Abstract
Aims
Midazolam is given intravenously for induction of anaesthesia and conscious sedation and by subcutaneous infusion in patients in palliative care units. The objective of the present study was to determine the absolute bioavailability of subcutaneous midazolam and its pharmacokinetics in young, healthy, male volunteers.
Methods
Eighteen volunteers were given single doses of 0.1 mg kg−1 midazolam i.v. and s.c. after a wash-out period of 7–15 days in an open-label, randomized, cross-over study. Blood samples were collected up to 12 h post-infusion. Plasma concentrations of midazolam and of its two metabolites, 1′-OHM and 4-OHM, were assessed using an h.p.l.c.-MS method (LOQ 0.5 ng ml−1 for each analyte). Vital signs, cardiac parameters and oximetry were monitored. Local tolerance was determined and adverse events were also monitored.
Results
After s.c. infusion tmax and Cmax were 0.51 ± 0.18 h and 127.8 ± 29.3 ng ml−1 (mean ± s.d.), respectively. No statistically significant difference was detected in AUC(0,∞) after i.v. and s.c. administration. The mean (± s.d.) absolute bioavailability of subcutaneous midazolam was 0.96 (± 0.14) (CI 0.84, 1.03). Mean (± s.d.) t1/2 was similar after s.c. (3.2 (± 1.0) h) and i.v. infusion (2.9 (± 0.7) h), although a statistically significant difference was reached (P < 0.05). Mean CL and V of i.v. midazolam were 4.4 ± 1.0 ml min−1 kg−1 and 1.1 ± 0.2 l kg−1 (mean ± s.d.), respectively. Plasma concentrations of 1’-OHM were higher than those of 4-OHM. Few mild and transient adverse events were noted and there were no clinically significant effects on EEG, blood pressure and laboratory parameters.
Conclusions
This study has shown that subcutaneous midazolam has excellent bioavailability and that administration of midazolam by this route could be preferable when the intravenous route is inappropriate.
Keywords: bioavailability, intravenous, midazolam, pharmacokinetics, subcutaneous
Introduction
Midazolam (Hypnovel®, Roche) is a water soluble benzodiazepine which is characterized by rapid onset and short duration of action. Midazolam is largely used in clinical practice for induction of anaesthesia and for sedation of patients in intensive care units [1, 2]. Midazolam is rapidly and extensively metabolized almost exclusively by CYP3A isoforms [3,4] and its main metabolite is 1′-hydroxymidazolam (1′-OHM). Two other hydroxy metabolites 4-hydroxymidazolam (4-OHM) and 1′, 4-dihydroxymidazolam are also formed, although to a much lesser extent. The metabolites are eliminated as glucuronoconjugates and are thought not to be pharmacologically active. However, it has been suggested that the conjugate of 1′-OHM may contribute to sedation on accumulation in patients with renal failure [5].
The pharmacokinetics of midazolam have been evaluated following administration by various routes and under different subject conditions. Its bioavailability (F) has been shown to vary widely with the route of administration, with mean values of about 40%, 52%, 75%, 83% and 85% being reported for the oral [6, 7], rectal [8], buccal [9], nasal [10] and intramuscular [11] routes, respectively. In one study [12], interindividual oral bioavailability was reported to range from 31 to 72%, probably because of the involvement of CYP3A in midazolam metabolism. Large interindividual variation in both the content and activity of the CYP3A subfamily occur in humans [13]. The pharmacokinetics of midazolam are not influenced by age, although elderly subjects are more sensitive to its effects than young adults [14, 15]. Volume of distribution has been reported to be significantly higher and elimination half-life to be longer in obese subjects compared with control subjects of normal weight, whereas oral bioavailability does not differ between the two groups [16]. In addition, variability in midazolam pharmacokinetics can be accounted for, at least partially, by differences in renal [17] and liver [18] function.
In palliative care units, midazolam is commonly administered by subcutaneous infusion over a dose range of approximately 10–60 mg day−1, often associated with morphine [19]. Under conditions of continuous subcutaneous administration, a significant correlation was found between midazolam dose and steady-state plasma concentration [20]. Following an infusion of 20 mg day−1, a mean steady-state plasma concentration of 39 ng ml−1 was found (range 22–71 ng ml−1; n = 4). However, the absolute bioavailability and pharmacokinetics of midazolam after subcutaneous administration have not been reported.
