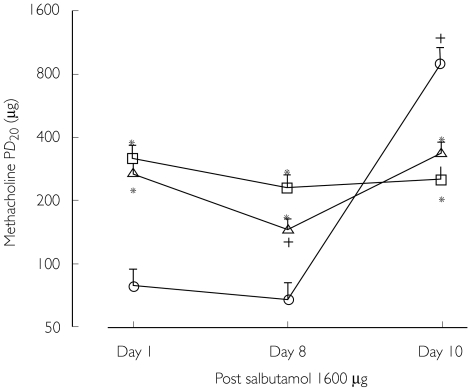
The potential for interaction with salbutamol was investigated further in a study of corticosteroid treated asthmatics, who after an initial 1 week β2-adrenocptor agonist free run-in period, were randomized to receive crossover treatments for 7 days with either placebo, salmeterol 50 µg twice daily or formoterol 12 µg twice daily [58]. Methacholine challenge performed after 7 days at 1 h showed a 2.1 fold shift with salmeterol and a 3.4 fold shift with formoterol, as compared with placebo pretreatment. Patients continued treatment for a further 2 days when a third methacholine challenge was performed 1 h after a single 1600 µg dose of salbutamol, having taken the last dose of randomized treatment 12 h previously. On this occasion, the salbutamol protection afforded after placebo pretreatment was 3.6 fold higher than after formoterol pretreatment and 2.7 fold higher than after salmeterol pretreatment (Figure 2). Thus, a high bolus dose of salbutamol did not overcome bronchoprotective subsensitivity in asthmatic patients receiving regular salmeterol or formoterol. In order to establish whether this interaction was due to subsensitivity per se or due to prolonged receptor occupancy by the long-acting β2-adrenoceptor agonist, a study of corticosteroid treated asthmatics was performed who were randomized to receive in double-blind crossover fashion single doses of inhaled placebo, formoterol 9 µg or salmeterol 50 µg, followed 12 h later by a single dose of 400 µg or 1600 µg or salbutamol, with methacholine challenge performed on each of six separate occasions 1 h after salbutamol administration [59]. There was a significant difference in salbutamol protection between low and high dose salbutamol after placebo, but not after formoterol or salmeterol. Moreover protection with high dose salbutamol after placebo was 1.6 fold higher than low dose salbutamol after formoterol, and 1.9 fold higher than low dose salbutamol after salmeterol. Thus, salmeterol and formoterol both caused a significant degree of in vivo antagonism of salbutamol induced bronchorelaxation in methacholine contracted bronchi, which could be caused by prolonged occupancy of airway β2-adrenoceptors by long-acting β2-adrenoceptor agonists, or possibly by early subsensitivity after single dose exposure.
Figure 2.
Protection against methacholine after the first (day 1) and fifteenth dose (day 8), and eighteenth dose (day 10 with salbutamol) of continuous treatment with placebo (○) and formoterol (□) or salmeterol (▵). * denotes P < 0.05 between active treatments and placebo. † denotes P < 0.05 among day 1, 8 or 10 of the same treatment. Taken from reference [58] with permission.
Van der Woude et al. also looked at methacholine induced bronchoconstriction in corticosteroid treated asthmatics, but evaluated the acute bronchorelaxant effect of salbutamol at the end of the methacholine challenge, showing blunting of salbutamol recovery in patients receiving twice daily salmeterol 100 µg or formoterol 18 µg compared with placebo [60]. The immediate bronchodilating effect of salbutamol in the first 3 min relative to the lowest FEV1 after methacholine, amounted to an increase of 26% after placebo, 14% after formoterol and 12% after salmeterol. Finally, in patients who exhibited exercise induced bronchoconstriction, a comparison was made of fluticasone 100 µg twice daily alone, fluticasone with salmeterol 50 µg twice daily or fluticasone with montelukast 10 mg once daily [61]. The recovery over 30 min after salbutamol administration was evaluated following a standardized exercise challenge, showing blunting and delay of salbutamol protection in patients treated with the combination of fluticasone and salmeterol.
These findings together indicate a significant blunting of the acute response to salbutamol used as reliever therapy in patients taking long-acting β2-adrenoceptor agonists, which is evident after single and chronic dosing, in the presence of low and high bronchomotor tone. It is likely that this phenomenon is due to the occurrence of subsensitivity as well as prolonged receptor occupancy by the long-acting β2-adrenoceptor agonist. In the presence of increased bronchomotor tone, the subsensitivity of airway β2-adrenoceptors is not overcome by administering a higher dose of salbutamol, suggesting that receptor occupancy or uncoupling of the β2-adrenoceptor complex may have occurred.
Effects of corticosteroids on subsensitivity
Corticosteroids are known to reverse the process of β2-adrenoceptor agonist induced down-regulation, by increasing β2-adrenoceptor gene transcription, which may occur rapidly within 2 h of administration [62, 63]. Intravenous infusion of corticosteroid in rats protects against down-regulation of pulmonary β2-adrenoceptors induced by isoprenaline [64]. In patients with asthma, high dose inhaled fluticasone propionate 2 mg daily did not have any significant facilitatory effects on peripheral blood lymphocyte β2-adrenoceptor parameters, in contrast to significant suppression of early morning plasma cortisol [65, 66]. This was in contrast to the effects of a single dose of oral prednisolone 50 mg which produced increased lymphocyte β2-adrenoceptor binding density and increased cyclic-AMP response to isoprenaline, as well as producing suppression of plasma cortisol. This suggested a dissociation in systemic sensitivity between the effects of an inhaled corticosteroid on adrenal suppression and lymphocyte β2-adrenoceptor regulation with fluticasone propionate. In healthy volunteers, concomitant administration of prednisolone 15 mg daily and inhaled formoterol 24 µg twice daily, produced protection against subsensitivity of systemic β2-adrenoceptor responses to inhaled salbutamol, although prednisolone did not prevent ex vivo down-regulation of peripheral blood lymphocyte β2-adrenoceptors despite causing significant cortisol suppression [67]. In other study of healthy volunteers, concomitant therapy with high dose inhaled budesonide 1.2 mg twice daily with inhaled formoterol 24 µg twice daily prevented desensitization of cardiac β2-adrenoceptor response to salbutamol [68].
