Abstract
Aims
The primary aims of these two single-centre, randomized, evaluator-blind, placebo/positive-controlled, parallel-group studies were to evaluate the potential for pharmacodynamic and pharmacokinetic interaction between ezetimibe 0.25, 1, or 10 mg and simvastatin 10 mg (Study 1), and a pharmacodynamic interaction between ezetimibe 10 mg and simvastatin 20 mg (Study 2). Evaluation of the tolerance of the coadministration of ezetimibe and simvastatin was a secondary objective.
Methods
Eighty-two healthy men with low-density lipoprotein cholesterol (LDL-C) ≥130 mg dl−1 received study drug once daily in the morning for 14 days. In Study 1 (n = 58), five groups of 11–12 subjects received simvastatin 10 mg alone, or with ezetimibe 0.25, 1, or 10 mg or placebo. In Study 2 (n = 24), three groups of eight subjects received simvastatin 20 mg alone, ezetimibe 10 mg alone, or the combination. Blood samples were collected to measure serum lipids in both studies. Steady-state pharmacokinetics of simvastatin and its β-hydroxy metabolite were evaluated in Study 1 only.
Results
In both studies, reported side-effects were generally mild, nonspecific, and similar among treatment groups. In Study 1, there were no indications of pharmacokinetic interactions between simvastatin and ezetimibe. All active treatments caused statistically significant (P < 0.01) decreases in LDL-C concentration vs placebo from baseline to day 14. The coadministration of ezetimibe and simvastatin caused a dose-dependent reduction in LDL-C and total cholesterol, with no apparent effect on high-density lipoprotein cholesterol (HDL-C) or triglycerides. The coadministration of ezetimibe 10 mg and simvastatin 10 mg or 20 mg caused a statistically (P < 0.01) greater percentage reduction (mean −17%, 95% CI −27.7, −6.2, and −18%, −28.4, −7.4, respectively) in LDL-C than simvastatin alone.
Conclusions
The coadministration of ezetimibe at doses up to 10 mg with simvastatin 10 or 20 mg daily was well tolerated and caused a significant additive reduction in LDL-C compared with simvastatin alone. Additional clinical studies to assess the efficacy and safety of coadministration of ezetimibe and simvastatin are warranted.
Keywords: cholesterol absorption inhibitor, drug interaction, ezetimibe, hypercholesterolaemia, pharmacodynamics, pharmacokinetics, simvastatin
Introduction
Hypercholesterolaemia is an important risk factor for coronary artery disease, a major cause of death in the United States and other industrialized countries [1]. The reduction of elevated serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) reduces the risk of coronary artery disease, resulting in a decrease in cardiovascular morbidity and mortality [2–6]. Serum cholesterol is derived from biosynthesis (endogenous pathway) and intestinal uptake (exogenous pathway) of dietary and biliary cholesterol [7]. Drug therapy with cholesterol-lowering medications, particularly 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins), is effective in reducing the risk for cardiovascular disease and stroke in subjects who do not have adequate reduction in lipid levels after dietary modification [4, 6, 8]. Statins, which modulate only endogenous cholesterol, inhibit biosynthesis of cholesterol, deplete intracellular pools, and enhance removal of plasma LDL-C [9] leading to significant reductions of serum LDL-C [8–13]. However, not all subjects respond to statin treatment [14, 15]. Combination therapy of two or occasionally three hypolipidaemic drugs may be required to meet target LDL-C blood levels recommended by the European Second Joint Task Force [16] and the U.S. National Cholesterol Education Program (NCEP) [17]. Combinations of drugs that act by different mechanisms can provide additive effects in LDL-C reduction [15, 18]. However, the utility of current combination therapies with statins and niacin or bile acid sequestrants is limited by difficulties with side effects and, consequently, with compliance [19].
Ezetimibe (SCH 58235) is the first of the selective cholesterol absorption inhibitors, drugs that prevent the absorption of cholesterol by inhibiting the passage of dietary and biliary cholesterol across the intestinal wall [20, 21]. After oral administration ezetimibe is rapidly absorbed, extensively conjugated to its pharmacologically active [21] glucuronide conjugate, and slowly eliminated with evidence of significant enterohepatic recycling [22]. The efficacy of ezetimibe was evaluated in phase II [23] and phase III [24] trials in subjects with primary hypercholesterolaemia who were maintained on an NCEP Step I or stricter diet [25]. The results of these studies show that ezetimibe significantly (P < 0.05) reduced TC and LDL-C from baseline compared with placebo, with favourable effects on high density lipoprotein cholesterol (HDL-C) and triglycerides (TG) [23, 24].
Despite the diversity of available cholesterol-lowering therapies, a significant proportion of the hypercholesterolaemic population is not attaining the recommended target cholesterol levels [26–29]. Thus, there is a continued search for effective, better-tolerated drugs or combinations of drugs for the treatment of patients with hypercholesterolaemia. Results of preclinical studies in hypercholesterolaemic dogs have demonstrated that ezetimibe synergistically reduces plasma cholesterol levels when coadministered with HMG-CoA reductase inhibitors without evidence of liver or skeletal muscle toxicity [30, 31]. Therefore, it was hypothesized that ezetimibe might enhance the LDL-C-lowering effects of simvastatin in humans. Two pilot multiple-dose studies were conducted in otherwise healthy hypercholesterolaemic subjects in order to test this hypothesis. Study 1 evaluated the effect of different doses of ezetimibe on the pharmacokinetics of simvastatin 10 mg day−1 and assessed the tolerability and pharmacodynamic effect of the coadministration. Study 2 was conducted to obtain additional pharmacodynamic and tolerability data with the higher approved starting dose of simvastatin (20 mg day−1) coadministered with ezetimibe 10 mg day−1.
Methods
Study design
Two randomized, evaluator-blind, multiple-dose, parallel-group studies were conducted at the same research centre. Both studies followed identical protocols unless otherwise indicated. Before the initiation of either study, the protocol and statement of informed consent were approved by the clinical site's regional government ethics committee (Ethik Kommission bei der Landersärztekammer Baden-Württemberg, Stuttgart, Germany), and written informed consent was obtained from each volunteer.