The purpose of this study was to determine the bioavailability and characterize the pharmacokinetics of midazolam after subcutaneous and intravenous injection in young healthy male volunteers.
Methods
Subjects
Nineteen healthy male volunteers between 18 and 31 years of age (24.2 ± 3 years, mean ± s.d.; body mass index 18–30 kg m−2) participated in this study. One subject dropped out after the subcutaneous injection (first sequence) because of withdrawal of consent. None of the subjects had clinical evidence of significant gastrointestinal, renal, respiratory, endocrine, haematological, neurological, psychiatric or cardiovascular system abnormalities. Subjects who had a known or suspected history of alcohol or drug misuse or a positive urine drug screen were excluded from entry, as were those who had donated blood within 3 months of entry or those excluded according to the ‘Fichier National des Volontaires’ (French National Database for Volunteers). Subjects who reported smoking 10 cigarettes day−1 or more, who were excessive consumers of caffeine or methylxanthine drinks or who had taken medicines, with the exception of paracetamol, within 2 weeks of the start of the study were also excluded.
The study was conducted in accordance with the principles stated in the Declaration of Helsinki, in compliance with the Good Clinical Practice and ICH Guidelines and in agreement with the French law (loi Huriet) for clinical trials without direct benefit. The study protocol was approved by the Institutional Ethics Committee, CCPPRB (Comité Consultatif des Personnes se Prêtant à la Recherche Biomédicale), of Brest. All subjects gave written informed consent prior to participation.
Protocol
This was an open-label, randomized, two-period cross-over study. All volunteers were screened by medical history, ECG, laboratory and physical examination and urine drug analysis. In each study period, subjects were admitted to the study site 12 h before drug administration. Each treatment period was 24 h in duration from midazolam administration. A drug-free washout period of 7–15 days was observed between the two sequences. On each study period, baseline measurements (routine physical examination, weight and vital signs) were obtained upon admission. A urine drug screen was also performed. The following morning, after a 12 h fast, subjects were given a 0.1 mg kg−1 dose of midazolam (1 ml vial dosed 5 mg ml−1 available in France) by intravenous (antecubital vein) or subcutaneous (abdominal area) injection over 5 min in both cases using an electric infusion pump. After drug administration, the subjects remained fasting from food and fluids for a further 3 h. Arterial blood pressure and heart rate were monitored pre-dose, at the end of infusion, every 5 min from the end of the infusion up to 40 min, then at 50 min, 1, 1.5, 2, 2.5, 3, 4, 6 and 12 h post-infusion. Respiratory rate was monitored post-dose, at the end of the infusion, then 15, 30 and 45 min, 1, 1.5, 2, 3, 4 and 12 h post-infusion. Cardiac monitoring and oxymetry were performed continuously from pre-dose up to 4 h post-dose. Local tolerance was determined at the end of the infusion and 1 and 4 h post-infusion. Following drug administration, blood samples were collected at specific intervals for the next 12 h. The subjects were discharged from the study site the following morning after clinical examination, vital signs and ECG determination and laboratory investigations except for serology and urine drug screen. The occurrence of adverse events was monitored throughout the study period. For each subject, an intensive care practitioner was present from the start of injection up to 2 h post-injection.
Sample collection and analysis
Venous blood samples (5 ml) were collected prior to drug administration, at the end of infusion and then 5, 10, 20, 30, 40, 50 min and 1, 1.25, 1.5, 1.75, 2, 3, 4, 6, 8, 10 and 12 h after the end of infusion for the intravenous route. The same time course for sample collection was followed for the subcutaneous administration except that blood was not collected at 5 min and 1.75 h. Blood was collected into lithium, heparinized Vacutainer tubes, centrifuged immediately (1730 g, 10 min) at 4 °C and two 2 ml aliquots of plasma were placed in polypropylene tubes and stored at −20 °C until assayed.
