Abstract
Aims
We investigated the repeatability of the forearm blood flow response to intra-arterial infusion of endothelin-1 (ET-1), assessed by venous occlusion plethysmography.
Methods
In eight healthy men (aged 18–50 years), on four separate occasions, ET-1 (2.5 or 10 pmol min−1) was infused for 120 min via a 27 SWG cannula sited in the brachial artery of the nondominant arm. Each dose level was administered twice on consecutive visits. The dose order was randomized. Results are expressed as percentage change from baseline at 120 min (mean ± s.e. mean).
Results
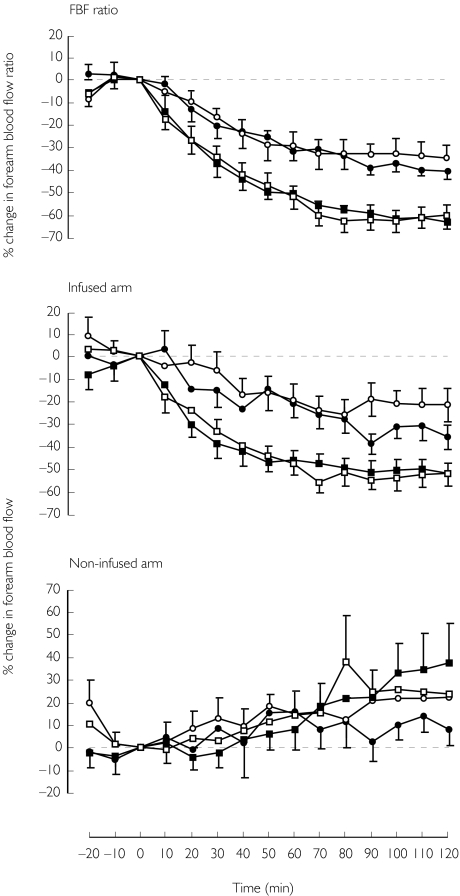
ET-1 caused significant vasoconstriction (P < 0.0001 anova) at both doses (38 ± 3%, 2.5 pmol min−1 and 62 ± 3%, 10 pmol min−1; mean visit 1 and 2). There was no difference in the response to either dose on repeated challenge. Responses appeared to be less variable when expressed as percentage change in the ratio of blood flow (infused:noninfused) in both arms than as percentage change in blood flow in the infused arm alone, as indicated by repeatability coefficients (15% vs 21%, 2.5 pmol min−1 and 11% vs 13%, 10 pmol min−1; ratio vs infused arm alone).
Conclusions
We have shown dose-dependent vasoconstriction in the forearm vascular bed to intra-arterial infusion of ET-1 and that this response is less variable when expressed as percentage change in the ratio of forearm blood flow than percentage change in the infused arm. These data should also provide useful information to determine the power of early clinical pharmacology studies investigating the activity of endothelin receptor antagonists.
Keywords: endothelin-1, forearm plethysmography, repeatability, vasoconstriction
Introduction
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide generated by the endothelium [1]. Its vasoconstrictor and vasopressor effects [1, 2] are predominantly mediated via the ET-1 selective, vascular smooth muscle cell ETA receptor [3], although nonisopeptide selective ETB receptors [4] situated on vascular smooth muscle cells [5] may contribute [6, 7]. In contrast, the ETB receptor, situated on endothelial cells, mediates vasodilatation through generation of nitric oxide [8] and prostacyclin [9].
The importance of ET-1 as an endogenous mediator of vascular tone has been confirmed by forearm [10–12] and systemic [11, 13] vasodilatation in response to local and systemic administration of endothelin receptor an-tagonists, respectively. As a consequence of its potent vasoconstrictor and growth promoting properties, ET-1 has been implicated in the pathophysiology of diseases such as hypertension, heart failure and renal failure [14, 15], leading to the rapid development of endothelin receptor antagonists as potential vasodilator treatments for cardiovascular disease [16]. Some of these compounds are currently being investigated in clinical trials [11, 17, 18].
Venous occlusion plethysmography coupled with brachial artery infusion provides a valuable method for the assessment of pharmacological and physiological vasoactive properties of locally active doses of potentially vasoactive compounds [19]. Indeed, vasoconstriction of the forearm vascular bed to local intra-arterial infusion of ET-1 has previously been demonstrated by venous occlusion plethysmography [2, 7, 10] and inhibition of this response has been used to assess the efficacy of endothelin receptor antagonists in early clinical trials [11, 20]. However, although there are published data describing the repeatability of this technique with other vasoactive agents or stimuli in forearm resistance vessels in vivo [21–24], there are currently no data specifically with ET-1.