Study 1 (n =58) was a placebo-controlled study in which subjects (11–12 per group) were randomized and received one of the following five treatments: simvastatin 10 mg with placebo; simvastatin 10 mg with ezetimibe 0.25 mg, 1 mg, or 10 mg; or placebo alone. For Study 2 (n =24), subjects (8 per group) were randomized and received one of the following three treatments: simvastatin 20 mg with ezetimibe 10 mg, simvastatin 20 mg with placebo, or ezetimibe 10 mg with placebo.
Subjects were stabilized as outpatients on an NCEP Step I diet [25] for ≥7 days, followed by an inpatient confinement period of 16 days to ensure compliance. After an overnight fast of ≥10 h, study treatments were administered orally with 200 ml of noncarbonated, room-temperature water, once daily in the morning for 14 consecutive days. Fasting (except for water) continued until standardized meals were served 2 h after dosing and at appropriate times during the day.
Subjects
All subjects had to satisfy the following inclusion criteria: a screening serum LDL-C of ≥130 mg dl−1; be in good health based on medical history, physical examination, electrocardiogram (ECG) results, and routine laboratory tests. In Study 1, subjects who had previously received ezetimibe were excluded; however, 10 subjects who had participated in Study 1 (> 60 days earlier) were allowed to enrol in Study 2. Exclusion criteria included: drug abuse, infectious disease within 4 weeks of starting the study; use of prescription drugs within 2 weeks; receiving investigational drugs within 60 days; and smoking >10 cigarettes per day.
Measurements
Tolerability
Physical examinations were conducted during screening and on day 15. Blood and urine samples were collected for routine laboratory tests before the first dose (day −1, baseline) and at the conclusion of the study (day 15). Blood samples were collected more frequently before dosing on days 3, 7, and 10 for monitoring signs of muscle and liver injury (i.e. alanine transaminase [ALT], aspartate transaminase, γ-glutamyl transferase [GGT], creatine phosphokinase [CPK], and alkaline phosphatase). ECGs were obtained during screening, before dosing on days 1 and 7, and at follow-up on day 15. Vital signs (blood pressure, heart rate, respiratory rate, and oral body temperature) were monitored during screening, daily before treatment administration and at follow-up on day 15. Subjects were continually observed and questioned for possible adverse events.
Pharmacodynamics
In both studies, fasting (> 10 h) blood samples were collected for serum lipid profiles (LDL-C, TC, HDL-C, and TG) just before dosing on days 1, 7, 14, and 15. Baseline values were day 1 predose concentrations.
Pharmacokinetics
Blood samples for determination of plasma simvastatin and β-hydroxysimvastatin concentrations were collected prior to the first and last dose (0 h on day 14) and at 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after the last dose of study treatment. Each plasma sample was frozen at −70° C or below until shipped to the analytical facility (Phoenix International Life Sciences, Int., Saint-Laurent, Quebec, Canada, [now MDS Pharma Services, Inc.]).
For determination of plasma ezetimibe concentration, a blood sample was collected at 1 h after the last dose on day 14. Plasma was separated by centrifugation and immediately frozen at −20° C or below until shipped to the analytical facility.
Analytical methods
Lipoproteins
Lipid concentrations (TC, LDL-C, HDL-C, and TG) were determined by direct quantitative assay methods (enzymatic colorimetric tests) using validated commercial assay kits (Boehringer Mannheim Systems, Boehringer Mannheim GmbH, Mannheim, Germany) on an automated analyser (HITACHI 747–100). The assays were performed by the investigators' clinical laboratory (Arztelaborgemeinschaft Freiburg, a subsidiary of Bioscentia, GmbH, Freiburg, Germany).
The factors for conversion of the lipid values to Système International (SI) units are 0.02586 (e.g. 200 mg dl−1×0.02586=5.17 mmol l−1) for TC, LDL-C, and HDL-C, and 0.01129 for TG (e.g. 150 mg dl−1×0.01129=1.69 mmol l−1) [33].
Simvastatin and β-hydroxysimvastatin
Plasma simvastatin and β-hydroxysimvastatin concentrations were determined using a validated liquid chromatography method with a tandem mass spectrometric detection assay (LC-MS/MS). The procedures had a lower limit of quantification (LOQ) of 0.100 ng ml−1 and a linear range of 0.100–10.0 ng ml−1 for both simvastatin and β-hydroxysimvastatin. Precision (%CV) and accuracy (%Bias) of the lowest calibration curve standards were 10.6 and −3.0%, respectively, for simvastatin and 11.5 and −5.9%, respectively, for β-hydroxysimvastatin.
Ezetimibe and total ezetimibe
Plasma ezetimibe and total ezetimibe (ezetimibe plus ezetimibe-glucuronide) concentrations were determined using validated LC-MS/MS assays. These assays had lower LOQ of 0.02 and 0.25 ng ml−1 plasma for ezetimibe and total ezetimibe, respectively, and the linear ranges were 0.02–20 ng ml−1 and 0.25–250 ng ml−1, respectively. Precision (%CV) and accuracy (%Bias) of the lowest calibration curve standards were 5.6 and 0.5%, respectively, for ezetimibe and 4.7 and −4.6%, respectively, for total ezetimibe.
The selectivity of the assays was demonstrated during the method validations. For all analytes measured, there was no significant endogenous interference at the retention times of the analytical (> 20% of the LOQ) or the internal standard (IS) (> 5% of the mean response for the IS) following processing of at least 6 lots of blank plasma.