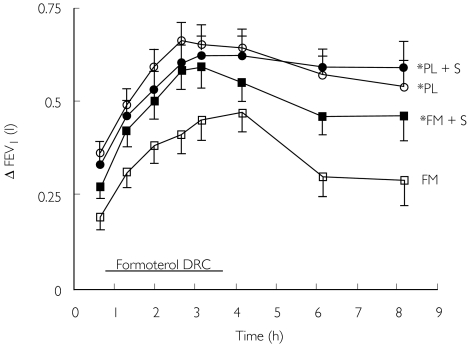
The more clinically relevant question is what happens to asthmatics who are receiving regular long-acting inhaled β2-adrenoceptor agonists in terms of airway β2-adrenoceptor responsiveness following acute administration of systemic or inhaled corticosteroid. Patients with moderate persistent asthma who were receiving inhaled corticosteroids, were randomized to receive 4 weeks of either inhaled placebo or formoterol 24 µg twice daily in a double-blind crossover design [69]. A dose–response curve and duration time profile for formoterol was produced at 3 weeks, 1 h after administration of placebo injection and tablets, and at 4 weeks 1 h after administration of oral prednislone 50 mg tablets together with intravenous hydrocortisone injection 200 mg. There was a significant rightward shift in the bronchodilator dose–response curve to inhaled formoterol after pretreatment with inhaled formoterol as compared to placebo. This shift was rapidly reversed by administration of systemic steroid and was also mirrored by effects on peripheral blood lymphocyte β2-adrenoceptors (Figure 3). This finding was similar to the previous nonrandomized and controlled study by Ellul-Mikallef et al. where intravenous prednisolone restored the bronchodilator response to a single 200 µg dose of isoprenaline, given 60 min after injection in 10 patients who had previously been shown to be nonresponsive to isoprenaline [70]. Holgate et al. in a study of normal subjects showed reversal of the salbutamol dose–response at 16 h after administration of intravenous hydrocortisone [71].
Figure 3.
Bronchodilator dose–response curve (DRC) to formoterol and time profile after last dose for change in FEV1, after regular treatment with placebo (PL) and formoterol (FM), with or without acute administration of systemic corticosteroid (S). * denotes a significant (P < 0.05) difference vs formoterol alone for area under curve. Taken from reference [69] with permission.
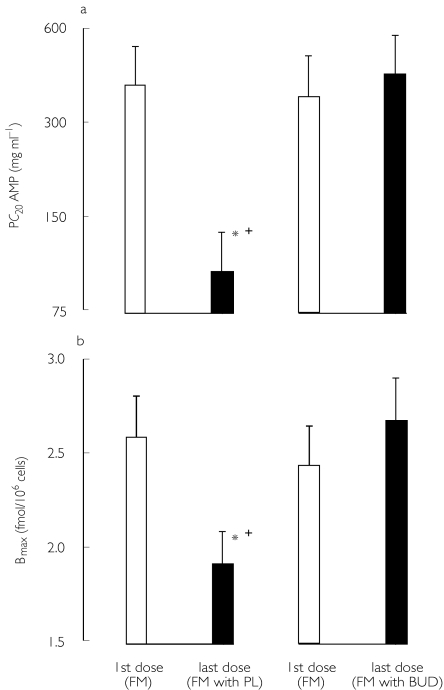
An evaluation was made to compare the effects of acute administration of a bolus dose of intravenous or inhaled corticosteroid on bronchodilator responsiveness. Patients with moderate to severe persistent asthma who were taking inhaled corticosteroids were evaluated in a placebo controlled randomized double-blind crossover design [72]. Patients received treatment for 2 weeks with placebo or formoterol 18 µg twice daily, followed by a bronchodilator dose–response to salbutamol, measured 2 h after bolus administration of placebo, intravenous hydrocortisone 200 µg or inhaled budesonide 1.6 mg. There was a significant rightward shift of the salbutamol bronchodilator dose–response curve induced by formoterol alone compared with placebo, along with partial reversal by inhaled or intravenous corticosteroid administration. In a further study, the acute effects of a high dose of inhaled corticosteroid were evaluated on the bronchoprotective effects of inhaled formoterol [73]. Patients with mild to moderate asthma or receiving inhaled corticosteroids were randomized in a double-blind crossover design to receive two separate 1 week treatment period with formoterol 24 µg twice daily, along with a single bolus dose of placebo or budesonide 1600 µg taken in conjunction with the last dose of each randomized treatment, with adenosine monophosphate challenge performed 2 h after the first and last dose of formoterol (Figure 4). Peripheral blood lymphocyte β2-adrenoceptor binding density was also measured before and after treatment with formoterol. The results showed significant desensitization for the protective effect of formoterol against adenosine monophosphate challenge in association with significant down-regulation of lymphocyte β2-adrenoceptor binding density, which were both rapidly reversed 2 h after administration of inhaled budesonide. This observation suggests the possibility that high dose inhaled corticosteroids should be administered as soon as possible along with β2-adrenoceptor agonists during an acute episode of bronchoconstriction. Whether this effect can be achieved using for example a combination budesonide/formoterol combination inhaler in the setting of early phase of an acute asthma attack, requires to be investigated.