In order to assess the repeatability of the forearm blood flow response to intra-arterial infusion of ET-1 in vivo, we compared the response to infusion of two locally active doses of ET-1, administered on separate occasions, in the forearm resistance vessels of healthy men. Each dose level was administered twice, on consecutive visits, and the dose order was randomized. We also compared methods of data presentation of the forearm blood flow data to assess which method is more reliable in this setting.
Methods
Subjects
Eight healthy men (aged between 18 and 50 years), were recruited to the study, which was conducted with the approval of the local research ethics committee and with the written informed consent of each subject. All subjects abstained from vasoactive medication in the 2 weeks before each study and from alcohol, caffeine-containing drinks, and tobacco from at least 12 h before each study. Each subject fasted for at least 3 h before any measurements were taken.
Drugs
Endothelin-1 (Calbiochem, Nottingham, UK) was administered by continuous infusion, via the brachial artery, for 120 min at a rate of 2.5 or 10 pmol min−1 according to the study randomization. The infusion rate was kept constant at 1 ml min−1 for both dose levels. We have previously assessed the effects of ET-1 at a locally active dose of 5 pmol min−1 for 60–90 min [10, 11, 20]. The doses selected for the current study allowed assessment of the repeatability of the response to ET-1 at a lower (2.5 pmol min−1) and a higher (10 pmol min−1) dose than previously used, to provide further information on the threshold of effect, the dose–response and tolerability.
All dilutions were prepared in 0.9% saline (Baxter Healthcare Ltd, Thetford, UK) from sterile stock solutions on the day of the study.
Intra-arterial infusion
The brachial artery of the nondominant arm was cannulated under local anaesthetic (1% lignocaine; Astra Pharmaceuticals, Kings Langley, England) with a 27 SWG steel cannula (Cooper's Needle Works, Birmingham, UK) attached to a 16G epidural catheter (Portex Ltd, Hythe, Kent, UK). The cannulae are provided nonsterile and were sterilized by the hospital CSSD department (Western General Hospital, UK).
Infusions were administered from 50 ml plastic syringes (Becton-Dickinson, UK) connected directly to the epidural catheter. The infusion rate was kept constant at 1 ml min−1 throughout.
Measurements
Forearm blood flow
The response to intra-arterial infusion was assessed by measurement of forearm blood flow in both the infused and noninfused forearms by venous occlusion plethysmography using mercury-in-silastic strain gauges securely applied around the widest part of the forearm [19]. The hands were excluded from the circulation during measurements through inflation of wrist cuffs to 220 mmHg. Upper arm cuffs were intermittently inflated to 40 mmHg for the first 10 s in every 15 s to prevent temporarily venous outflow from the forearm and thus obtain plethysmographic recordings. Recordings of forearm blood flow were made over 3 min periods at 10 min intervals.
Venous occlusion plethysmography was performed using a dual channel strain gauge plethysmograph (Hokanson, USA) and calibration was achieved using the internal standard of the Hokanson plethysmography unit. The voltage output was transferred from the plethysmograph to a Macintosh personal computer (Classic II, Apple Computer Inc, Cupertino, CA) using a MacLab analogue-to-digital converter and Chart software (v. 3.2.8; both from AD Instruments, Castle Hill, NSW, Australia).
Blood pressure and heart rate
Blood pressure and heart rate were measured in the noninfused arm using a well-validated semiautomated noninvasive method [25]. Blood pressure was measured immediately after forearm blood flow to avoid any effect on these measurements of the venous congestion caused by this procedure.
Study design
In a single-blind, randomized, two way crossover study, the local effects of two dose levels of ET-1 were investigated in eight healthy men. On four separate occasions, each separated by at least 1 week, subjects received an intra-arterial infusion of ET-1 (2.5 pmol min−1 or 10 pmol min−1) for 120 min. Each dose level was administered twice, on consecutive visits and the dose order randomized. Subjects were blinded to the dose administered.
