Abstract
Aims
To define by amalgamation of data obtained in contemporaneous case-control studies, the risks associated with individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) according to doses used.
Methods
Meta-analysis of individual patient data from three retrospective case-control studies using similar data collection protocols was carried out in hospitals in Catalonia, England, Scotland and Sweden. 2472 cases of upper gastrointestinal bleeding and 5877 controls were studied. Main outcome measures were risks associated with individual NANSAIDs according to dose used and the period of time for which they were given.
Results
Ibuprofen showed the lowest odds ratio (OR = 1.7; 95% confidence interval 1.1, 2.5), followed by diclofenac (4.9; 3.3, 7.1), indomethacin (6.0; 3.6, 10.0), naproxen (9.1; 6.0–13.7), piroxicam (13.1; 7.9–21.8) and ketoprofen (34.9; 12.7, 96.5). Striking dose–response relationships were seen with four to eight-fold increases in risk within conventionally used dose ranges for all except ketoprofen, where numbers were too few to allow dose analysis. Across the class, risk was highest during the first week of use (11.7; 6.5, 21.0), decreased thereafter with continuing use (5.6; 4.6, 7.0), and fell to 3.2 (2.1, 5.1) 1 week after discontinuing use. Concurrent use of more than one NANSAID substantially increased risk.
Conclusions
The risk of upper gastrointestinal bleeding with NANSAIDs varies twenty-fold depending on the drug, and by three to seven-fold depending on the dose chosen. Risk is maximal during the first week and decreases thereafter. Paracetamol (acetaminophen) is not associated with upper gastrointestinal bleeding at any dose and should be the first-line analgesic wherever possible.
Keywords: diclofenac, dose–response, ibuprofen, indomethacin, ketoprofen, meta-analysis of individual patient data, naproxen, non-aspirin nonsteroidal anti-inflammatory drugs, paracetamol (acetaminophen), piroxicam, upper gastrointestinal bleeding
Introduction
Overt upper gastrointestinal (UGI) haemorrhage is recognized to be causally associated with exposure to nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) [1–3]. There is substantial evidence that the underlying mechanism for this effect is by inhibition of cyclo-oxygenase-1 (COX-1) activity in the UGI mucosa [4]. The role of paracetamol, a weak inhibitor of COX, in causing serious UGI bleeding has not been established.
Individual studies have shown marked differences in their estimates of the overall relative risk of UGI bleeding with aspirin and other nonselective NANSAIDs [5, 6]. This heterogeneity may be due to differences in other risk factors amongst the populations studied, patterns of use, or methodology. The differences in the estimated rank order of toxicity from study to study may also reflect random variation as well as differences in the characteristics of treated groups. We present the results of a meta-analysis of individual patient data in which information from three large studies has been combined whilst taking account of variables including age, sex, prior ulcer history, social habits, use of particular NANSAIDs and their doses and patterns of use.
In contrast to qualitative systematic review, which, in essence, itemizes and comments on available data, meta-analysis uses statistical techniques to combine either summarized outcome results, or individual data themselves. The latter method has the advantage that, provided similar designs and criteria were employed in studies, there can be enhanced power to detect significant differences inapparent in the separate studies, for contrasts which could not otherwise have been made effectively.
Methods
Raw data were obtained from the principal investigators of three contemporary case-control studies [1–3]. The data from the studies by Langman et al.[2] and Laporte et al.[3] were complete and as published. The Swedish data were a subset of the study by Kaufman et al.[1]. They had, however, been augmented by the inclusion of additional Swedish cases and controls as data collection had continued after completion of the original study. Throughout this paper, the three studies will be referred to as the British [2], Catalan [3] and Swedish [1] ones.
All three studies included, as far as possible, consecutive incident cases of acute UGI haemorrhage presenting at hospital with haematemesis or melaena and the diagnosis confirmed by endoscopy, radiology or surgery. The British study additionally included subjects who had collapsed due to a condition that was subsequently found to be an acute UGI bleed.