Figure 4.
Effects of regular formoterol (FM) after the first or last dose of treatment. Effects are shown for (a) the provocative concentration of adenosine monophosphate required to produce a 20% fall in FEV1 (PC20 AMP) or (b) peripheral blood lymphocyte β2-adrenoceptor binding density (Bmax). * denotes a significant difference between the first and last dose of formoterol, whilst cross denotes significant difference between the last dose of formoterol plus placebo vs the last dose of formoterol with budesonide. Taken from reference [73] with permission.
Effects of β2-adrenoceptor polymorphism
Single nucleotide polymorphisms at positions 16 and 27 of the β2-adrenoceptor are common in asthmatic and nonasthmatic subjects and may have modifying effects on the asthmatic phenotype as well as on the response to β2-adrenoceptor agonists [74]. In vitro studies in transfected cell lines have shown that the gycline-16 form of the receptor is more prone to agonist promoted down-regulation and subsensitivity than the arginine-16 form [75, 76]. The glutamic acid-27 form, which is in linkage disequilibrium with the glycine-16 form of the receptor, confers relative protection against down-regulation and subsensitivity as compared with the glutamine-27 form. The homozygous glycine-16 genotype occurs in approximately 40% of patients, whilst the arginine-16 genotype occurs in approximately 10% of patients [77, 78]. Increased bronchial hyperresponsiveness and nocturnal asthma have been shown to be associated with the homozygous glycine-16 genotype [78, 79].
In terms of modifying the response to β2-adrenoceptor agonist therapy, Turki and colleagues showed that high doses of inhaled metaproterenol produced profound down-regulation of alveolar macrophage and bronchial epithethial β2-adrenoceptors in normal volunteers who had the homozygous glycine-16 genotype [80]. Lima et al. showed a significantly reduced bronchodilator response to 8 mg of oral salbutamol in patients with the homozygous or heterozygous glycine-16 genotype as compared with patients with the homozygous arginine genotype [81]. Martinez and colleagues showed that children with the homozygous glycine-16 genotype were significantly less likely to respond to a bolus dose of 200 µg salbutamol as compared with those with the homozygous arginine-16 genotype [82]. There are however, conflicting findings to suggest that the homozygous arginine-16 genotype may be associated with worse control in patients taking regular short-acting β2-adrenoceptor agonists. Israel et al. showed that patients with the homozygous arginine-16 genotype who were regularly using salbutamol had a significantly lower morning peak expiratory flow than patients with the same genotype who used salbutamol on an as needed basis, whilst there was no decline in peak flow associated with regular salbutamol in patients with the homozygous glycine-16 genotype [83]. In another retrospective analysis, patients with the homozygous arginine-16 genotype were more susceptible to asthma exacerbations during regular treatment with salbutamol but not salmeterol [84]. According to the dynamic model of receptor kinetics the glycine-16 genotype would be more prone to down regulation from endogenous catectolamines as compared to the arginine-16 genotype. Consequently, patients who possess the arginine-16 genotype would be more susceptible to the effects of exogenous β2-adrenoceptor agonists as their receptors have not yet been down regulated. However, these findings with regular salbutamol are of little clinical relevance as current guidelines do not advocate the use of short-acting β2-adrenoceptor agonists on a regular basis.
There are relatively few clinical studies which have looked at the effect of β2-adrenoceptor polymorphisms in patients taking long-acting β2-adrenoceptor agonists. Sixty patients with stable mild to moderate asthma were evaluated having being washed out of short or long-acting β2-adrenoceptor agonists, followed by measurement of peripheral blood lymphocyte β2-adrenoceptor cyclic AMP response to isoprenaline and the acute protective effect of a single dose of inhaled formoterol 18 µg against methacholine challenge, with a posthoc analysis of β2-adrenoceptor genotype [85]. The results showed that polymorphisms at position 16 or 27 did not influence stimulated coupling of lymphocyte β2-adrenoceptors or the degree of functional antagonism exhibited by inhaled formoterol. This in turn suggested that a single dose of β2-adrenoceptor agonist when used on demand would afford equal bronchoprotection regardless of genetic polymorphism. This of course does not address the more important question of whether genetic polymorphism of β2-adrenoceptors determines response to regular treatment. A retrospective analysis of β2-adrenoceptor polymorphisms was made of patients who were taking regular inhaled formoterol or terbutaline, which showed that bronchoprotective subsensitivity occurred readily in response to short or long-acting β2-adrenoceptor agonist exposure, irrespective of polymorphisms at position 16 or 27, although the study was not properly powered to compare genotypes [86].