Forearm blood flow studies
Subjects rested recumbent throughout each study in a quiet temperature-controlled room (23–25 °C). Strain gauges and arm cuffs were applied and a cannula was sited in the brachial artery of the nondominant arm. Saline (0.9%) was infused for at least 30 min, during which three measurements of forearm blood flow were made. ET-1 was then infused for 120 min. Forearm blood flow, and blood pressure and heart rate recordings, were made at 10 min intervals throughout.
Statistical analysis
Plethysmographic data listings were extracted from data files and forearm blood flows calculated for individual venous occlusion cuff inflations using a template spreadsheet (Excel 5.0; Microsoft Ltd, Wokingham, UK). Recordings made in the first 60 s after wrist cuff inflation were not used for analysis because of the transient instability in blood flow that this causes [26]. Blood flow in both forearms was obtained from the mean of the last five consecutive recordings of each measurement period. Baseline blood flow was taken as the last measurement during the saline infusion, before the start of the ET-1 infusion. Forearm blood flow results are expressed as the percentage change from baseline in the ratio of blood flow between the infused and noninfused arms [19]. We also expressed the results as percentage change in forearm blood flow in the infused arm alone, to compare this with our standard method of data presentation. The area under the curve (AUC) was calculated for both methods to present a summary statistic for the overall response.
The repeatability of each method was assessed by the method of Bland & Altman [27] using Student's t distribution. In brief, the mean response and the mean difference between the responses on each visit are compared and the repeatability coefficient calculated according to the recommendations of the British Standards Institution [27]. Power calculations were performed using the standard deviation and the mean response, as a percentage change, for visit 1 for each dose, to estimate the sample sizes required to detect a shift in the response at 60, 90 and 120 min; and the AUC for (0, 60 min), (30, 60 min), (0, 90 min), (60, 90 min), (0, 120 min) and (90, 120 min); for each dose for 80 or 90% power to detect a predetermined difference (of 10–100%) with significance accepted at the 5% level.
All results are expressed as mean ± standard error of the mean (s.e. mean). Blood pressure, heart rate and baseline measurements were compared using the Student's paired t-test. Forearm blood flow data were examined by repeated-measures analysis of variance (anova) (Excel 5.0, Microsoft Ltd, Wokingham, UK). Statistical significance was accepted at the 5% level.
Results
All eight subjects successfully completed the study (age range: 18–50 years, mean 33 ± 3 years). There were no significant differences between baseline measurements on each of the study visits. There was no significant change in blood pressure or heart rate at the end of each infusion (Table 1). There was a trend for the blood flow in the noninfused arm to increase with time (Figure 1). Although this trend reached significance over 120 min (P = 0.02) for one of the visits (10 pmol min−1, visit 1) when the data were expressed as percentage change in blood flow, there was no significant change in forearm blood flow (absolute values) in the noninfused arm at the end of each infusion (Table 1).
Table 1.
Mean arterial pressure (MAP), heart rate (HR) and forearm blood flow (FBF) at baseline and 120 min after the start of each infusion. Values are mean ± s.e.mean.
| Endothelin-1 infusion | ||||
|---|---|---|---|---|
| 2.5 pmol min−1 | 10 pmol min−1 | |||
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |
| MAP (mmHg) | ||||
| Basal | 94 ± 2 | 92 ± 3 | 91 ± 3 | 90 ± 2 |
| 120 min | 94 ± 3 | 98 ± 4 | 93 ± 4 | 94 ± 3 |
| HR (beats min−1) | ||||
| Basal | 62 ± 4 | 64 ± 5 | 61 ± 5 | 63 ± 4 |
| 120 min | 58 ± 4 | 64 ± 7 | 62 ± 4 | 63 ± 4 |
| FBF (ml 100 ml−1 min−1) | ||||
| Control arm | ||||
| Basal | 3.4 ± 0.3 | 3.3 ± 0.4 | 3.1 ± 0.3 | 3.5 ± 0.3 |
| 120 min | 3.7 ± 0.5 | 3.9 ± 0.3 | 4.1 ± 0.3 | 4.4 ± 0.5 |
| Infused arm | ||||
| Basal | 4.0 ± 0.4 | 3.9 ± 0.7 | 3.6 ± 0.4 | 3.8 ± 0.5 |
| 120 min | 2.5 ± 0.3 | 3.2 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.2 |
Figure 1.