The three studies selected their controls in different ways. They all matched controls to cases by age and sex but the Catalan selected hospital controls, the Swedish selected community controls, and the British study included both. The British and Catalan studies selected their hospital controls from acute medical inpatients with conditions thought to be unrelated to intake of analgesics. In Britain, a community control was selected for each case from the register of the same general practice by selecting the next person on the alphabetical list who fulfilled the matching criteria. In Sweden community controls were selected from computerized population registers. In the published account of the British study, no difference in results was found between the hospital and community controls, and they were amalgamated into a single control population. Consequently hospital and community controls were amalgamated in this meta-analysis.
In all three studies, information about drug exposure was obtained by direct questioning of cases and controls using trained interviewers with structured questionnaires. Interview results were verified by the British and Swedish by checking them against hospital and GP records. In the Catalan study interview results were verified by questioning relatives of cases and controls. The British study included subjects aged 60 years or over, whereas the other two studies included subjects of all ages. All studies excluded subjects with gastric carcinoma. Subjects with oesophageal varices were excluded in the Spanish and Catalan studies, whereas the British included them if the varices were not bleeding and if there was evidence of a gastric or duodenal lesion. The Swedish excluded subjects with a history of UGI bleeding, and those who had used histamine H2-receptor antagonists or anticoagulants in the month before admission. The Catalan study excluded patients whose information was deemed unreliable.
Individual patient data from each of the three case-control studies were entered into a single database. In the Swedish study, for some subjects, the dose of the NANSAID taken had not been entered. The Swedish National Formulary (FASS) was used to ascertain whether the drug was only available in one dose, and if so, this was entered manually. In the British study, the matching codes for one centre had been stolen and the published results had been based on an unmatched analysis. However, the matching was regained by examining the age, sex and date of entry to the study for each subject, and following the matching rules specified in the study protocol. It was therefore possible to include all the British data in a matched analysis.
The variables recorded in each study were examined to identify similarities and differences. If an effect was measured in one study, but not in the others, it was not used. All studies had recorded information on the diagnosis of the case, age, sex, previous history of UGI problems and consumption of NANSAIDs, paracetamol, aspirin, anticoagulants, corticosteroids, ulcer medication, alcohol, caffeine and cigarettes. To amalgamate data across studies it was necessary to arrange the information in a common form. This was relatively straightforward for UGI history and ulcer medication as all three studies had recorded directly comparable information, but more challenging for alcohol, smoking and caffeine. The distributions of alcohol, smoking and caffeine use were considered separately for each study. Subjects who did not drink/smoke were classified as nonusers and those who did drink/smoke were classified as users. Users were classified as low, medium or high depending on whether they were in the bottom, middle or top third of the distribution of users in that study. This classified users relative to their peers and made some allowance for national differences in consumption. It did mean, however, that absolute consumption for individuals in a given class was not consistent between the studies.
The dose of each drug, taken each day for 28 days before the index day, was recorded in both the Spanish and Swedish studies. The British coded the total dose taken in the week before the bleed as low, medium or high, based on the range of doses recommended in the British National Formulary. From the Spanish and Swedish data, the total dose of drug taken in the week before bleeding or index day was divided by the number of days on which the drug was taken. This gave an average daily dose. Subjects were then categorized, by dose, using the dose bands adopted by the British study.
The data were analysed by conditional logistic regression using EGRET [9], and the results were replicated using STATA [10]. Matching was maintained within sets. Subjects were categorized by whether they had taken a low, medium or high dose of diclofenac, ibuprofen, indomethacin, ketoprofen, naproxen, piroxicam or paracetamol in the week before the bleed (or index day for controls). No other NANSAID had been taken by sufficient subjects to allow quantification of its effect on serious UGI bleeding. Any subject who had taken more than one NANSAID, who had taken a NANSAID concurrently with aspirin, who had taken a NANSAID other than those mentioned above, or for whom it was not known whether a NANSAID had been taken, was excluded. Cases or controls who had no matched partner after this exclusion were also removed from the analysis. The conditional logistic regression model included terms for use of aspirin, anticoagulants, smoking, and history of gastrointestinal problems. Alcohol, caffeine, use of ulcer medication and use of corticosteroids were not included in the model as they were found to have no significant effect and because of their ambiguous relationship to UGI bleeding in previous studies.