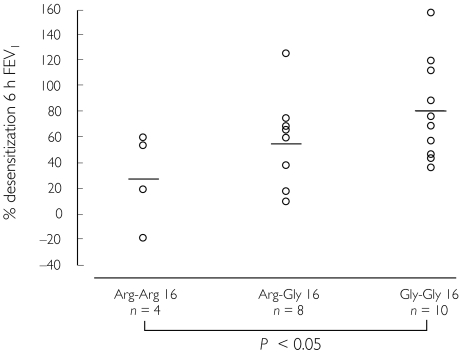
In a study of 22 moderate persistent asthmatics receiving inhaled corticosteroid, the degree of bronchodilator desensitization was evaluated in patients receiving formoterol 24 µg twice daily for 4 weeks as compared with placebo in crossover fashion [87]. This showed that the homozygous glycine-16 genotype conferred greater susceptibility to bronchodilator desensitization after regular formoterol as compared with the homozygous arginine-16 genotype, with the heterozygous-16 genotype response being in between (Figure 5). In a prospective study of corticosteroid treated mild to moderate persistent asthmatics expressing the homozygous glycine-16 genotype, patients were randomized to receive treatments for 1 week in double-blind crossover fashion comprising placebo, formoterol 9 µg twice daily or salmeterol 50 µg twice daily, with methacholine challenges performed at baseline after washout, as well as 12 h after the first and last dose [88]. For bronchodilator effects on FEV1, significant improvements were seen with both formoterol and salmeterol after the first but not the last dose. For methacholine protection, there were significant but small effects after the first and last doses with both drugs, which amounted to less than a one doubling dose shift in hyperresponsiveness. It was also interesting that there was no difference in chronic dosing effects after the last dose when comparing the two drugs, particularly as formoterol has a much higher degree of intrinsic agonist activity, which might be expected to produce greater down-regulation and subsensitivity in genetically susceptible patients.
Figure 5.
Individual scatterplot with mean values for the degree of bronchodilator desensitization after regular formoterol, according to β2-adrenoceptor genotype as position 16. Data are shown for FEV1 response. Taken from reference [87] with permission.
This also begs a question as to whether in genetically susceptible patients who express the homozygous glycine-16 genotype, the use of a leukotriene receptor antagonist, such as montelukast, might be a better option than a regular long-acting β2-adrenoceptor agonist, such as formoterol. In a randomized double-blind crossover design of corticosteroid receiving mild to moderate persistent asthmatics, add-on therapy with either montelukast 10 mg once daily or formoterol 9 µg twice daily was given for 2 weeks, with measurements made at the end of the dosing interval (12 h for formoterol and 24 h for montelukast), for lung function and protection against adenosine monophosphate challenge [89]. For effects on the forced mid expiratory flow rate, the first but not the last dose of formoterol conferred significant bronchodilator effects, whilst neither dose of montelukast produced significant improvements. For the primary outcome variable of adenosine monophosphate challenge, there was a significant improvement with the first but not the last dose of formoterol, whilst montelukast conferred sustained improvements amounting to a one doubling dilution shift after both the first and last dose. Neither drug significantly improved domiciliary peak expiratory flows, whilst the last dose of montelukast but not formoterol produced a significant reduction in peripheral blood eosinophil count. This suggests that in genetically susceptible patients who would be expected to fare worse with a long-acting β2-adrenoceptor agonist, the alternative option of a leukotriene receptor antagonist would seem to be the logical choice, where a significant additive effect was seen to inhaled corticosteroid in terms of bronchial hyperresponsiveness and eosinophil suppression. Further prospective large scale studies are needed with long-acting β2-adrenoceptor agonists as add-on therapy to inhaled corticosteroids to compare the glycine-16 and arginine-16 genotypes, in terms of commonly used endpoints such as lung function, symptom control and exacerbation rates. This may be feasible using buccal mouth washes from large multicentre studies where long-term effects of long-acting β2-adrenoceptor agonists are being evaluated.
Conclusions and the way forward
Current asthma management guidelines have driven the increasing use of fixed dose combination inhalers containing an inhaled corticosteroid with long-acting β2-adrenoceptor agonist. Since long-acting β2-adrenoceptor agonists do not exhibit any meaningful in vivo anti-inflammatory activity, their effects on exacerbations are due solely to sustained bronchodilator activity resulting in airway smooth muscle stabilization, which are complementary to the anti-inflammatory properties of inhaled corticosteroids. Increasing the dose of inhaled corticosteroid has a greater effect on exacerbations, while adding in a long-acting β2-adrenoceptor agonist confers greater improvements in lung function, symptoms and a relatively smaller effect on exacerbations. However, increasing the corticosteroid dose may in some cases be associated with increased systemic adverse effects.
β2–adrenoceptor down-regulation and associated subsensitivity to long-acting β2-adrenoceptor agonists is a predictable pharmacological phenomenon which occurs despite concomitant inhaled corticosteroid therapy and is determined by β2-adrenoceptor polymorphisms at codon 16. More long-term data are required to further characterize the clinical importance of β2-adrenoceptor polymorphisms in terms of asthma control parameters including exacerbations. As a consequence of β2-adrenoceptor subsensitivity and prolonged receptor occupancy, the rescue response to reliever therapy with salbutamol may be blunted by long-acting β2-adrenoceptor agonists, especially in the presence of increased bronchomotor tone as might occur during an acute episode of bronchoconstriction.
As the nature of the asthmatic disease process is for a varying degree of airway inflammation and bronchomotor tone over time, there may be a rationale for using a long-acting β2-adrenoceptor agonist with a fast onset of action (such as formoterol) in the same way as a short-acting β2-adrenoceptor agonist, for on demand reliever therapy, rather than unnecessarily exposing a patient to year round regular twice daily therapy, which is not always required. However subsensitivity occurs even when using long-acting β2-adrenoceptor agonists on a once daily basis. Administering the two components separately permits greater flexibility to adjust the dose of inhaled corticosteroid up and down throughout the year, which is not possible with a fixed dose combination inhaler. Most patients with mild to moderate asthma can be controlled using monotherapy with a low to medium dose of inhaled corticosteroid, which is considerably cheaper than adding in a long-acting β2-adrenoceptor agonist.