Plasma concentrations of midazolam and of its metabolites 1′-OHM and 4-OHM were measured by a specific and selective high-performance liquid chromatography-tandem mass spectrometry method [21] with substantial modifications: a Symmetry C18, 5 µm (50 × 1 mm i. d.) column (Waters, France) was used; the mobile phase, delivered at a flow rate of 45 µl min−1 at room temperature was a gradient of acetonitrile in 2 mm ammonium formate (20% acetonitrile for 0.5 min increasing to 70% in 3 min, stable at 70% for 1 min, increasing to 90% in 0.5 min, stable at 90% for 2.5 min then decreasing to 20% in 1 min). Detection was performed using an API 2000 tandem mass spectrometer (Sciex, Concord, Canada) equipped with a Turboionspray® source. Data acquisition was made in the positive ion mode with the following settings; ionspray voltage 5800 V, nebulizer temperature 325 °C, declustering potential optimized for each analyte (midazolam + 86 V, 1′-OHM + 66 V, 4-OHM + 76 V, internal standard (I.S.) methylclonazepam + 20 V). Mass spectometry detection was carried out in the multiple reaction monitoring mode using one transition for each analyte: midazolam 326.1 → 291.2, 1′-OHM 342.1 → 324.2, 4-OHM 342.1 → 325.0, I.S. 330.1 → 284.2. Sample preparation was also modified to allow 4-OHM extraction with good recovery. Briefly, 500 µl plasma, 50 µl of a 1 mg l−1 solution of internal standard in deionized water and 200 µl of 0.5 m carbonate-hydrogencarbonate pH 9.5 buffer were successively added in glass tubes. After vortex mixing, the mixture was deposited on Extrelut NT 1 extraction cartridges (Merck, Darmstadt, Germany) and left to percolate for 15 min at room temperature. Elution was performed using 6 ml of diethyl ether/2-propanol (98: 2; v/v) mixture, the organic phase was evaporated to dryness and dissolved in 100 µl acetonitrite/pH 3 ammonium acetate (40: 60; v/v). Midazolam, 1′-OHM, 4-OHM and methylclonazepam were supplied by Roche, Basel, Switzerland.
The within-day and between-day precision and accuracy as well as linearity were evaluated over the 0.5–500 ng ml−1 concentration range. The within-day and between-day coefficients of variation were lower than 15% at all concentrations. Concentrations were interpolated from calibration curves shown to be linear from 0.5 ng ml−1−500 ng ml−1. The limit of detection defined as the concentration giving a chromatographic peak of at least three times the average background noise was 0.2 ng ml−1. The limit of quantification (LOQ) was set at 0.5 ng ml−1 for midazolam and its two metabolites.
Pharmacokinetic analysis
The maximum drug concentration in plasma (Cmax) and the time to Cmax (tmax) were obtained directly from the concentration-time data. For subjects with more than one peak, tmax was defined as the time at which the highest peak occurred. The terminal rate constant (λz) and corresponding terminal plasma half-life (t1/2) were calculated following inspection of the data. The terminal phase was assessed subjectively over the final four to six sampling points with a measured concentration equal or above the limit of quantification. The terminal rate constant was determined by linear least squares regression (WinNonlin V.I.I.) using logarithmically transformed points in the terminal phase. The area under the plasma concentration curve (AUC) for the time at which the final measurable concentration was obtained (AUC(0,t)) was calculated by the linear trapezoidal rule with t0 at the time of starting the infusion. The AUC from the final time point to time infinity (AUC(t,∞)) was estimated as the ratio of the final measurable concentration to λz. The total area under the concentration-time curve (AUC(0,∞)) was calculated by addition of AUC(0,t) to (AUC(t,∞). The terminal plasma half-life (t1/2) was calculated from (ln2)/λz. The absolute subcutaneous bioavailability (F) was calculated from:
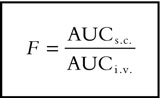 |
Clearance (CL) and the volume of distribution (V) after intravenous administration were calculated from Dose/AUC(0,∞) and CL/λz, respectively.
Number of subjects and statistical analysis
The main purpose of this study was to determine the absolute bioavailability of subcutaneous midazolam. In the absence of relevant pharmacokinetic data on midazolam given subcutaneously, the estimation of the number of subjects to be included in this study was based on data obtained after intravenous infusion. From data published in references [6] [9], and [12], the mean value (and standard deviation s.d.) of AUC for the 0.1 mg kg−1 intravenous dose of midazolam was estimated as being 340 ± 110 ng ml−1 h. Assuming a bilateral 95% confidence interval (CI) and a 15% precision (that is to say 51 ng ml−1 h) for AUC after intravenous midazolam and a similar precision for the AUC value after subcutaneous midazolam, the number of subjects (n) for the determination of a mean AUC value with 5% α risk is 18 (n = 4 × 1102 × 1.962/(2 × 51)2 [22]).