Pharmacokinetic analysis
Individual plasma simvastatin and β-hydroxysimvastatin concentrations were used to estimate pharmacokinetic parameters using model-independent methods [32]. The maximum plasma concentration (Cmax) and tmax were the observed values. The areas under the plasma concentration-time curve (AUCs) from time 0 to the time of final quantifiable sample [AUC(tf)], and from time 0–24 h after dosing [AUC(0,24 h)] were calculated with the linear trapezoidal method. The terminal rate constant (λz) was calculated as the negative of the slope of the log:linear terminal portion of the plasma concentration-time curve using linear regression. The terminal half-life (t1/2) was calculated as 0.693/λz. Total body clearance (CL/F) was calculated as:
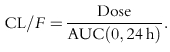 |
The apparent volume of distribution (Vd/F) was calculated as:
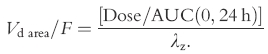 |
No pharmacokinetic analysis was performed for the ezetimibe data, since only a single sample was collected.
Statistical analysis
For both studies, summary statistics including mean, standard deviation or standard error, and coefficient of variation were provided for the demographic and pharmacodynamic data. For Study 1, concentration data at each time point, and the derived pharmacokinetic parameters were also summarized using descriptive statistics. An analysis of variance (anova) model with one factor (treatment) was performed on the original scale and log-transformed Cmax and AUC values to evaluate the effect of treatment on the pharmacokinetics of simvastatin and β-hydroxysimvastatin. The relative oral bioavailability of simvastatin and β-hydroxysimvastatin was expressed as the Cmax and AUC ratio from each treatment based on log-transformed data. Ninety percent confidence intervals (90% CI) for these estimates of relative bioavailability and the power to detect a 20% difference between treatment means for an α level of 0.05 (two-tailed) were computed.
For both studies, actual values, changes from baseline and percentage changes from baseline for lipid parameters LDL-C, TC, HDL-C, and TG were evaluated. anova models extracting treatment effect were performed to compare the treatment groups at baseline, day 7, day 14, endpoint (the last observed LDL-C after day 1 and up to day 14), and day 15. In Study 1, pairwise comparisons of each treatment group vs placebo and each of the three simvastatin/ezetimibe combination arms vs simvastatin alone were tested using the least square mean procedures. In Study 2, pairwise comparisons were tested using the least square mean procedures. Ninety-five percent confidence intervals (95% CI) were calculated for the mean difference between each pair of treatments using the pooled residual error and associated degrees of freedom from the anova. In addition, percentage changes in LDL-C were categorized as follows:<10%, 10% to 25%, 25% to <35%, 35% to <50%, and ≥50%; the distribution of subjects in each category was tabulated. Pairwise comparisons were tested using the least square mean procedures.
Results
Demographics
In Study 1, 58 male white subjects between 20 and 50 years old with a body mass index (BMI) ranging from 19 to 31 were enrolled (Table 1). In Study 2, 24 male white subjects between 22 and 49 years old with a BMI between 19 and 27 were enrolled (Table 2). In both studies, baseline LDL-C values were generally similar among treatment groups (Tables 1 and 2).
Table 1.
Demographic profile and baseline LDL-C values of treatment group (Study 1).
| SIM 10 mg + EZE 0.25 mg (n = 11) | SIM 10 mg + EZE 1 mg (n = 12) | SIM 10 mg + EZE 10 mg (n = 12) | SIM 10 mg (n = 12) | Placebo (n = 11) | |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean | 36.0 | 36.1 | 38.0 | 34.1 | 37.1 |
| s.e. mean | 1.5 | 2.3 | 2.1 | 2.3 | 2.8 |
| Median | 37 | 36 | 41 | 34 | 33 |
| Range | 28–44 | 20–47 | 24–46 | 21–49 | 26–50 |
| Baseline weight (kg) | |||||
| Mean | 75.8 | 73.3 | 74.8 | 72.8 | 72.3 |
| s.e. mean | 2.7 | 3.0 | 2.6 | 1.9 | 2.7 |
| Median | 78 | 71 | 73 | 75 | 68 |
| Range | 55–89 | 55–90 | 63–97 | 60–81 | 62–88 |
| Baseline LDL-C (mg dl−1) | |||||
| Mean | 177.1 | 177.3 | 171.4 | 167.5 | 164.0 |
| s.e. mean | 12.7 | 12.5 | 7.3 | 8.9 | 6.6 |
| Median | 164 | 174 | 166 | 166 | 161 |
| Range | 132–282 | 130–275 | 135–219 | 108a–228 | 135–203 |
SIM, simvastatin; EZE, ezetimibe; s.e. mean, standard error; LDL-C, low-density lipoprotein cholesterol.
This subject met the LDL-C entry criterion of ≥130 mg dl−1 at screening.
Table 2.
Demographic profile and baseline LDL-C values of treatment groups (Study 2).
| SIM 20 mg + EZE 10 mg (n = 8) | SIM 20 mg + EZE placebo (n = 8) | EZE 10 mg + EZE placebo (n = 8) | |
|---|---|---|---|
| Age (years) | |||
| Mean | 38.0 | 37.6 | 33.3 |
| s.e. mean | 2.9 | 2.7 | 2.0 |
| Median | 38 | 36 | 33 |
| Range | 22–49 | 27–47 | 26–43 |
| Baseline weight (kg) | |||
| Mean | 70.3 | 81.0 | 72.3 |
| s.e. mean | 2.9 | 2.0 | 2.9 |
| Median | 72 | 81 | 74 |
| Range | 57–79 | 72–89 | 60–82 |
| Baseline LDL-C (mg dl−1) | |||
| Mean | 183.4 | 157.8 | 168.5 |
| s.e. mean | 11.9 | 6.4 | 9.5 |
| Median | 182 | 159 | 165 |
| Range | 147–221 | 134–181 | 135–197 |
SIM, simvastatin; EZE, ezetimibe; s.e. mean, standard error; LDL-C, low-density lipoprotein cholesterol.
Tolerability
In Study 1, 57 of the 58 subjects enrolled successfully completed the study. One subject in the simvastatin 10 mg plus ezetimibe 10 mg group discontinued treatment on day 11 because of pain and swelling of the wrist considered possibly related to study drug. All 24 subjects enrolled in Study 2 successfully completed the study.