Results
The three studies provided information on 2472 cases and 5877 controls. The British subjects were the oldest, with the distribution of ages in the Catalan and Swedish studies being very similar to each other (Table 1). All studies had a higher proportion of females than males. The British had the largest proportion of subjects with a UGI history. A dose–response relationship was found between smoking and serious UGI bleeding, although the odds ratios were small (Table 2). The odds ratio of having a serious UGI bleed among subjects with a previous history of UGI symptoms was 4.7 (95% CI 4.1, 5.5). A small risk was found for use of anticoagulants.
Table 1.
Descriptive statistics of the three individual studies.
| Statistic | British [2] | Catalan [3] | Swedish [1] |
|---|---|---|---|
| Number of subjects | 3245 | 3501 | 1603 |
| Cases (%) | 1131 | 875 | 466 |
| Controls (%) | 2114 | 2626 | 1137 |
| Age (years) | |||
| Minimum | 60 | 7 | 20 |
| Maximum | 103 | 95 | 94 |
| Mean | 74 | 58 | 64 |
| s.d. | 7.8 | 17.1 | 15.2 |
| Sex | |||
| Males (%) | 1811 (55.8%) | 2405 (68.7%) | 851 (53.1%) |
| Females (%) | 1434 (44.2%) | 1096 (31.3%) | 752 (46.9%) |
| History of UGI problems | |||
| Yes (%) | 1307 (40.3%) | 1257 (35.9%) | 0 |
| No (%) | 1841 (56.7%) | 2244 (64.1%) | 1603 (100%) |
| Missing (%) | 97 (3.0%) | 0 | 0 |
Table 2.
Odds ratios (ORs) for upper gastrointestinal (UGI) bleeding with cigarette smoking, anticoagulant use and previous UGI history.
| Variable | Cases | Controls | OR(*) | 95% CI |
|---|---|---|---|---|
| Cigarette smoking | ||||
| None | 1558 | 3877 | 1.0 | – |
| Low | 213 | 516 | 1.1 | 0.9, 1.4 |
| Medium | 247 | 487 | 1.4 | 1.2, 1.8 |
| High | 243 | 460 | 1.6 | 1.3, 1.9 |
| Missing | 20 | 11 | – | – |
| Anticoagulants | ||||
| Not taken | 2209 | 5219 | 1.0 | – |
| Taken | 72 | 132 | 1.4 | 1.0, 2.1 |
| GI history | ||||
| Absent | 1125 | 4042 | 1.0 | – |
| Present | 1129 | 1250 | 4.7 | 4.1, 5.5 |
| Missing | 27 | 59 | – | – |
Adjusted for use of paracetamol, diclofenac, ibuprofen, indomethacin, ketoprofen, naproxen, piroxicam and aspirin.
Individual analgesics and the effect of dose
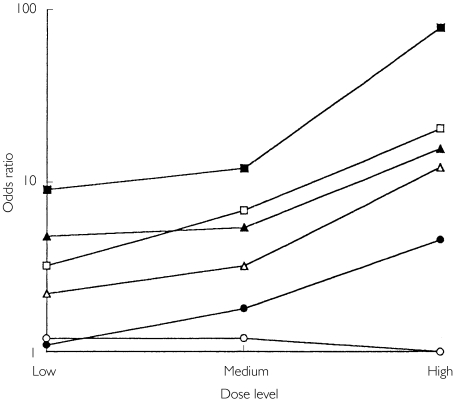
For individual NANSAIDs, the estimated order of the odds ratios (from highest to lowest) was: ketoprofen > piroxicam > naproxen > indomethacin > diclofenac > ibuprofen (Table 3 and Figure 1). Paracetamol had a lower odds ratio than any NANSAID. Striking dose–response relationships were found for all NANSAIDs (Table 4 and Figure 1) apart from ketoprofen for which too few subjects had taken more than one dose for the effect of dose to be estimated. The odds ratios for all doses of NANSAIDs (apart from low doses of diclofenac and low, moderate or high doses of ibuprofen) were significantly greater than unity. Piroxicam was associated with the highest odds ratio at all doses. When log odds ratio is plotted against dose for each drug (Figure 1), the resulting lines are essentially parallel. By exploration of models it was found that fit was improved at a probability of P < 0.001 by terms to the effect that all drugs were not equivalent, and that dose effects were linear with parallel lines. Fit was not significantly improved by inserting terms for nonparallelism.