Response of forearm blood flow to local intra-arterial infusion of ET-1; mean percentage change in forearm blood flow ratio (infused arm:noninfused arm), forearm blood flow (infused arm) and forearm blood flow (noninfused arm), ± s.e. mean for all; (2.5 pmol min−1; visit 1, s; visit 2, d and 10 pmol min−1; visit 1, □; visit 2, ▪).
Although there were no reported adverse events with either dose of ET-1, some skin blanching was noted in some volunteers with the higher dose of ET-1 (10 pmol min−1). These effects were not formally evaluated and did not cause symptoms at the end of the study or thereafter.
Forearm blood flow
(i) Forearm blood flow ratio (FBFR)
Forearm vasoconstriction was indicated by a reduction in the ratio of forearm blood flow in response to both doses of ET-1 at each visit (P < 0.0001, all visits), the response to ET-1 (10 pmol min−1) was significantly greater than that to ET-1 (2.5 pmol min−1) (P < 0.0001) (Figure 1). The response to ET-1 was slow in onset and appeared to plateau at around 60 min. There was no significant difference between the responses on visit 1 and visit 2 for either dose. The repeatability coefficient for 10 pmol min−1 was lower than that for 2.5 pmol min−1 (Table 2), indicating that the response to 10 pmol min−1 was the more repeatable.
Table 2.
Data for percentage change in forearm blood flow (FBF) ratio, blood flow in the infused arm for 30, 60, 90 and 120 min following the start of each infusion with AUC, repeatability coefficients (Rep coeff) and 95% confidence intervals (CI).
| FBF ratio (infused:noninfused arm) | FBF infused arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ET-1 (pmol min−1) | Time (min) | Mean % change (visit 1 and visit 2) | Mean difference (visit 1 – visit 2) | Rep coeff (%) | 95% CI range (% change) | Mean % change (visit 1 and visit 2) | Mean difference (visit 1 – visit 2) | Rep coeff (%) | 95% CI range (% change) |
| 2.5 | 30 | −19 | −4 | 15 | −25, −13 | −11 | −9 | 20 | −23, +1 |
| 60 | −30 | −2 | 14 | −36, −24 | −20 | −2 | 19 | −28, −12 | |
| 90 | −36 | −6 | 14 | −40, −32 | −28 | −20 | 19 | −35, −21 | |
| 120 | −38 | −6 | 15 | −44, −32 | −29 | −14 | 21 | −37, −21 | |
| AUC | −3064 | −201 | 1411 | −3500, −2628 | −2222 | −697 | 1774 | −2914, −1530 | |
| 10 | 30 | −36 | −3 | 16 | −43, −29 | −36 | −6 | 15 | −45, −27 |
| 60 | −51 | 2 | 10 | −57, −45 | −47 | 1 | 12 | −54, −40 | |
| 90 | −61 | 3 | 8 | −68, −54 | −53 | 4 | 12 | −59, −47 | |
| 120 | −62 | −3 | 11 | −68, −56 | −52 | 1 | 13 | −59, −45 | |
| AUC | −5534 | 96 | 1003 | −6008, −5060 | −4957 | 83 | 1259 | −5488, −4426 | |
The AUC was calculated for each dose and each visit (P < 0.0001, all visits) (Table 2). There was no significant difference between the responses on visit 1 and visit 2 for either dose. Again, the repeatability coefficient for 10 pmol min−1 was lower than for 2.5 pmol min−1 (Table 2).
(ii) Infused arm only (FBFI)
There was a significant reduction in blood flow in the infused arm in response to both doses of ET-1 at each visit (P < 0.0001, all visits) (Figure 1). Although there was no significant difference between the response to ET-1 (10 pmol min−1) on visit 1 and visit 2 (P = 0.7), the difference between the response to ET-1 (2.5 pmol min−1) on visit 1 and visit 2 was significant (P = 0.03). The repeatability coefficient for 10 pmol min−1 was lower than that for 2.5 pmol min−1 (Table 2).
The AUC was calculated for each dose and each visit (P < 0.0001, all visits) (Figure 1). There was no significant difference between the responses on visit 1 and visit 2 for either dose. Again, the repeatability coefficient for 10 pmol min−1 was lower than that for 2.5 pmol min−1 (Table 2).
(iii) Non-infused arm only (FBFNI)
Although there was no significant change in forearm blood flow (absolute values) at the end of the infusion (Table 1), when expressed as a percentage change from baseline, the change on visit 1 for the 10 pmol min−1 dose was significant over 120 min (P = 0.02; anova, one way). This change was not significant up to and including the 90 min timepoint for this visit (P > 0.1; anova, one way), or for the other visits over 120 min.