There were no serious adverse events, or clinically significant changes or trends in vital signs, ECGs, or clinical laboratory tests (particularly in the enzymes assessing muscle and liver injury) with any of the treatments. The incidence of adverse events was similar among treatment groups, with no evidence of dose-related increases. Overall (i.e. Study 1 and Study 2 data combined) 34 subjects (41%) reported treatment-emergent adverse events, most commonly headache (8/82; 10%), flatulence (6/82; 7%), and viral infections (5/82; 6%). Less common adverse events included loose stools (3/82; 4%) and diarrhoea (3/82; 4%). Most adverse events were mild to moderate in intensity.
In Study 1, two subjects complained of myalgia; one subject had received simvastatin 10 mg plus ezetimibe 10 mg, and the other had received placebo. Neither subject had increased CPK levels or any other laboratory test abnormalities associated with their adverse event or at any other time during the study. Two subjects in the Study 2, both in the simvastatin 20 mg group, had transient increases in ALT values of >1.5–3×the upper limit of normal. Of these two subjects, one subject had a concurrent viral illness, and the other had complained of a moderate headache for which he had been treated with acetaminophen (paracetamol) 500 mg.
Pharmacokinetics
Mean AUC and Cmax values for simvastatin and β-hydroxysimvastatin were similar (P >0.32) among all treatment groups, with one notable pharmacokinetic outlier in the simvastatin 10 mg plus ezetimibe 0.25 mg treatment group whose high values affected the group mean (Table 3). The relative oral bioavailability of simvastatin and β-hydroxysimvastatin after the coadministration of ezetimibe with simvastatin compared with simvastatin alone ranged from 96% (90% CI 63, 146%) to 138% (90% CI 80, 236%). Thus, ezetimibe has no apparent effect on the pharmacokinetics of simvastatin or β-hydroxysimvastatin. The increases in plasma ezetimibe and total ezetimibe concentrations at 1 h after study drug administration were dose related.
Table 3.
Mean (s.d.) pharmacokinetic parameters for simvastatin and β-hydroxysimvastatin on day 14 (Study 1).
| Parameter | SIM 10 mg+EZE 0.25 mg (n = 11) | SIM 10 mg+EZE 1 mg (n = 12) | SIM 10 mg+EZE 10 mg (n = 11) | SIM 10 mg (n = 12) |
|---|---|---|---|---|
| Simvastatin | ||||
| Cmax (ng ml−1) | 2.94 (1.84) | 2.38 (1.32) | 2.65 (1.65) | 2.36 (1.09) |
| tmaxa (h) | 1 (0.5–2) | 1 (0.5–2) | 1 (0.5-2) | 1 (0.5-3) |
| AUC(0,24 h) (ng ml−1 h) | 8.07 (4.67) | 7.82 (6.15) | 8.42 (7.02) | 6.82 (3.29) |
| t1/2 (h) | 3.76b (1.85) | 2.78c (1.98) | 3.46b (2.11) | 3.52c (3.06) |
| CL/F (kg−1) (ml min−1 kg−1) | 362 (172) | 455 (262) | 502 (509) | 448 (283) |
| Vd/F (kg−1) (l kg−1) | 111b (94) | 85.4c (44) | 112b (76) | 116c (109) |
| β-Hydroxysimvastatin | ||||
| Cmax (ng ml−1) | 0.90 (1.03) | 0.63 (0.34) | 0.62 (42) | 0.57 (0.26) |
| tmaxa (h) | 3 (1–8) | 3.5 (0.5–8) | 3 (0.5–6) | 4 (1–8) |
| AUC(0,24 h) (ng ml−1 h) | 9.10 (11.72) | 5.64 (4.85) | 5.55 (3.95) | 5.10 (2.63) |
| t1/2 (h) | 6.20d (4.80) | 5.15d (1.88) | 4.69e (1.64) | 4.91d (2.00) |
s.d., standard deviation; SIM, simvastatin; EZE, ezetimibe; Cmax, maximum plasma concentration; tmax, time to reach Cmax; AUC, area under the plasma concentration-time curve; t1/2, terminal half-life associated with the terminal slope of the semilogarithmic plasma concentration-time curve; CL/F, apparent oral clearance where F represents bioavailability and CL represents total clearance; Vd, volume of distribution.
Median (range).
n = 10,
n = 11,
n = 8,
n = 7.
Pharmacodynamics
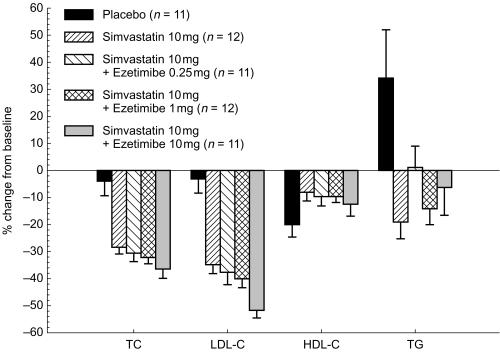
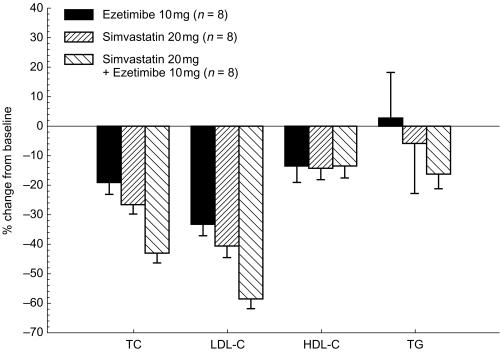
In both studies, all active treatments caused statistically significant (P < 0.01) decreases in LDL-C vs placebo from baseline to day 7 and day 14 (Figures 1 and 2). By day 14 in Study 1, simvastatin 10 mg alone reduced LDL-C by 35%; coadministration of ezetimibe at doses of 0.25, 1, or 10 mg augmented the reduction of LDL-C in a dose-dependent manner. For the group receiving ezetimibe 10 mg with simvastatin, LDL-C was reduced by a mean of 52.4%, with the actual mean value reduced to 82.9 mg dl−1 (Table 4). There was an incremental change of −17.0% (95% CI −27.7, −6.2) for the ezetimibe 10 mg plus simvastatin 10 mg group compared with the simvastatin alone group (P < 0.01) (Figure 1). In Study 2, by day 14 the administration of ezetimibe 10 mg alone reduced LDL-C by 33.6%; and simvastatin 20 mg alone decreased LDL-C by 40.8% below baseline (Figure 2). The coadministration of ezetimibe 10 mg and simvastatin 20 mg reduced LDL-C by a mean of 58.7% (actual 77.0 mg dl−1); an incremental mean change of −17.9% (95% CI −28.4, −7.4) more than the group receiving simvastatin alone (P < 0.01) (Figure 2).