Table 3.
Odds ratios (ORs) and 95% confidence intervals (95% CI) of serious upper gastrointestinal bleeding with individual nonaspirin nonsteroidal anti-inflammatory drugs and acetaminophen.
| Drug | Level | Cases | Controls | OR (95% CI) (*) |
|---|---|---|---|---|
| Paracetamol | Not taken | 1794 | 4564 | 1.0 |
| Taken | 487 | 787 | 1.2 (1.1, 1.5) | |
| Diclofenac | Not taken | 2202 | 5281 | 1.0 |
| Taken | 79 | 70 | 4.9 (3.3, 7.1) | |
| Ibuprofen | Not taken | 2228 | 5246 | 1.0 |
| Taken | 53 | 105 | 1.7 (1.1, 2.5) | |
| Indomethacin | Not taken | 2223 | 5316 | 1.0 |
| Taken | 58 | 35 | 6.0 (3.6, 10.0) | |
| Ketoprofen | Not taken | 2242 | 5346 | 1.0 |
| Taken | 39 | 5 | 34.9 (12.7, 96.5) | |
| Naproxen | Not taken | 2180 | 5303 | 1.0 |
| Taken | 101 | 48 | 9.1 (6.0, 13.7) | |
| Piroxicam | Not taken | 2205 | 5322 | 1.0 |
| Taken | 76 | 29 | 13.1 (7.9, 21.8) |
All odds ratios are relative to nonuse of any NANSAID or aspirin. All odds ratios are adusted for use of aspirin use of anticoagulants, smoking, and history of UGI problems.
Figure 1.
Dose–response relationships for the risks (odds ratios) of upper gastrointestinal bleeding with individual nonaspirin nonsteroidal anti-inflammatory drugs (▪, piroxicam, □ indomethacin, ▴ naproxen, ▵ diclofenac, • ibuprofen, ○ paracetamol).
Table 4.
Estimates of the effect of dose (in the week before the index day) on the odds ratios (ORs) and their 95% confidence intervals (95% CI) of upper gastrointestinal bleeding.
| Variable | Level (*) | Cases | Controls | OR (95% CI) (†) |
|---|---|---|---|---|
| Paracetamol | < 2000 | 351 | 593 | 1.2 (1.0, 1.4) |
| 2000–3999 | 96 | 150 | 1.2 (0.8, 1.7) | |
| ≥ 4000 | 31 | 42 | 1.0 (0.5, 1.9) | |
| Diclofenac | < 75 | 8 | 15 | 2.2 (0.8, 5.8) |
| 75–149 | 39 | 41 | 3.2 (1.9, 5.5) | |
| ≥ 150 | 27 | 13 | 12.2 (5.6, 26.7) | |
| Ibuprofen | < 1200 | 22 | 58 | 1.1 (0.6, 2.0) |
| 1200–1799 | 18 | 39 | 1.8 (0.8, 3.7) | |
| ≥ 1800 | 6 | 6 | 4.6 (0.9, 22.3) | |
| Indomethacin | ≤ 50 | 9 | 10 | 3.2 (1.1, 9.5) |
| 51–149 | 39 | 21 | 6.8 (3.6, 12.9) | |
| ≥ 150 | 10 | 2 | 20.4 (4.2, 99.7) | |
| Naproxen | < 500 | 6 | 5 | 4.8 (1.3, 18.1) |
| 500–999 | 31 | 24 | 5.4 (2.9, 9.9) | |
| ≥ 1000 | 61 | 17 | 15.6 (8.1, 30.2) | |
| Piroxicam | ≤ 10 | 7 | 3 | 9.0 (2.1, 39.2) |
| 11–20 | 54 | 25 | 12.0 (6.5,22.1) | |
| ≥ 21 | 14 | 1 | 79.0 (9.9, 631.8) |
All doses in mg day−1.