There was no significant difference between the percentage change in the noninfused arm on visit 1 and visit 2 for the 10 pmol min−1 dose. However the difference between the percentage change on visit 1 and visit 2 for the 2.5 pmol min−1 dose reached statistical significance (P = 0.047).
(iv) Power calculations
The sample sizes estimated from the power calculations were smaller when data were represented as a percentage change in the ratio of forearm blood flow rather than as percentage change in the infused arm alone (Tables 3 and 4).
Table 3.
Power calculations estimating the sample sizes required to detect a 10, 25, 33, 50, 75 and 100% shift in the response, as percentage change (i) at 120 min, (ii) AUC(0, 120 min) and (iii) AUC(90, 120 min); for each dose with a power of 90% and 80% and significance accepted at 5%.
| (i) | |||||
|---|---|---|---|---|---|
| FBF ratio | FBF infused arm | ||||
| ET-1 (pmol min−1) | % shift (120 min) | 90% power | 80% power | 90% power | 80% power |
| 2.5 | 10 | 132 | 99 | 389 | 293 |
| 25 | 22 | 16 | 63 | 47 | |
| 33 | 13 | 10 | 36 | 27 | |
| 50 | 6 | ≥ 5 | 16 | 12 | |
| 75 | ≥ 5 | ≥ 5 | 7 | 6 | |
| 100 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
| 10 | 10 | 38 | 29 | 122 | 92 |
| 25 | 6 | ≥ 5 | 20 | 15 | |
| 33 | ≥ 5 | ≥ 5 | 12 | 9 | |
| 50 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
| 75 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
| 100 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
| (ii) | |||||
|---|---|---|---|---|---|
| FBF ratio | FBF infused arm | ||||
| ET-1 (pmol min−1) | % shiftAUC (0, 120 min) | 90% power | 80% power | 90% power | 80% power |
| 2.5 | 10 | 180 | 136 | 1093 | 823 |
| 25 | 29 | 22 | 175 | 132 | |
| 33 | 17 | 13 | 101 | 76 | |
| 50 | 8 | 6 | 44 | 33 | |
| 75 | ≥ 5 | ≥ 5 | 20 | 15 | |
| 100 | ≥ 5 | ≥ 5 | 11 | 9 | |
| 10 | 10 | 33 | 25 | 144 | 108 |
| 25 | 6 | ≥ 5 | 23 | 18 | |
| 33 | ≥ 5 | ≥ 5 | 14 | 10 | |
| 50 | ≥ 5 | ≥ 5 | 6 | ≥ 5 | |
| 75 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
| 100 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
| (iii) | |||||
|---|---|---|---|---|---|
| FBF ratio | FBF infused arm | ||||
| ET-1 (pmol min−1) | % shiftAUC (90, 120 min) | 90% power | 80% power | 90% power | 80% power |
| 2.5 | 10 | 83 | 63 | 368 | 277 |
| 25 | 13 | 10 | 59 | 45 | |
| 33 | 8 | 6 | 34 | 26 | |
| 50 | ≥ 5 | ≥ 5 | 15 | 12 | |
| 75 | ≥ 5 | ≥ 5 | 7 | ≥ 5 | |
| 100 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
| 10 | 10 | 50 | 38 | 130 | 98 |
| 25 | 8 | 6 | 21 | 16 | |
| 33 | ≥ 5 | ≥ 5 | 12 | 9 | |
| 50 | ≥ 5 | ≥ 5 | 6 | ≥ 5 | |
| 75 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
| 100 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | |
Table 4.