All pharmacokinetic parameters were summarized using descriptive statistics. These parameters (except tmax and t1/2) were log-transformed and subjected to analysis of variance (except tmax). A mixed model was used with factors of subject, sequence, period and way of administration. Bioequivalence was evaluated by examining whether the 90% CI of the AUC ratio was within the 20% bioequivalence interval 0.8–1.25. For analyses conducted on a logarithmic scale, point estimates and differences between routes of administration resulted in geometric means, and ratio estimates were back-transformed. tmax was analysed using the Wilcoxon rank sum test.
Results
There were no clinically significant changes in any safety assessment during the course of the study. Regarding oximetry, 13 desaturation episodes were noted in four subjects of which nine occurred after intravenous injection. In one subject, three episodes lasted more than 1 min (5, 6 and 8 min). Local tolerance to subcutaneous administration was generally good. In one subject, moderate erythema was observed, which disappeared in less than 1 h. Mild erythema and local pain were noted, more frequently (9 vs 2 subjects) after subcutaneous injection. Headache and palpitation of mild intensity were also reported, each in one subject. All these effects except for palpitation were considered to be related to midazolam.
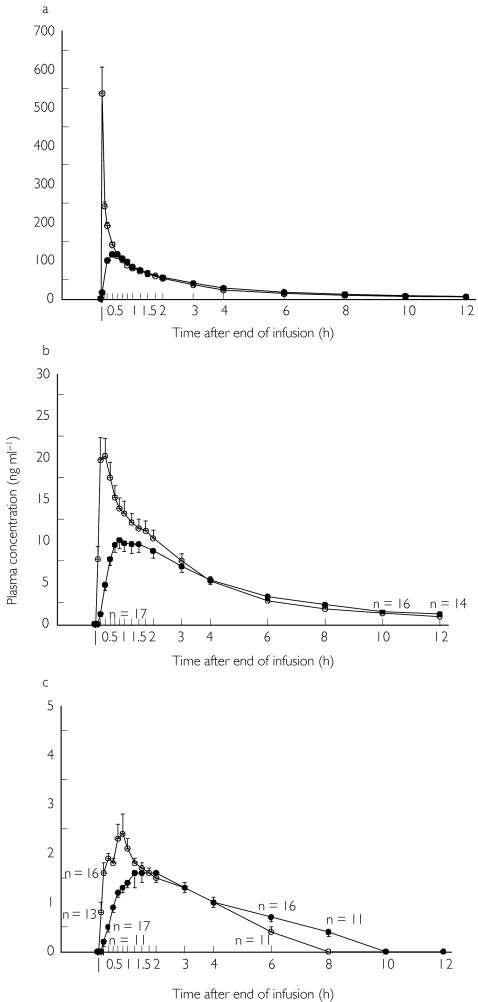
The mean (s.e. mean) plasma concentration vs time curves for midazolam and its two metabolites 1′-OHM and 4-OHM after intravenous and subcutaneous infusions are shown in Figure 1. Midazolam concentrations were above the LOQ at all sampling times for both routes of administration. For 1′-OHM, more than half of the plasma concentrations were below the LOQ at the end of the intravenous and subcutaneous infusions. Therefore, the mean value at this time was taken as zero. For 4-OHM, the mean plasma concentration was also taken as zero for the same reason at the end of the infusion and at 8, 10 and 12 h post-dose for the intravenous route, and at the end of perfusion and 10 min, 10 h and 12 h post-dose for the subcutaneous route. For the time points at which more than half but not all plasma concentrations were determinable, the mean plasma concentration value was calculated assuming zero for the samples with plasma concentrations below the LOQ. Mean (± s.d.) AUC(0,∞) values after intravenous and subcutaneous injection were 65.6 (± 27.8) and 61.2 (± 28.7) ng ml−1 h for 1′-OHM and 11.7 (± 3.5) and 12.6 (± 4.2) ng ml−1 h for 4-OHM, respectively.