All odds ratios are relative to nonuse of any NANSAID or aspirin and are adjusted for use of aspirin, ketoprofen and anticoagulants, smoking, and history of UGI problems.
This suggests that there is a difference between drugs, even when dose is taken into account, with a rank order of toxicity (from highest to lowest) of: piroxicam, indomethacin = naproxen, diclofenac, ibuprofen. No dose–response effect was found with paracetamol, and the magnitude of the odds ratios at each dose level was small (1.1–1.2).
Duration of use
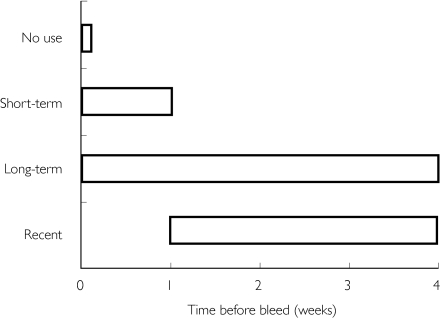
Three types of NANSAID users were defined (Figure 2): short-term users (who took a NANSAID in the week before the bleed, but not in the 2–4 weeks before that); continuing [long-term] users (who took a NANSAID in the week before the bleed and in the 2–4 weeks before that); and recent users (who took a NANSAID in the 2–4 weeks before the bleed but not in the week before the bleed). The estimated risk for short-term use of a NANSAID at any dose is 11.7 (Table 5). The risk of bleeding for continuing users is lower (5.6), and the risk for recent users is lower still (3.2). There was no evidence of differential use of individual NANSAIDs amongst the three categories.
Figure 2.
Schematic representation of long-term, short-term and recent use of nonaspirin nonsteroidal anti inflammatory drugs.
Table 5.
Odds ratios (ORs) and 95% confidence intervals (95% CIs) for short-term, continuing and recent use of nonaspirin nonsteroidal anti-inflammatory drugs.
| Type of NANSAID use | Cases | Controls | OR (95% CI) |
|---|---|---|---|
| Short-term | 53 | 22 | 11.7 (6.5, 21.0) |
| Continuing | 353 | 268 | 5.6 (4.6, 7.0) |
| Recent | 52 | 59 | 3.2 (2.1, 5.1) |
All odds ratios are relative to nonuse of any NANSAID or aspirin and are adjusted for use of aspirin, ketoprofen and anticoagulants, smoking, and history of UGI problems. One control took a NANSAID in the week before bleed, but it was not known if they took it before that.
Multiple use of nonsteroidal anti-inflammatory drugs
The total number of different nonsteroidal anti-inflammatory drugs (NSAIDs), taken in the week before the bleed (or index day for controls), was calculated for each subject. Aspirin, and all NANSAIDs were included in this calculation. Risks increased strikingly as more drugs were taken (Table 6) but it was not possible to analyse formally the number of drugs taken, dose and drug together. No evidence could be found to suggest that those on more drugs were on higher doses.
Table 6.
The odds ratios (ORs) and their 95% confidence intervals (95%CIs) of serious upper gastrointestinal bleeding in patients using one or more nonsteroidal anti-inflammatory drugs.
| Number of NSAIDs taken | Cases | Controls | OR (*) | 95% CI |
|---|---|---|---|---|
| 1 | 1159 | 1075 | 4.9 | (4.4, 5.6) |
| 2 | 112 | 50 | 10.7 | (7.3, 15.7) |
| 3 or more | 9 | 1 | 60.0 | (6.9, 525.9) |
All odds ratios are relative to nonuse of any NANSAID or aspirin and are adjusted for use of anticoagulants, smoking, and history of UGI problems.