The spouse and mother of the author have stockholdings in GlaxoSmithKline who make salmeterol and fluticasone/salmeterol combination. The author has received financial support for clinical trials involving formoterol from Novartis and AstraZeneca. The Asthma and Allergy Research Group have received occasional financial support for postgraduate meetings from AstraZeneca and GlaxoSmithKline, and second-hand computer equipment from GlaxoSmithKline.
References
- 1.Lipworth BJ. Modern drug treatment of chronic asthma. Br Med J. 1999;318:380–384. doi: 10.1136/bmj.318.7180.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The British Guidelines on asthma management. 1995 review and position statement. Thorax. 1997;52(Suppl 1):S1–S21. [Google Scholar]
- 3.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999;159:941–955. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 4.Sears MR, Taylor DR, Print CG, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–1396. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- 5.Spitzer WO, Suissa S, Ernst P, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326:501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 6.Chapman KR, Kesten S, Szalai JP. Regular vs as-needed inhaled salbutamol in asthma control. Lancet. 1994;343:1379–1382. doi: 10.1016/s0140-6736(94)92520-8. [DOI] [PubMed] [Google Scholar]
- 7.Dennis SM, Sharp SJ, Vickers MR, et al. Regular inhaled salbutamol and asthma control: the TRUST randomised trial. Therapy Working Group of the National Asthma Task Force and the MRC General Practice Research Framework. Lancet. 2000;355:1675–1679. doi: 10.1016/s0140-6736(00)02238-8. [DOI] [PubMed] [Google Scholar]
- 8.Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salmeterol with albuterol in the treatment of mild-to- moderate asthma. N Engl J Med. 1992;327:1420–1425. doi: 10.1056/NEJM199211123272004. [DOI] [PubMed] [Google Scholar]
- 9.Lundback B, Rawlinson DW, Palmer JB. Twelve month comparison of salmeterol and salbutamol as dry powder formulations in asthmatic patients. European Study Group. Thorax. 1993;48:148–153. doi: 10.1136/thx.48.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet. 1994;344:219–224. doi: 10.1016/s0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- 11.Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med. 1996;153:1481–1488. doi: 10.1164/ajrccm.153.5.8630590. [DOI] [PubMed] [Google Scholar]
- 12.Van Noord JA, Schreurs AJ, Mol SJ, Mulder PG. Addition of salmeterol versus doubling the dose of fluticasone propionate in patients with mild to moderate asthma. Thorax. 1999;54:207–212. doi: 10.1136/thx.54.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condemi JJ, Goldstein S, Kalberg C, Yancey S, Emmett A, Rickard K. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Salmeterol Study Group. Ann Allergy Asthma Immunol. 1999;82:383–389. doi: 10.1016/s1081-1206(10)63288-7. [DOI] [PubMed] [Google Scholar]
- 14.Gardiner PV, Ward C, Booth H, Allison A, Hendrick DJ, Walters EH. Effect of eight weeks of treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. Am J Respir Crit Care Med. 1994;150:1006–1011. doi: 10.1164/ajrccm.150.4.7921429. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JA, Bradding P, Britten KM, et al. The long-acting beta2-agonist salmeterol xinafoate. effects on airway inflammation in asthma. Eur Respir J. 1999;14:275–282. doi: 10.1034/j.1399-3003.1999.14b07.x. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Ward C, Thien F, et al. An anti-inflammatory effect of salmeterol, a long-acting beta (2) agonist, assessed in airway biopsies and bronchoalveolar lavage in asthma. Am J Respir Crit Care Med. 1999;160:1493–1499. doi: 10.1164/ajrccm.160.5.9811052. [DOI] [PubMed] [Google Scholar]
- 17.Wallin A, Sandstrom T, Soderberg M, et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. Am J Respir Crit Care Med. 1999;159:79–86. doi: 10.1164/ajrccm.159.1.9801007. [DOI] [PubMed] [Google Scholar]
- 18.Wilson SJ, Wallin A, Della-Cioppa G, Sandstrom T, Holgate ST. Effects of budesonide and formoterol on NF-kappaB, adhesion molecules, and cytokines in asthma. Am J Respir Crit Care Med. 2001;164:1047–1052. doi: 10.1164/ajrccm.164.6.2010045. [DOI] [PubMed] [Google Scholar]
- 19.Kips JC, O'Connor BJ, Inman MD, Svensson K, Pauwels RA, O'Byrne PM. A long-term study of the anti-inflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. Am J Respir Crit Care Med. 2000;161:996–1001. doi: 10.1164/ajrccm.161.3.9812056. [DOI] [PubMed] [Google Scholar]
- 20.Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 21.McIvor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. Am J Respir Crit Care Med. 1998;158:924–930. doi: 10.1164/ajrccm.158.3.9802069. [DOI] [PubMed] [Google Scholar]
- 22.Calhoun WJ, Hinton KL, Kratzenberg JJ. The effect of salmeterol on markers of airway inflammation following segmental allergen challenge. Am J Respir Crit Care Med. 2001;163:881–886. doi: 10.1164/ajrccm.163.4.2001060. [DOI] [PubMed] [Google Scholar]