Power calculations estimating the sample sizes required to detect a 25 and 50% shift in the response, as percentage change at 60 min, 90 min and 120 min and percentage change in AUC(0, 60 min), (30, 60 min), (0, 90 min), (60, 90 min), (0, 120 min) and (90, 120 min), for each dose with a power of 90% and significance accepted at 5%.
| ET-1(pmol min−1) | FBF method | % shift | AUC (0, 60 min) | AUC (30, 60 min) | 60 min | AUC (0, 90 min) | AUC (60, 90 min) | 90 min | AUC (0, 120 min) | AUC (90, 120 min) | 120 min |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | Ratio | 25 | 116 | 59 | 30 | 52 | 22 | 20 | 29 | 13 | 22 |
| 50 | 29 | 15 | 8 | 13 | 6 | ≥ 5 | 8 | ≥ 5 | 6 | ||
| Infused arm | 25 | 726 | 369 | 165 | 296 | 92 | 165 | 175 | 59 | 6 | |
| 50 | 182 | 93 | 42 | 74 | 23 | 42 | 44 | 15 | ≥ 5 | ||
| 10 | Ratio | 25 | 42 | 15 | ≥ 5 | 13 | 8 | 12 | 6 | 8 | 6 |
| 50 | 11 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 5 | ||
| Infused arm | 25 | 59 | 31 | 27 | 34 | 22 | 27 | 23 | 21 | 15 | |
| 50 | 15 | 8 | 7 | 9 | 6 | 7 | 6 | 6 | ≥ 5 |
Discussion
We have demonstrated dose-dependent vasoconstriction in the forearm vascular bed of healthy men in response to infusion of locally active doses of ET-1 (2.5 and 10 pmol min−1), consistent in evolution and magnitude with previous responses to ET-1 (1 and 5 pmol min−1) [2, 7, 11]. We investigated the response to each dose of ET-1 on two separate occasions and found responses to each dose to be similar on both visits.
Forearm blood flow can be measured in both arms and it has been suggested that data from studies with locally active doses of drugs are best represented as percentage change in the ratio of forearm blood flow in the infused and noninfused arm [19, 22, 28, 29]. However, some investigators prefer to report the effects in the infused arm without including blood flow data from the noninfused arm [24, 30]. It is likely that, where the responses are relatively small, and particularly in the case of constrictors, the response is more accurately expressed as a percentage change in the ratio of forearm blood flow. On the other hand, when responses are potentially of a greater magnitude, particularily with vasodilator effects [24], it may be more useful to express the response as a percentage change in the infused arm and use the effects in the noninfused arm only to confirm that there are no systemic drug effects.
We examined the response to ET-1 when expressed as percentage change in the ratio of forearm blood flow and as percentage change in the infused arm alone, to identify which is better suited for future studies investigating this response. Although both methods demonstrated significant reduction in blood flow for all visits (Figure 1), the repeatability coefficients were consistently lower for the blood flow ratio than for blood flow in the infused arm (Table 2) indicating that the data are less variable when presented as percentage change in forearm blood flow ratio. This translates into a need for a lower sample size to achieve the same power, using blood flow ratios rather than changes in the infused arm alone (Table 3), suggesting that, at least in the case of responses to ET-1, presentation of the response as a ratio is more robust.
Summarizing the forearm blood flow responses as areas under the curve (AUCs) allows presentation of responses as single values and may reduce the effect of single unexpected variations in blood flow on an overall assessment of the response. Again, the AUC data for the blood flow ratio appeared more robust than the AUC for the infused arm only with the response more repeatable and with consistently smaller sample sizes needed for future studies investigating this response. Interestingly, the sample sizes were smaller for AUC for the last 30 min of the response than for the AUC for the entire infusion period, for both the ratio and infused arm data (Table 4), probably because the response is more completely developed by this time.
In the current study there was a trend for the blood flow to increase in the noninfused arm, with significant change detected over 120 min on one of the study visits (10 pmol min−1, visit 1). It is important to note that, although changes were observed in the noninfused arm, these changes were not significant up to 90 min. Therefore, limiting the duration of the intra-arterial infusion to 90 min or less might avoid this problem. This increase in blood flow may simply be a time-dependent effect. However, as these effects were more pronounced with the higher dose level of ET-1 (10 pmol min−1), it is possible that the changes described in the noninfused arm are a breakthrough of systemic effects. Previous investigations of forearm blood flow responses to ET-1 have demonstrated vasodilatation in response to low dose (∼0.05–0.2 pmol min−1) infusion of ET-1 [31, 32]. Therefore the small increase in blood flow observed in the noninfused arm during infusion of ET-1 (10 pmol min−1) in the current study may have resulted from a small increase in ET-1 concentrations in the noninfused arm. Alternatively, this increase in forearm blood flow could reflect splanchnic vasoconstriction with the higher dose of ET-1. Although no changes were seen in blood pressure in the noninfused arm during infusion of ET-1, the increase in blood flow in the noninfused arm could result from an increase in blood flow to the forearm vascular bed in response to a degree of systemic vasoconstriction in more important vascular beds. Indeed, similar effects have been described in studies with systemic infusion of angiotensin II, where forearm vasodilatation was demonstrated in response to high dose infusion of angiotensin II [33].