Discussion
By combining individual data from three large observational studies, we have been able to show important differences in the risks of UGI bleeding between, and within (at different doses), individual NANSAIDs. Overall the data shows a twenty-fold variation in risks of UGI haemorrhage amongst the NANSAIDs studied between ketoprofen (most toxic) and low dose ibuprofen (least toxic). Variation in calculated risk between studies can be marked, and may reflect a variety of factors. These could include different dosages customarily employed the play of chance in small numbers studied and differential exposure to risk factors. Examination of risk can also hindered by the use of comparators which include all NANSAIDs, except the one under consideration, or by standardization on ibuprofen [1, 11, 12].
Other factors may also apply. Thus in the recent study of Rodriguez et al.[13] calculated risk associated with NANSAID use was generally lower than as found by us. It is impossible to tell whether this reflects systematic differences between outcome in case-control and cohort studies, adjustments possible in our data for nonprescribed drug use and social factors, or reductions in dosage of NSAIDs consequent on appreciation of risks reported by us and others in the past [1–3, 5, 11, 14, 15].
We found that, irrespective of dose, diclofenac is less prone to cause UGI toxicity than naproxen, or piroxicam, but more so than ibuprofen. Our results show similar patterns of ordering for risk with NANSAID used to those indicated by Henry et al. in their collaborative meta-analysis [16], necessarily given the use of the same large studies. In addition there is consistent evidence of dose–response relationships for the drugs considered, except paracetamol. Five main NANSAIDS, diclofenac, ibuprofen, indomethacin, naproxen, and piroxicam, accounted for, respectively, 78, 81 and 92% of UK, Swedish and Spanish case exposure, but most of that for ibuprofen was in the UK [80%]. Ketoprofen, a clear outlier in the analysis, was taken by subjects in all three study countries, with 55% in the UK. It follows that conclusions for ibuprofen were mainly dependent on findings in one country, but that those for the other main NSAIDs considered, and ketoprofen, were not.
The markedly higher risks of UGI bleeding with ketoprofen in the present investigation (OR 34.9, 95% CI 12.7, 96.5) compared with that found by Savage et al.[14] (OR 2.4, 95% CI 1.0, 5.9) may be related to dose since 89.5% of cases and 80% of controls in the present meta-analysis were taking doses greater than the recommended maximum (≥ 200 mg day−1). The more recent data of Rodriguez et al.[13], indicating a risk for ketoprofen of the same order as for naproxen are difficult to reconcile, but moderated doses in recent prescribing may be important.
As seen in Figure 1, risk increased with dose so that at high dose ibuprofen (≥ 1800 mg day−1) may be indistinguishable from low dose indomethacin (≤ 50 mg day−1) or naproxen (< 500 mg day−1), or medium dose diclofenac (75–149 mg day−1). Uncertainties due to small numbers prevent firm conclusions about the likely actual risks with high dosages of individual drugs. However, modelling indicates that the best fit is obtained on assumptions which take into account differences between individual drugs and increasing dose.
The present study suggests strongly the relative gastrointestinal safety of paracetamol. In the absence of coadministration of NANSAIDs, there was no evidence of any increased risk of UGI bleeding (Table 4) with any dose. Moreover, there were no interactions with NANSAIDs. This finding conflicts with the results of Savage et al.[14] and Rodriguez et al.[13]. The former observed a gradient of risk of UGI bleeding with paracetamol in patients exposed to NANSAIDs. This is likely to have been due to selection bias since, in that study, a prior history of UGI symptoms was an exclusion criterion for cases but not for controls. The latter have suggested that increasing doses of paracetamol are associated with raised risk,with an interaction with NANSAID use. However, their study, which did not seem to have the examination of paracetamol as a primary objective, is incomplete, lacking information on OTC purchases, which are particularly likely to be made by individuals under pensionable age in the UK, who cannot usually obtain prescription drugs free, and being data-base-derived has limited power to take account of severity of illness driving prescribers to enhance analgesic treatment. Evidence of significant association is modest, and the possibility of post hoc rationalization from the data seems likely.