- 23.Aziz I, Wilson AM, Lipworth BJ. Effects of once-daily formoterol and budesonide given alone or in combination on surrogate inflammatory markers in asthmatic adults. Chest. 2000;118:1049–1058. doi: 10.1378/chest.118.4.1049. [DOI] [PubMed] [Google Scholar]
- 24.Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma. Br Med J. 2000;320:1368–1373. doi: 10.1136/bmj.320.7246.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eickelberg O, Roth M, Lorx R, et al. Ligand-independent activation of the glucocorticoid receptor by beta2- adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- 26.Lemanske RF, Sorkness CA, Mauger EA, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–2603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 27.Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–2593. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 28.Tattersfield AE, Lofdahl CG, Postma DS, et al. Comparison of formoterol and terbutaline for as-needed treatment of asthma: a randomised trial. Lancet. 2001;357:257–261. doi: 10.1016/S0140-6736(00)03611-4. [DOI] [PubMed] [Google Scholar]
- 29.O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392–1397. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa M, Mak JC, Barnes PJ. Effect of short- and long-acting beta 2-adrenoceptor agonists on pulmonary beta 2-adrenoceptor expression in human lung. Eur J Pharmacol. 1996;318:123–129. doi: 10.1016/s0014-2999(96)00769-8. [DOI] [PubMed] [Google Scholar]
- 31.Lipworth BJ, Struthers AD, McDevitt DG. Tachyphylaxis to systemic but not to airway responses during prolonged therapy with high dose inhaled salbutamol in asthmatics. Am Rev Respir Dis. 1989;140:586–592. doi: 10.1164/ajrccm/140.3.586. [DOI] [PubMed] [Google Scholar]
- 32.Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. Am J Med. 1994;97:29–37. doi: 10.1016/0002-9343(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 33.Newnham DM, Grove A, McDevitt DG, Lipworth BJ. Subsensitivity of bronchodilator and systemic beta-2-adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax. 1995;50:497–504. doi: 10.1136/thx.50.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor BJ, Aikman SL, Barnes PJ. Subsensitivity to the non-bronchodilator effects of inhaled beta 2-agonists in asthma. N Engl J Med. 1992;327:1204–1208. doi: 10.1056/NEJM199210223271704. [DOI] [PubMed] [Google Scholar]
- 35.Cockcroft DW, Swystun VA, Bhagat R. Interaction of inhaled beta 2 agonist and inhaled corticosteroid on airway responsiveness to allergen and methacholine. Am J Respir Crit Care Med. 1995;152:1485–1489. doi: 10.1164/ajrccm.152.5.7582281. [DOI] [PubMed] [Google Scholar]
- 36.Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-term effects of a long-acting beta 2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992;327:1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- 37.Booth H, Bish R, Walters J, Whitehead F, Walters EH. Salmeterol tachyphylaxis in steroid treated asthmatic subjects. Thorax. 1996;51:1100–1104. doi: 10.1136/thx.51.11.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verberne AA, Hop WC, Creyghton FB, et al. Airway responsiveness after a single dose of salmeterol and during four months of treatment in children with asthma. J Allergy Clin Immunol. 1996;97:938–946. doi: 10.1016/s0091-6749(96)80068-6. [DOI] [PubMed] [Google Scholar]
- 39.Meijer GG, Postma DS, Mulder PG, van Aalderen WM. Long-term circadian effects of salmeterol in asthmatic children treated with inhaled corticosteroids. Am J Respir Crit Care Med. 1995;152:1887–1892. doi: 10.1164/ajrccm.152.6.8520751. [DOI] [PubMed] [Google Scholar]
- 40.Kalra S, Swystun VA, Bhagat R, Cockcroft DW. Inhaled corticosteroids do not prevent the development of tolerance to the bronchoprotective effect of salmeterol. Chest. 1996;109:953–956. doi: 10.1378/chest.109.4.953. [DOI] [PubMed] [Google Scholar]
- 41.Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest. 1995;108:1235–1239. doi: 10.1378/chest.108.5.1235. [DOI] [PubMed] [Google Scholar]
- 42.Drotar DE, Davis EE, Cockcroft DW. Tolerance to the bronchoprotective effect of salmeterol 12 hours after starting twice daily treatment. Ann Allergy Asthma Immunol. 1998;80:31–34. doi: 10.1016/S1081-1206(10)62935-3. [DOI] [PubMed] [Google Scholar]
- 43.Ramage L, Lipworth BJ, Ingram CG, Cree IA, Dhillon DP. Reduced protection against exercise induced bronchoconstriction after chronic dosing with salmeterol. Respir Med. 1994;88:363–368. doi: 10.1016/0954-6111(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 44.Nelson JA, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden ER., Jr Effect of long-term salmeterol treatment on exercise-induced asthma. N Engl J Med. 1998;339:141–146. doi: 10.1056/NEJM199807163390301. [DOI] [PubMed] [Google Scholar]
- 45.Giannini D, Carletti A, Dente FL, et al. Tolerance to the protective effect of salmeterol on allergen challenge. Chest. 1996;110:1452–1457. doi: 10.1378/chest.110.6.1452. [DOI] [PubMed] [Google Scholar]
- 46.Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. Am J Med. 1998;104:431–438. doi: 10.1016/s0002-9343(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 47.Aziz I, Tan KS, Hall IP, Devlin MM, Lipworth BJ. Subsensitivity to bronchoprotection against adenosine monophosphate challenge following regular once-daily formoterol. Eur Respir J. 1998;12:580–584. doi: 10.1183/09031936.98.12030580. [DOI] [PubMed] [Google Scholar]