Figure 1.
Mean (s.e. mean) % change from baseline in serum lipids (Study 1).
Figure 2.
Mean (s.e. mean) % change from baseline in serum lipids (Study 2).
Table 4.
Mean (s.e. mean) serum lipid concentrations (mg dl−1) on day 14 after oral administration of simvastatin, ezetimibe, the coadministration of simvastatin and ezetimibe, or placebo (Studies 1 and 2).
| Treatment | Day | LDL-C | TC | HDL-C | TG |
|---|---|---|---|---|---|
| Study 1 | |||||
| Placebo, n = 11 | Baseline | 164.0 (6.6) | 241.5 (7.9) | 49.7 (3.5) | 118.5 (10.4) |
| Day 14 | 158.3 (9.4) | 229.4 (9.9) | 39.0 (2.8) | 146.1 (13.0) | |
| SIM 10 mg, n = 12 | Baseline | 167.5 (8.9) | 247.3 (7.3) | 44.8 (2.5) | 138.7 (12.1) |
| Day 14 | 108.4 (6.5) | 176.8 (7.5) | 41.0 (2.5) | 110.6 (11.4) | |
| SIM 10 mg + EZE 0.25 mg, n = 11 | Baseline | 177.1 (12.7) | 257.9 (13.6) | 41.4 (2.8) | 134.6 (17.0) |
| Day 14 | 109.3 (10.8) | 177.3 (9.9) | 36.9 (2.3) | 127.1 (12.4) | |
| SIM 10 mg + EZE 1 mg, n = 12 | Baseline | 177.3 (12.5) | 267.0 (12.8) | 43.5 (3.3) | 167.7 (17.4) |
| Day 14 | 104.5 (6.8) | 180.1 (8.7) | 38.8 (2.3) | 144.3 (19.1) | |
| SIM 10 mg + EZE 10 mg, n = 12 | Baseline | 171.4 (7.3) | 245.1 (9.5) | 43.5 (2.1) | 126.4 (7.1) |
| Day 14a | 82.9 (6.5) | 152.3 (7.3) | 37.3 (2.1) | 121.5 (17.2) | |
| Study 2 | |||||
| SIM 20 mg, n = 8 | Baseline | 157.8 (6.4) | 242.1 (9.0) | 44.3 (1.9) | 153.8 (12.4) |
| Day 14 | 93.0 (6.2) | 176.1 (7.2) | 37.6 (1.7) | 134.9 (13.7) | |
| EZE 10 mg, n = 8 | Baseline | 168.5 (9.5) | 243.8 (10.4) | 45.5 (3.5) | 134.4 (13.7) |
| Day 14 | 112.4 (9.6) | 197.4 (14.1) | 38.8 (3.1) | 148.6 (41.1) | |
| SIM 20 mg + EZE 10 mg, n = 8 | Baseline | 183.4 (11.9) | 265.3 (14.0) | 46.6 (2.5) | 151.9 (20.8) |
| Day 14 | 77.0 (9.7) | 150.9 (12.8) | 39.8 (1.8) | 124.6 (17.5) | |
s.e. mean, standard error; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; SIM, simvastatin; EZE, ezetimibe.
n = 11.
Serum TC was reduced on day 14 after simvastatin alone (Figures 1 and 2) and ezetimibe alone (Figure 2). In both studies, coadministration of simvastatin and ezetimibe was associated with a greater reduction in serum TC than simvastatin alone (Figures 1 and 2), and the magnitude of reduction was related to the ezetimibe dose (Figure 1). A trend toward decreasing serum HDL-C occurred in all groups (Figures 1 and 2); however, none of these changes was statistically significant (P >0.05). In Study 1, serum TG concentrations were unchanged or decreased in the active treatment groups whereas they increased in the placebo group (Figure 1); none of the changes was statistically significant. In Study 2, a trend toward a decrease in serum TG in the ezetimibe 10 mg plus simvastatin 20 mg group compared with the monotherapy group was not statistically significant (P >0.05) (Figure 2).
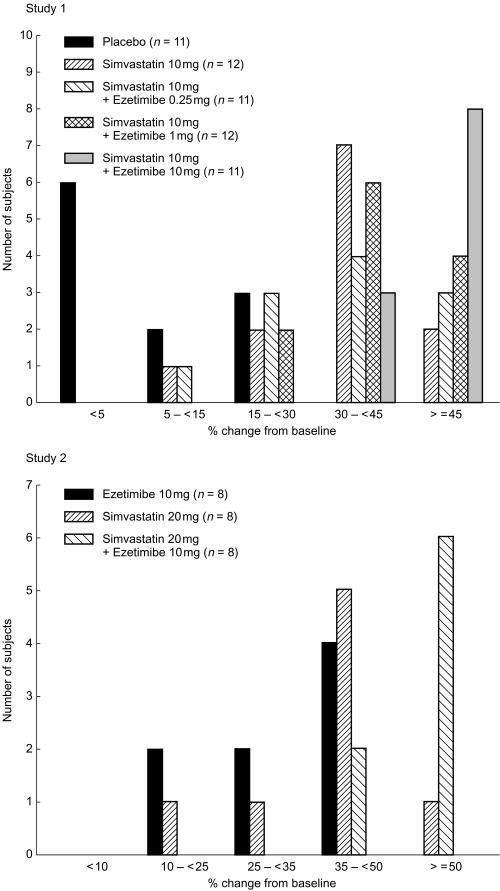
In Study 1, 8 of the 11 subjects who received simvastatin 10 mg with ezetimibe 10 mg achieved a ≥50% reduction in LDL-C by day 14, compared with 1 of 12 subjects receiving simvastatin alone, 3 of 11 subjects receiving simvastatin 10 mg plus ezetimibe 0.25 mg, and 2 of 12 subjects treated with simvastatin 10 mg plus ezetimibe 1 mg (Figure 3). In Study 2, 1 of the 8 subjects treated with simvastatin 20 mg alone and none of 8 subjects treated with ezetimibe 10 mg alone achieved a ≥50% reduction in LDL-C by day 14, compared with 6 of the 8 subjects in the coadministration group (Figure 3).