Figure 1.
Plasma concentration-time curves of midazolam (1a) and its metabolites 1′-OHM (1b) and 4-OHM (1c) following intravenous (○) and subcutaneous (•) administration of a 0.1 mg kg−1 dose of midazolam (mean ± s.e.mean). When the analyte was not quantifiable in all samples, the number with concentrations higher than the LOQ (0.5 mg ml−1) is given.
Pharmacokinetic parameters for midazolam are displayed in Table 1. Midazolam was rapidly absorbed after subcutaneous infusion with a tmax value of 0.51 h (± 0.18). Cmax was 577.7 (± 261.6) ng ml−1 after intravenous injection and 127.8 (± 29.3) ng ml−1 for the subcutaneous route. The terminal plasma half-life t1/2 was 2.9 (± 0.7) h and 3.2 (± 1.0) h for the intravenous and subcutaneous infusion, respectively. Compared with the intravenous route, Cmax was statistically lower (P < 0.0001) after subcutaneous administration, whereas tmax and t1/2 were statistically higher for the latter route of administration (P < 0.0001 and P < 0.05, respectively). In contrast, no statistically significant difference was detected in AUC(0,∞) after intravenous and subcutaneous administration. The absolute bioavailability of subcutaneous midazolam was found to be 96% (min 70%, max 122%, CV 14.9%, CI 84%, 103%). Mean clearance and volume of distribution following intravenous midazolam were found to be 4.4 ± 1.0 ml min−1 kg−1 and 1.1 ± 0.2 l kg−1, respectively. Determination of the 90% CI of the geometric mean AUC(0,∞) s.c./AUC(0,∞) i.v. ratio showed bioequivalence of the subcutaneous and intravenous administration of a 0.1 mg kg−1 dose of midazolam in healthy volunteers (ratio 0.95; CI 0.83, 1.01 within the CI limits 0.80, 1.25 for a 20% bioequivalence).
Table 1.
Pharmacokinetic parameters for midazolam after intravenous and subcutaneous administration of a 0.1 mg kg−1 dose to healthy volunteers (n = 18) over 5 min. Values are geometric means (± s.d.).
| Pharmacokinetic parameters | i.v. route | s.c. route |
|---|---|---|
| Cmax (ng ml−1) | 557.7 (261.6) | 127.8 (29.3)*** |
| tmax (h) | 0.10 (0.03) | 0.51 (0.18)*** |
| t1/2 (h) | 2.9 (0.7) | 3.2 (1.0)* |
| AUC (0,t) (ng ml−1 h) | 383.8 (81.9) | 350.4 (71.8) |
| AUC (0,∞) (ng ml−1 h) | 400.1 (93.7) | 379.1 (88.2) |
| F (%) | – | 96 (14) |
| CL (ml min−1 kg−1) | 4.4 (1.0) | – |
| V (l kg−1) | 1.1 (0.2) | – |
Statistically significant difference between the two ways of administration was established using anova (Cmax, t1/2) or Wilcoxon rank sum test (tmax)
P < 0.05
P < 0.0001.
Discussion
Use of a high-performance liquid chromatography-tandem mass spectrometry method allowed the detection of midazolam plasma concentrations up to 12 h after intravenous and subcutaneous injection of a 0.1 mg kg−1 dose. A mean value for Cmax of 127.8 ng ml−1 was found after subcutaneous midazolam, which was markedly higher than that reported previously (27 ng ml−1) [23] using half this dose. The mean time to maximum plasma concentration for midazolam (tmax) after subcutaneous infusion was about 31 min, equal or similar to values reported after oral (22–55 min [6, 7]), rectal (31 min [8]), buccal (30 min [9]), intramuscular (20 min [11]) and intranasal (20–25 min [24, 25]) administration to adults. A shorter tmax value, 10 min, was found after intranasal midazolam in children [26]. The terminal half-life following intravenous and subcutaneous midazolam was similar (2.9 h i.v. vs 3.2 h s.c.) and in line with most reported values whatever the route of administration (range: 1.7–3.7 h). AUC(0,∞) after intravenous infusion (400 mg ml−1 h) was in good agreement with values reported previously [6, 8, 12] (range 270–367 ng ml−1 h). Mean values for clearance (4.4 ml min−1 kg−1) and volume of distribution (1.2 l kg−1) for intravenous midazolam in this study are in the range reported previously (CL 4.1–6.4 ml min−1 kg−1 [6–12, 14, 15, 25, 27], V 0.7–1.3 l kg−1 [6–8,12, 14, 15, 27, 28] in young healthy volunteers. In one study [16], however, CL and V after 5 mg kg−1 intravenous midazolam were outside this range with values of 8.6 ml min−1 kg−1 and 1.7 l kg−1, respectively. Taken together, the present pharmacokinetic data are in good agreement with those previously reported for midazolam.