- 48.Simons FE, Gerstner TV, Cheang MS. Tolerance to the bronchoprotective effect of salmeterol in adolescents with exercise-induced asthma using concurrent inhaled glucocorticoid treatment. Pediatrics. 1997;99:655–659. doi: 10.1542/peds.99.5.655. [DOI] [PubMed] [Google Scholar]
- 49.Villaran C, O'Neill SJ, Helbling A, et al. Montelukast versus salmeterol in patients with asthma and exercise- induced bronchoconstriction. Montelukast/Salmeterol Exercise Study Group. J Allergy Clin Immunol. 1999;104:547–553. doi: 10.1016/s0091-6749(99)70322-2. [DOI] [PubMed] [Google Scholar]
- 50.Edelman JM, Turpin JA, Bronsky EA, et al. Oral montelukast compared with inhaled salmeterol to prevent exercise- induced bronchoconstriction. A randomized, double-blind trial. Exercise Study Group. Ann Intern Med. 2000;132:97–104. doi: 10.7326/0003-4819-132-2-200001180-00002. [DOI] [PubMed] [Google Scholar]
- 51.Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Evaluation of salmeterol or montelukast as second-line therapy for asthma not controlled with inhaled corticosteroids. Chest. 2001;119:1021–1026. doi: 10.1378/chest.119.4.1021. [DOI] [PubMed] [Google Scholar]
- 52.Grove A, Lipworth BJ. Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet. 1995;346:201–206. doi: 10.1016/s0140-6736(95)91265-7. [DOI] [PubMed] [Google Scholar]
- 53.Fuglsang G, Vikre-Jorgensen J, Agertoft L, Pedersen S. Effect of salmeterol treatment on nitric oxide level in exhaled air and dose–response to terbutaline in children with mild asthma. Pediatr Pulmonol. 1998;25:314–321. doi: 10.1002/(sici)1099-0496(199805)25:5<314::aid-ppul5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 54.Wilding P, Clark M, Coon JT, et al. Effect of long-term treatment with salmeterol on asthma control: a double blind, randomised crossover study. Br Med J. 1997;314:1441–1446. doi: 10.1136/bmj.314.7092.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson HS, Berkowitz RB, Tinkelman DA, Emmett AH, Rickard KA, Yancey SW. Lack of subsensitivity to albuterol after treatment with salmeterol in patients with asthma. Am J Respir Crit Care Med. 1999;159:1556–1561. doi: 10.1164/ajrccm.159.5.9807128. [DOI] [PubMed] [Google Scholar]
- 56.Lipworth BJ, Jackson C. Lack of subsensitivity to albuterol after treatment with salmeterol in patients with asthma. Am J Respir Crit Care Med. 1999;160:2125–2126. doi: 10.1164/ajrccm.160.6.16062. [DOI] [PubMed] [Google Scholar]
- 57.Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the bronchoprotective effect of a long-acting inhaled beta 2-agonist. Am J Respir Crit Care Med. 1996;154:1603–1607. doi: 10.1164/ajrccm.154.6.8970342. [DOI] [PubMed] [Google Scholar]
- 58.Lipworth BJ, Aziz I. A high dose of albuterol does not overcome bronchoprotective subsensitivity in asthmatic subjects receiving regular salmeterol or formoterol. J Allergy Clin Immunol. 1999;103:88–92. doi: 10.1016/s0091-6749(99)70530-0. [DOI] [PubMed] [Google Scholar]
- 59.Aziz I, Lipworth BJ. In vivo effect of albuterol on methacholine-contracted bronchi in conjunction with salmeterol and formoterol. J Allergy Clin Immunol. 1999;103:816–822. doi: 10.1016/s0091-6749(99)70425-2. [DOI] [PubMed] [Google Scholar]
- 60.Van der Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting beta2 agonists. Thorax. 2001;56:529–535. doi: 10.1136/thorax.56.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storms WW, Bird S, Firriolo KM, Edelman JM. The effect of rescue short-acting beta-agonist bronchodilatation in patients on montelukast or salmeterol. J Allergy Clin Immunol. 2001;107:S316. [Google Scholar]
- 62.Hadcock JR, Williams DL, Malbon CC. Physiological regulation at the level of mRNA. analysis of steady-state of specific mRNAs by DNA-excess solution hybridization. Am J Physiol. 1989;256:C457–C465. doi: 10.1152/ajpcell.1989.256.3.C457. [DOI] [PubMed] [Google Scholar]
- 63.Mak JCW, Nishikawa M, Barnes PJ. Glucocorticosteroids increase receptor transcription in human lung. Am J Physiol. 1995;12:L41–L46. doi: 10.1152/ajplung.1995.268.1.L41. [DOI] [PubMed] [Google Scholar]
- 64.Mak JC, Nishikawa M, Shirasaki H, Miyayasu K, Barnes PJ. Protective effects of a glucocorticoid on downregulation of pulmonary beta 2-adrenergic receptors in vivo. J Clin Invest. 1995;96:99–106. doi: 10.1172/JCI118084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan KS, Grove A, Cargill RI, McFarlane LC, Lipworth BJ. Effects of inhaled fluticasone propionate and oral prednisolone on lymphocyte beta 2-adrenoceptor function in asthmatic patients. Chest. 1996;109:343–347. doi: 10.1378/chest.109.2.343. [DOI] [PubMed] [Google Scholar]
- 66.Tan KS, McFarlane LC, Lipworth BJ. Effects of oral and inhaled corticosteroid on lymphocyte β2-adrenoceptor function in asthmatic patients. Br J Clin Pharmacol. 1997;44:565–568. doi: 10.1046/j.1365-2125.1997.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan KS, McFarlane LC, Lipworth BJ. Concomitant administration of low-dose prednisolone protects against in vivo beta2-adrenoceptor subsensitivity induced by regular formoterol. Chest. 1998;113:34–41. doi: 10.1378/chest.113.1.34. [DOI] [PubMed] [Google Scholar]