Figure 3.
Distribution of subjects based on the magnitude of LDL-C reduction achieved at study endpoint (day 14) (Studies 1 and 2).
Discussion
Ezetimibe is a novel cholesterol absorption inhibitor that has been shown to be well tolerated and to significantly decrease LDL-C and TC in patients with primary hypercholesterolaemia, with favourable effects on HDL-C and TG [23, 24]. The mean percentage reduction in directly measured LDL-C at study endpoint (i.e. after 8 or 12 weeks of treatment) for ezetimibe 10 mg day−1 was consistently in the range of 16% to 19% [23, 24]. Simvastatin is a marketed HMG-CoA reductase inhibitor which has been shown to significantly decrease serum LDL-C levels and reduce the risk of overall and coronary heart disease mortality in patients with hypercholesterolaemia [4, 34]. In animal models the coadministration of ezetimibe and simvastatin has been shown to cause a much greater reduction in LDL-C than would be expected by the sum of the effects of either drug alone. The potential for a pharmacodynamic interaction between ezetimibe and simvastatin would offer significant clinical benefits provided that the combination was safe and well tolerated. Study 1 was conducted as an initial proof-of-principle study to confirm the preclinical observations and Study 2 was conducted to obtain additional pharmacodynamic and tolerability data with the higher approved starting dose of simvastatin before conducting large clinical trials. In both of these studies treatment was administered for 14 days since data from Phase II trials [23] had indicated that the maximum LDL-C-lowering effect of ezetimibe was achieved by 2 weeks and was sustained throughout treatment.
The results from these two pilot studies show that the coadministration of ezetimibe and simvastatin was well tolerated, with no evidence of increased incidence of adverse events or increases in clinical laboratory tests indicative of liver or skeletal muscle toxicity. The coadministration of combination therapy caused significantly greater reductions in LDL-C than simvastatin alone. In these studies, adding ezetimibe 10 mg day−1 to simvastatin 10 or 20 day−1 demonstrated additional LDL-C reductions of 17–18%, similar to reductions one might expect from titrating three times with a statin (e.g. from 10 mg to 80 mg of simvastatin) [35]. Unlike the preclinical findings, the incremental reduction in mean LDL-C achieved by the coadministration of ezetimibe 10 mg and simvastatin 10 or 20 mg appears to be additive rather than synergistic. This pharmacodynamic interaction is similar to the mean 14% additional reduction in LDL-C reported by the coadministration of simvastatin and cholestyramine in hypercholesterolaemic patients [36].
Results from Study 1 indicate that the coadministration of ezetimibe had no significant effect on the pharmacokinetics of simvastatin and β-hydroxysimvastatin. Since most pharmacokinetic drug interactions with simvastatin are primarily mediated through the induction or inhibition of the cytochrome P450 isoenzyme 3A4 [37–39], these results are consistent with ezetimibe's demonstrated lack of effect on this enzyme [40]. In our study there was considerable intersubject variability in both simvastatin and β-hydroxysimvastatin concentrations (Table 3), but this is not unexpected for this drug [38, 39, 41]. The mean Cmax and AUC values for these two analytes were similar among active treatments, with one notable pharmacokinetic outlier for β-hydroxysimvastatin in the simvastatin 10 mg plus ezetimibe 0.25 mg treatment group whose high values affected the group mean (Table 3). Despite these uncharacteristically high simvastatin and β-hydroxysimvastatin values, this individual did not report any adverse events, and his safety laboratory test results were normal, although he did achieve the highest reduction of LDL-C (−60.2% on day 14) in his treatment group. In this study ezetimibe pharmacokinetics were not fully characterized since after oral administration of ezetimibe 0.25 and 1 mg day−1 plasma drug concentrations for ezetimibe and total ezetimibe were expected to be too low relative to the assay LOQ to adequately characterize a pharmacokinetic profile. Consequently, plasma samples for ezetimibe and total ezetimibe concentrations were only measured at 1 h post dose to coincide with the anticipated Cmax of total ezetimibe [42, 43]. The results indicate that plasma ezetimibe and total ezetimibe concentrations increased in a dose-related manner. The mean plasma ezetimibe and total ezetimibe concentrations (3.23 and 92.4 ng ml−1, respectively) achieved at 1 h after the administration of simvastatin 10 mg plus ezetimibe 10 mg are consistent with those observed after the administration of ezetimibe 10 mg alone in previous studies [44, 45], suggesting no effect of simvastatin on ezetimibe pharmacokinetics.
In our studies there was a general trend for serum HDL-C concentrations to decrease and TG to increase during treatment (Table 4, Figures 1 and 2). This is likely the result of volunteer confinement, dietary changes (the subjects' diet was altered from one predominantly higher in percentage of fat and lower in carbohydrates to one higher in carbohydrates and lower in fat), and restricted physical activity. Similar results have been observed in other inpatient studies in healthy subjects with hypercholesterolaemia [46–48]. These observations are consistent with published data on the effects of low-fat, carbohydrate-rich diets on serum lipoprotein levels [49].