Evidence (Table 5) that the risk of UGI bleeding is greater at the start of NANSAID therapy could be attributed to ‘depletion of susceptibles’, or to greater initial compliance with prescribed regimes. Findings are similar to those of others, studying NANSAID [11, 13], or aspirin use [17]. A significant increase in the risk of UGI bleeding in patients with a past history of UGI problems, has been detected by others [11], although the influence of current Helicobacter pylori infection on the risk of ulcer complications is uncertain [18–20].
The reasons why risk has appeared to persist, albeit at a reduced frequency, after exposure to NANSAIDs has ceased [12] are unclear. Part of the effect might be artefactual in that patients may remain continuing occasional takers of NANSAIDs from earlier unused supplies. Secondly, the persistent risk in takers in the Dundee study after drug prescription ceased might be related to the use of patients as their own controls, initial prescription having taken place at a low point in the individual risk cycle.
Data on newer NANSAIDS would be well-worth having, but use has been too small to allow examination in practice [13].
Meta-analyses of observational data have potential drawbacks. Those based on summary statistics are obviously limited by the information provided within individual studies. Furthermore, few adjustments for differences in potential sources of bias or confounding are possible because of the manner in which aggregated data are normally presented. The meta-analysis described here, however, is based on individual data from three large studies chosen with care. Each was carried out in a European population, within comparable periods of time. Methods of data collection were similar (direct questioning of patients by trained staff) and it was possible to analyse potential confounders using a uniform approach, and to obtain information on over-the-counter drug use. Studies not chosen for examination include those which have been data-base-derived and/or without community controls, with almost all being smaller than those we chose. For all these reasons the base from which we started seems robust. Matching within study sets was maintained because of the difficulty of interpreting apparent effects where [say] Swedish controls were matched with UK cases.
Other meta-analyses of gastrointestinal disease following NANSAID exposure have depended on summary statistics of published data [8, 16], allowing the use of more data-sets, and hence larger case numbers, but with uncertainties associated from combining observational data not necessarily collected in the same way, and often including smaller data-sets.
The main finding of our study is of a gradient of risk for all NANSAIDs examined, except paracetamol, and with evidence of fundamentally identical dose associated patterns. This is in keeping with actions through a common mechanistic pathway. The observed level of risk is higher than seen by some, though the ordering is generally similar to that found elsewhere, with the exception of high recorded risk for ketoprofen. It is not possible to determine the reasons. The increased UGI toxicity of NANSAIDs, taken together, is concordant with the results of García Rodríguez et al.[13] and Henry et al.[11], and is to be expected when effects are due to the total amount of COX-1 inhibition. The results strongly reinforce advice to use nonselective NANSAIDs singly, in the lowest possible doses, and choosing the drug with the best risk profile initially.
This project was supported by CEC Biomed Project Numbers G-9Z0011-GB and PL92 1556.