The absolute bioavailability (F) of subcutaneous midazolam was found to be high (96%), with wide although acceptable interindividual variations. F after subcutaneous midazolam is clearly higher than after oral (range of mean value 36–52% [6, 7, 12, 16, 28], buccal (74.5% [9]), rectal (52% [8]) administration and intramuscular injection (85% [11]). Large variations in F between 50% [25] to 83% [10] have been reported after intranasal administration. Because of its high bioavailability, subcutaneous administration of midazolam is an attractive alternative to the intravenous route particularly in palliative care. Finally, comparison of the geometric mean AUC(0,∞) values after subcutaneous and intravenous administration of a 0.1-mg kg−1 dose of midazolam showed bioequivalence.
In conclusion, the pharmacokinetic data presented in this study demonstrate a high bioavailability and reproducible plasma concentrations after subcutaneous midazolam and suggest preferential use of this method of administration in patients in whom the intravenous route is not appropriate.
References
- 1.Reves JG, Fragen J, Vinik H, Greenblatt J. Midazolam: pharmacology and uses. Anesthesiol. 1985;62:310–324. [PubMed] [Google Scholar]
- 2.Drummond SH, Peterson GM, Galloway JG, Keefe PA. National survey of drug use in palliative care. Palliative Med. 1996;10:119–124. doi: 10.1177/026921639601000206. [DOI] [PubMed] [Google Scholar]
- 3.Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol. 1989;36:89–96. [PubMed] [Google Scholar]
- 4.Ghosal A, Satoh H, Thomas PE, Bush E, Moore D. Inhibition and kinetics of cytochrome P4503A activity in microsomes from rat, human and cDNA-expressed human cytochrome P450. Drug Metab Dispos. 1996;24:940–947. [PubMed] [Google Scholar]
- 5.Bauer TM, Ritz R, Haberthür C, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet. 1995;346:145–147. doi: 10.1016/s0140-6736(95)91209-6. [DOI] [PubMed] [Google Scholar]
- 6.Smith MT, Eadie MJ, O'Rourke Brophy T. The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol. 1981;19:271–278. doi: 10.1007/BF00562804. [DOI] [PubMed] [Google Scholar]
- 7.Allonen H, Ziegler G, Klotz U. Midazolam kinetics. Clin Pharmacol Ther. 1981;30:653–661. doi: 10.1038/clpt.1981.217. [DOI] [PubMed] [Google Scholar]
- 8.Clausen TG, Wolff J, Hansen PB, et al. Pharmacokinetics of midazolam and α-hydroxy-midazolam following rectal and intravenous administration. Br J Clin Pharmacol. 1988;25:457–463. doi: 10.1111/j.1365-2125.1988.tb03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwagmeier R, Alincic S, Striebel HW. Midazolam pharmacokinetics following intravenous and buccal administration. Br J Clin Pharmacol. 1998;46:203–206. doi: 10.1046/j.1365-2125.1998.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Björkman S, Rigemar G, Idvall J. Pharmacokinetics of midazolam given as an intranasal spray to adult surgical patients. Br J Anaesth. 1997;79:575–580. doi: 10.1093/bja/79.5.575. [DOI] [PubMed] [Google Scholar]
- 11.Crevoisier C, Eckert M, Heizmann P, Thurneysen DJ, Ziegler WH. Relation entre l’effet clinique et la pharmacocinétique du midazolam après administration i. v. et i m. Arzneim/Drug Res. 1981;31:2211–2215. [PubMed] [Google Scholar]
- 12.Heizmann P, Eckert M, Ziegler WH. Pharmacokinetics and bioavailability of midazolam in man. Br J Clin Pharmacol. 1983;16(Suppl 1):43S–49S. doi: 10.1111/j.1365-2125.1983.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450 – Structure, Mechanism and Biochemistry. New York: Plenum Press; 1995. pp. 473–535. [Google Scholar]