There are several limitations to our studies. One is that the study population consisted of white males and so the conclusions, strictly speaking, only apply to that population, although the pharmacokinetics, LDL-C response, and safety profile of ezetimibe have been shown to be independent of gender and race [42, 50]. Another limitation is that the number of subjects was small, and the studies were not powered to show safety and tolerability. Consequently, our conclusions regarding tolerability of the coadministration of ezetimibe and simvastatin are preliminary, and need to be confirmed in larger studies.
In summary, once-daily administration of ezetimibe (at doses up to 10 mg) coadministered with simvastatin (10 or 20 mg) to healthy subjects with hypercholesterolaemia was well tolerated and significantly reduced serum LDL-C and TC. Thus, coadministration of simvastatin and ezetimibe is an alternative to titrating to higher doses of simvastatin. Additional clinical studies to evaluate the efficacy and safety of the coadministration of ezetimibe and simvastatin are warranted.
This study was supported by Schering-Plough Research Institute, Kenilworth, New Jersey, USA.
References
- 1.Gensini GF, Comeglio M, Colella A. Classical risk factors and emerging elements in the risk profile for coronary artery disease. Eur Heart J. 1998;19(Suppl A):A53–A61. [PubMed] [Google Scholar]
- 2.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 3.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 4.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 5.Sacks RF, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 6.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and deaths with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 7.Beisiegel U. Lipoprotein metabolism. Eur Heart J. 1998;19(Suppl A):A20–A23. [PubMed] [Google Scholar]
- 8.Farnier M, Davignon J. Current and future treatment of hyperlipidemia: the role of statins. Am J Cardiol. 1998;82:3J–10J. doi: 10.1016/s0002-9149(98)00423-8. [DOI] [PubMed] [Google Scholar]
- 9.Witztum JL. Drugs used in the treatment of hyperdyslipidemias. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 9. New York: McGraw-Hill International Book Co; 1996. pp. 875–897. [Google Scholar]
- 10.Blum CB. Comparison of the properties of four inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Am J Cardiol. 1994;73(Suppl):3D–11D. doi: 10.1016/0002-9149(94)90626-2. [DOI] [PubMed] [Google Scholar]
- 11.Nawrocki JW, Weiss SR, Davidson MH, et al. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler Thromb Vasc Biol. 1995;15:678–682. doi: 10.1161/01.atv.15.5.678. [DOI] [PubMed] [Google Scholar]
- 12.Lea AP, McTavish D. Atorvastatin: a review of its pharmacology and therapeutic potential in the management of hyperlipidemias. Drugs. 1997;53:828–847. doi: 10.2165/00003495-199753050-00011. [DOI] [PubMed] [Google Scholar]
- 13.Malinowski JM. Atorvastatin: a hydroxymethylglutaryl-coenzyme A reductase inhibitor. Am J Health-Syst Pharm. 1998;55:2253–2267. doi: 10.1093/ajhp/55.21.2253. [DOI] [PubMed] [Google Scholar]
- 14.Moghadasian MH. Clinical pharmacology of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Life Sci. 1999;65:1329–1337. doi: 10.1016/s0024-3205(99)00199-x. [DOI] [PubMed] [Google Scholar]
- 15.Tikkanen MJ. Statins: within-group comparisons, statin escape and combination therapy. Curr Opin Lipidol. 1996;7:385–388. doi: 10.1097/00041433-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Wood D, De Backer G, Faergeman O, et al. Prevention of coronary heart disease in clinical practice: recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Eur Heart J. 1998;19:1434–1503. doi: 10.1053/euhj.1998.1243. [DOI] [PubMed] [Google Scholar]
- 17.Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults. Summary of the Second Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) JAMA. 1993;269:3015–3023. [PubMed] [Google Scholar]
- 18.Schectman G, Hiatt J. Dose–response characteristics of cholesterol-lowering drug therapies: implications for treatment. Ann Intern Med. 1996;125:990–1000. doi: 10.7326/0003-4819-125-12-199612150-00011. [DOI] [PubMed] [Google Scholar]
- 19.Schectman G, Hiatt J. Drug therapy for hypercholesterolemia in patients with cardiovascular disease factors limiting achievement of lipid goals. Am J Med. 1996;100:197–204. doi: 10.1016/s0002-9343(97)89459-4. [DOI] [PubMed] [Google Scholar]
- 20.van Heek M, France CF, Compton DS, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283:157–163. [PubMed] [Google Scholar]
- 21.van Heek M, Farley C, Compton DS, et al. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br J Pharmacol. 2000;129:1748–1754. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezzet F, Krishna G, Wexler D, Statkevich P, Kosoglou T, Batra V. A population pharmacokinetic model that describes multiple peaks due to enterohepatic recirculation of ezetimibe. Clin Ther. 2001;23:871–885. doi: 10.1016/s0149-2918(01)80075-8. [DOI] [PubMed] [Google Scholar]
- 23.Bays HE, Moore PB, Drehobl MA, et al. Effectiveness and tolerability of ezetimibe in subjects with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther. 2001;23:1209–1230. doi: 10.1016/s0149-2918(01)80102-8. [DOI] [PubMed] [Google Scholar]
- 24.Knopp RH, Gitter H, Truitt T, et al. Ezetimibe reduces low-density lipoprotein cholesterol: results of a Phase III, randomized, double-blind, placebo-controlled trial. Atherosclerosis. 2001;2:38. (Abstract). [Google Scholar]
- 25.Ernst ND, Cleeman J, Mullis R, Sooter-Bochenek J, Van Horn L. The National Cholesterol Education Program: implications for dietetic practitioners from the Adult Treatment Panel recommendations. J Am Diet Ass. 1988;88:1401–1408. [PubMed] [Google Scholar]
- 26.Marcelino JJ, Feingold KR. Inadequate treatment with HMG-CoA reductase inhibitors by health care providers. Am J Med. 1996;100:605–610. doi: 10.1016/s0002-9343(96)00011-3. [DOI] [PubMed] [Google Scholar]
- 27.Feely J, McGettigan P, Kelly A. Growth in use of statins after trials is not targeting to most appropriate patients. Clin Pharmacol Ther. 2000;67:438–441. doi: 10.1067/mcp.2000.105152. [DOI] [PubMed] [Google Scholar]
- 28.Gotto A, Pownall H. Manual of Lipid Disorders. 2. Baltimore: Williams & Wilkins; 1999. pp. 292–295. [Google Scholar]
- 29.National Institutes of Health. National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) Circulation. 1994;89:1329–1445. doi: 10.1161/01.cir.89.3.1333. [DOI] [PubMed] [Google Scholar]
- 30.Davis HR, van Heek M, Watkins RW, et al. The hypocholesterolemic activity of the potent cholesterol absorption inhibitor SCH58235 alone and in combination with HMG CoA reductase inhibitors. Drugs Affecting Lipid Metabolism (DALM); Houston, TX. (Abstract).