References
- 1.Kaufman DW, Kelly JP, Sheehan JE, et al. Nonsteroidal anti-inflammatory drug use in relation to major upper gastrointestinal bleeding. Clin Pharmacol Ther. 1993;53:485–494. doi: 10.1038/clpt.1993.55. [DOI] [PubMed] [Google Scholar]
- 2.Langman MJS, Weil J, Wainright P, et al. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:1075–1078. doi: 10.1016/s0140-6736(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 3.Laporte J-R, Carne X, Vidal X, Moreno V, Juan J. Upper gastrointestinal bleeding in relation to previous use of analgesics and non-steroidal anti-inflammatory drugs. Lancet. 1991;337:85–89. doi: 10.1016/0140-6736(91)90744-a. [DOI] [PubMed] [Google Scholar]
- 4.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;267:6610–6614. [PubMed] [Google Scholar]
- 5.Committee on Safety of Medicines. Relative safety of oral non-aspirin nonsteroidal anti-inflammatory drugs. Current Problems Pharmacovigilance. 1994;20:9. [Google Scholar]
- 6.Wright V. Historical overview of non-steroidal anti-inflammatory drugs. Br J Rheumatol. 1995;34(Suppl 1):2. doi: 10.1093/rheumatology/xxxiv.suppl_1.2. [DOI] [PubMed] [Google Scholar]
- 7.Rainsford KD. Aspirin and the Salicylates. Butterworths; 1984. [Google Scholar]
- 8.Belton KJ, Lewis SC, Matthew JNS, Rawlins MD. Systematic overview of upper gastrointestinal haemorrhage associated with non-steroidal anti-inflammatory drugs. In: Fracchia GN, editor. Perspectives in Pharmacotoxicology and Pharmacovigilance. Brussels: IOS Press; 1994. [Google Scholar]
- 9.Statistics and Epidemiology Research Corporation. EGRET. Seattle, Washington, USA: [Google Scholar]
- 10.Statacorp. Stata Statistical Software. College Station, TX: Stata Corporation; 1995. Release 4.0. [Google Scholar]
- 11.Henry D, Dobson A, Turner C. Variability in the risk of major gastrointestinal complications from nonaspirin nonsteroidal anti-inflammatory drugs. Gastroenterology. 1993;105:1078–1088. doi: 10.1016/0016-5085(93)90952-9. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald TM, Morant SV, Robinson GC, et al. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. Br Med J. 1997;315:1333–1337. doi: 10.1136/bmj.315.7119.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia Rodriguez LA, Hernandez-Dias S. Relative risk of upper gastrointestinal complications among users of acetaminophen and nonsteroidal anti-inflammatory drugs. Epidemiology. 2001;12:570–576. doi: 10.1097/00001648-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Savage RL, Moller PW, Ballantyne CL, Wells JE. Variation in the risk of peptic ulcer complications with nonsteroidal antiinflammatory drug therapy. Arthritis Rheumatism. 1993;36:84–90. doi: 10.1002/art.1780360114. [DOI] [PubMed] [Google Scholar]
- 15.Garcia Rodriguez LA, Walker AM, Perez Gutthann S. Nonsteroidal antiinflammatory drugs and gastrointestinal hospitalizations in Saskatchewan: a cohort study. Epidemiology. 1992;3:337–342. doi: 10.1097/00001648-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Henry DA, Lim LL-Y, Garcia-Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. Br Med J. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slattery J, Warlow CP, Shorrock CJ, Langman MJS. Risks of gastrointestinal bleeding during secondary prevention of vascular events with aspirin- analysis of gastrointestinal bleeding during the UK–TIA trial. Gut. 1995;37:509–511. doi: 10.1136/gut.37.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan FKL, Sung JJY, Chung SCS. Randomized controlled trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug drug therapy to prevent peptic ulcers. Lancet. 1997;350:975–979. doi: 10.1016/s0140-6736(97)04523-6. [DOI] [PubMed] [Google Scholar]
- 19.Cullen DJE, Hawkey GM, Greenwood DM, et al. Peptic ulcer bleeding in the elderly. relative roles of Helicobacter pylori and non-steroidal anti-inflammatory drugs. Gut. pp. 459–562. [DOI] [PMC free article] [PubMed]
- 20.Hawkey CJ, Tulassey Z, Szczepanski L, et al. Randomised controlled trial of Helicobacter pylori eradication in patients on non-steroidal anti-inflammatory drugs: HELP NSAIDs study. Lancet. 1997;350:1016–1021. doi: 10.1016/s0140-6736(98)04206-8. [DOI] [PubMed] [Google Scholar]
- 21.García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:769–772. doi: 10.1016/s0140-6736(94)91843-0. [DOI] [PubMed] [Google Scholar]