- 68.Aziz I, McFarlane LC, Lipworth BJ. Concomitant inhaled corticosteroid resensitises cardiac beta2- adrenoceptors in the presence of long-acting beta2-agonist therapy. Eur J Clin Pharmacol. 1998;54:377–381. doi: 10.1007/s002280050478. [DOI] [PubMed] [Google Scholar]
- 69.Tan KS, Grove A, McLean A, Gnosspelius Y, Hall IP, Lipworth BJ. Systemic corticosteriod rapidly reverses bronchodilator subsensitivity induced by formoterol in asthmatic patients. Am J Respir Crit Care Med. 1997;156:28–35. doi: 10.1164/ajrccm.156.1.9610113. [DOI] [PubMed] [Google Scholar]
- 70.Ellul-Micallef R, Fenech FF. Effect of intravenous prednisolone in asthmatics with diminished adrenergic responsiveness. Lancet. 1975;ii:1269–1271. doi: 10.1016/s0140-6736(75)90608-x. [DOI] [PubMed] [Google Scholar]
- 71.Holgate ST, Baldwin CJ, Tattersfield AE. Beta-adrenergic agonist resistance in normal human airways. Lancet. 1977;ii:375–377. doi: 10.1016/s0140-6736(77)90304-x. [DOI] [PubMed] [Google Scholar]
- 72.Lipworth BJ, Aziz I. Bronchodilator response to albuterol after regular formoterol and effects of acute corticosteroid administration. Chest. 2000;117:156–162. doi: 10.1378/chest.117.1.156. [DOI] [PubMed] [Google Scholar]
- 73.Aziz I, Lipworth BJ. A bolus of inhaled budesonide rapidly reverses airway subsensitivity and beta2-adrenoceptor down-regulation after regular inhaled formoterol. Chest. 1999;115:623–628. doi: 10.1378/chest.115.3.623. [DOI] [PubMed] [Google Scholar]
- 74.Hall IP. Beta2-adrenoceptor polymorphisms and asthma. Clin Exp Allergy. 1999;29:1151–1154. doi: 10.1046/j.1365-2222.1999.00660.x. [DOI] [PubMed] [Google Scholar]
- 75.Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- 76.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 77.Dewar JC, Wheatley AP, Venn A, Morrison JF, Britton J, Hall IP. Beta2-adrenoceptor polymorphisms are in linkage disequilibrium, but are not associated with asthma in an adult population. Clin Exp Allergy. 1998;28:442–448. doi: 10.1046/j.1365-2222.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- 78.Fowler SJ, Dempsey OJ, Sims EJ, Lipworth BJ. Screening for bronchial hyperresponsiveness using methacholine and adenosine monophosphate. Relationship to asthma severity and beta (2)-receptor genotype. Am J Respir Crit Care Med. 2000;162:1318–1322. doi: 10.1164/ajrccm.162.4.9912103. [DOI] [PubMed] [Google Scholar]
- 79.Turki J, Pak J, Green SA, Martin RJ, Liggett SB. Genetic polymorphisms of the beta 2-adrenergic receptor in nocturnal and non-nocturnal asthma. Evidence that Gly16 correlates with the nocturnal phenotype. J Clin Invest. 1995;95:1635–1641. doi: 10.1172/JCI117838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turki J, Green SA, Newman KB, Meyers MA, Liggett SB. Human lung cell beta 2-adrenergic receptors desensitize in response to in vivo administered beta-agonist. Am J Physiol. 1995;269:L709–L714. doi: 10.1152/ajplung.1995.269.5.L709. [DOI] [PubMed] [Google Scholar]
- 81.Lima JJ, Thomason DB, Mohamed MH, Eberle LV, Self TH, Johnson JA. Impact of genetic polymorphisms of the beta2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther. 1999;65:519–525. doi: 10.1016/S0009-9236(99)70071-8. [DOI] [PubMed] [Google Scholar]
- 82.Martinez FD, Graves PE, Baldini M, Solomon S, Erickson R. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J Clin Invest. 1997;100:3184–3188. doi: 10.1172/JCI119874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Israel E, Drazen JM, Liggett SB, et al. The effect of polymorphisms of the beta (2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 84.Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use. influence of beta (2) adrenoceptor polymorphism. Thorax. 2000;55:762–767. doi: 10.1136/thorax.55.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lipworth BJ, Hall IP, Tan S, Aziz I, Coutie W. Effects of genetic polymorphism on ex vivo and in vivo function of beta2-adrenoceptors in asthmatic patients. Chest. 1999;115:324–328. doi: 10.1378/chest.115.2.324. [DOI] [PubMed] [Google Scholar]
- 86.Lipworth BJ, Hall IP, Aziz I, Tan KS, Wheatley A. Beta2-adrenoceptor polymorphism and bronchoprotective sensitivity with regular short- and long-acting beta2-agonist therapy. Clin Sci. 1999;96:253–259. [PubMed] [Google Scholar]
- 87.Tan S, Hall IP, Dewar J, Dow E, Lipworth B. Association between beta 2-adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet. 1997;350:995–999. doi: 10.1016/S0140-6736(97)03211-X. [DOI] [PubMed] [Google Scholar]
- 88.Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous glycine-16 beta (2) -adrenoceptor polymorphism. Chest. 2000;118:321–328. doi: 10.1378/chest.118.2.321. [DOI] [PubMed] [Google Scholar]
- 89.Sims EJ, Lipworth BJ. Sustained protection with montelukast but not formoterol as add on therapy in asthmatics with the homozygous glycine-16 β2-adrenoceptor genotype. J Allergy Clin Immunol. 2002;109:S157. [Google Scholar]