- 31.Davis HR, Jr, Pula KK, Alton KB, Burrier RE, Watkins RW. The synergistic hypocholesterolemic activity of the potent cholesterol absorption inhibitor ezetimibe in combination with HMG-CoA reductase inhibitors in dogs. Metabolism. 2001;50:1234–1241. doi: 10.1053/meta.2001.26737. [DOI] [PubMed] [Google Scholar]
- 32.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker, Inc.; 1982. pp. 409–417. [Google Scholar]
- 33.Kratz A, Lewandrowski KB. Normal reference laboratory values. N Engl J Med. 1998;339:1063–1072. doi: 10.1056/NEJM199810083391508. [DOI] [PubMed] [Google Scholar]
- 34.Plosker GL, McTavish D Simvastatin. A reappraisal of its pharmacology and therapeutic efficacy in hypercholesterolemia. Drugs. 1995;50:334–363. doi: 10.2165/00003495-199550020-00009. [DOI] [PubMed] [Google Scholar]
- 35.Tuomilehta J, Guimaraes AC, Kettner H, et al. Dose–response of simvastatin in primary hypercholesterolemia. J Cardiovasc Pharmacol. 1994;24:941–949. doi: 10.1097/00005344-199424060-00012. [DOI] [PubMed] [Google Scholar]
- 36.Mölgaard J, Lundh BL, van Schenck H, Olsson AG. Long-term efficacy and safety of simvastatin alone and in combination therapy in treatment of hypercholesterolemia. Atherosclerosis. 1991;91:S21–S28. doi: 10.1016/0021-9150(91)90203-f. [DOI] [PubMed] [Google Scholar]
- 37.Neuvonen PJ, Kantola T, Kivisto KT. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin Pharmacol Ther. 1998;63:332–341. doi: 10.1016/S0009-9236(98)90165-5. [DOI] [PubMed] [Google Scholar]
- 38.Kantola T, Kivistö KT, Neuvonen PT. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 1998;64:177–182. doi: 10.1016/S0009-9236(98)90151-5. [DOI] [PubMed] [Google Scholar]
- 39.Gruer PJK, Vega JM, Mercuri MF, Dobrinska MR, Tobert JA. Concomitant use of cytochrome P450 3A4 inhibitors and simvastatin. Am J Cardiol. 1999;84:811–815. doi: 10.1016/s0002-9149(99)00442-7. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Statkevich P, Kosoglou T, et al. Effect of ezetimibe (SCH 58235) on the activity of drug metabolizing enzymes in vivo. Clin Pharmacol Ther. 2000;67:152. (Abstract). [Google Scholar]
- 41.Mauro VF. Clinical pharmacokinetics and practical applications of simvastatin. Clin Pharmacokin. 1993;24:195–202. doi: 10.2165/00003088-199324030-00002. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y, Statkevich P, Maxwell SE, et al. The effect of gender on the pharmacokinetics of SCH 58235, a cholesterol absorption inhibitor. AAPS Pharm Sci Supplement. 1999. pp. S–24. (Abstract).
- 43.Zhu Y, Statkevich P, Schuessler D, et al. Pharmacokinetics of ezetimibe in rats, dogs, and humans. AAPS Pharm Sci Supplement. 2000;2:2660. (Abstract). [Google Scholar]
- 44.Kosoglou T, Kakkar T, Statkevich P, et al. Multiple-dose safety and pharmacokinetics of ezetimibe in adolescent children. Clin Pharmacol Ther. 2001;69:P52. (Abstract). [Google Scholar]
- 45.Krishna G, Kosoglou T, Ezzet F, et al. Effect of cimetidine on the pharmacokinetics of ezetimibe. AAPS Pharm Sci Supplement. 2001. [Electronic (Abstract)]
- 46.Lipka LJ, Knopp RH, Gitter H, et al. Ezetimibe significantly lowers low-density lipoprotein cholesterol in subjects with primary hypercholesterolemia. Eur Heart J. 2001. p. 252. (Abstract).
- 47.Kosoglou T, Seiberling M, Statkevich P, et al. Pharmacodynamic interaction between the new selective cholesterol absorption inhibitor ezetimibe and atorvastatin. J Am Coll Cardiol. 2001;37(Suppl A):229A. (Abstract). [Google Scholar]
- 48.Kosoglou T, Guillaume M, Sun S, et al. Pharmacodynamic interaction between fenofibrate and the cholesterol absorption inhibitor ezetimibe. Atherosclerosis Suppl. 2001;2(2):38. (Abstract). [Google Scholar]
- 49.Kosoglou T, Seiberling M, Statkevich P, et al. Pharmacodynamic interaction between cerivastatin and the selective cholesterol absorption inhibitor ezetimibe. Eur Heart J. 2001. p. 252. (Abstract).
- 50.Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects (44564E) Proc Soc Exp Biol Med. 2000;225:191–199. doi: 10.1046/j.1525-1373.2000.22524.x. [DOI] [PubMed] [Google Scholar]