- 14.Platten H-P, Schweizer E, Dilger K, Mikus G, Klotz U. Pharmacokinetics and the pharmacodynamic action of midazolam in young and elderly patients undergoing tooth extraction. Clin Pharmacol Ther. 1998;63:552–560. doi: 10.1016/S0009-9236(98)90106-0. [DOI] [PubMed] [Google Scholar]
- 15.Geisslinger G, Dingemanse J, Schwilden H, Schüttler J. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther. 1999;65:630–639. doi: 10.1016/S0009-9236(99)90084-X. [DOI] [PubMed] [Google Scholar]
- 16.Greenblatt DJ, Abernathy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender and obesity on midazolam kinetics. Anesthesiol. 1984;61:27–35. [PubMed] [Google Scholar]
- 17.Oldenhof H, de Jong M, Steenhoek A, Janknegt R. Clinical pharmacokinetics of midazolam in intensive care patients, a wide interpatient variability? Clin Pharmacol Ther. 1988;43:263–269. doi: 10.1038/clpt.1988.31. [DOI] [PubMed] [Google Scholar]
- 18.Maitre PO, Buhrer M, Thomson D, Stanski DR. A three-step approach combining Bayesian regression and NONMEM population analysis: application to midazolam. J Pharmacokinet Biopharm. 1991;19:377–384. doi: 10.1007/BF01061662. [DOI] [PubMed] [Google Scholar]
- 19.Johanson GA. Midazolam in terminal care. Am J Hospice Palliative Care. 1993;10:13–14. doi: 10.1177/104990919301000105. [DOI] [PubMed] [Google Scholar]
- 20.Bleasel MD, Peterson GM, Dunne PF. Plasma concentrations of midazolam during continuous subcutaneous administration in palliative care. Palliative Med. 1984;8:231–236. doi: 10.1177/026921639400800307. [DOI] [PubMed] [Google Scholar]
- 21.Marquet P, Baudin O, Gaulier JM, et al. Sensitive and specific determination of midazolam and 1-hydoxymidazolam in human serum by liquid chromatography-electrospray mass spectrometry. J Chromatogr B. 1999;734:137–144. doi: 10.1016/s0378-4347(99)00340-0. [DOI] [PubMed] [Google Scholar]
- 22.Machin D, Campbell M, Fayers P, Pinol A, editors. Sample Size Tables for Clinical Studies. 2. Oxford: Blackwell Science; 1997. pp. 131–142. [Google Scholar]
- 23.Benett J, Nichols F, Rosenblum M, Condry J. Subcutaneous administration of midazolam: a comparison of the Bioject Jet Injector with the conventional syringe and needle. J Oral Maxilofac Surg. 1998;56:1249–1254. doi: 10.1016/s0278-2391(98)90601-2. [DOI] [PubMed] [Google Scholar]
- 24.Fukuta O, Braham RL, Yanase H, Kurosu K. Intranasal administration of midazolam: pharmacokinetic and pharmacodynamic properties and sedative potential. J Dent Child. 1997;64:89–98. [PubMed] [Google Scholar]
- 25.Burstein AH, Modica R, Hatton M, Forrest A, Gengo FM. Pharmacokinetics and pharmacodynamics of midazolam after intranasal administration. J Clin Pharmacol. 1997;37:711–718. doi: 10.1002/j.1552-4604.1997.tb04358.x. [DOI] [PubMed] [Google Scholar]
- 26.Walberg EJ, Willis RJ, Eckhert J. Plasma concentrations of midazolam in children following intranasal administration. Anesthesiol. 1991;74:233–235. doi: 10.1097/00000542-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Thummel KE, O'Shea D, Paine MF, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 28.Klotz U, Ziegler G. Physiologic and temporal variation in hepatic elimination of midazolam. Clin Pharmacol Ther. 1982;32:107–112. doi: 10.1038/clpt.1982.133. [DOI] [PubMed] [Google Scholar]