We have shown that the forearm blood flow response to intra-arterial infusion of ET-1, at two dose levels, is repeatable in between-day comparisons in healthy volunteers. These data support the use of the forearm blood flow response to ET-1 as a model to assess the effects of antagonists and inhibitors of the endothelin system in early clinical trials [10, 11, 20]. From our experience this model is most powerful when the response to intra-arterial infusion is assessed in a within-subject, placebo-controlled design, allowing the shift in individual dose–responses to be assessed with each study subject acting as his own control. Concerns over the binding of ET-1 to infusion lines and syringes and subsequent loss of activity have been raised, prompting dilution of ET-1 in colloid solutions by some investigators [32]. However, our standard method [7, 10, 11, 20] is to dilute ET-1 in saline and the current data have shown responses to be sustained and reproducible. We have also tested ET-1 activity using standard saline dilution and infusion techniques and found ∼80% recovery of the peptide in the final infusate after 75 min (unpublished data using our standard infusion devices: 79 ± 6%, n = 3). Therefore concerns over the stability of ET-1 diluted in saline in the current context appear to be unfounded.
The method described is generally well tolerated and repeat studies can be scheduled 5–7 days apart, allowing the effects of study drug to be investigated using a crossover design. This model is also useful in assessing any differences in responses to ET-1 between patients and healthy matched controls [34–36].
The characteristically slow onset of and sustained vasoconstriction to ET-1 [2, 7] precludes the construction of a full dose–response curve on a single visit. There is also the possibility of an accumulation of effect with subsequent increases in dose level. Indeed, early studies with ET-1 demonstrated significant adverse events including vomiting, sensation of heat and deep muscular pain following intra-arterial infusion of ET-1 in stepwise increases in dose from 5 × 10−11 to 5 × 10−8 mol min−1 with effects still evident 10 h following infusion [31]. In the current study, we observed skin blanching in the infused arm following infusion of ET-1, but only at the higher dose level. These effects have not been noted in our studies with ET-1 (5 pmol min−1) [10, 11, 20, 34, 35]. In addition, a small but significant increase in forearm blood flow was noted in the noninfused arm following infusion of ET-1 (10 pmol min−1), which could indicate threshold systemic effects at this dose level. Given these observations with ET-1 (10 pmol min−1), and our previous results with ET-1 (5 pmol min−1), we would suggest that the forearm blood flow response to intra-arterial infusion of ET-1 at 5 pmol min−1 for 90 min provides the most useful model for the assessment of effects of receptor antagonists or the responsiveness to ET-1 in patients. Based on power calculations from the current data, a sample size of eight should be sufficient to detect a 50% shift in AUC (60–90 min) for ET-1 (10 pmol min−1) and a 33% shift in AUC (60–90 min) for ET-1 (2.5 pmol min−1), with 90% power, when responses are expressed as a percentage change in the forearm blood flow ratio. Therefore, we would expect that a sample size of eight should be sufficient to detect a 33–50% shift in AUC (60, 90 min) for 5 pmol min−1, with 90% power, when responses are expressed as a percentage change in the forearm blood flow ratio.
It is important to acknowledge that our observations are confined to healthy men in the age range 18–50 years. Given the potentially teratogenic nature of endothelin antagonists [37, 38], it is unlikely and probably inadvisable that proof of concept studies would include women of childbearing potential. Although further assessment of the repeatability of the forearm blood flow response to intra-arterial infusion of ET-1 in elderly or postmenopausal subjects may be required, the results from the current study could serve as a guide for initial investigations.
In summary, the assessment of the forearm blood flow response to intra-arterial infusion of ET-1 provides a well tolerated and reliable model for the pharmacodynamic assessment of endothelin receptor antagonists at an early stage in drug development in a relatively small number of patients or controls [11, 20, 34–36] enabling identification of a pharmacologically effective dose range for use in early patient trials. The data from the current study should provide a valuable basis for protocol design in early clinical pharmacology studies investigating the activity of new endothelin receptor antagonists.
Acknowledgments
Fiona Strachan was supported by a Wellcome Trust project grant (PG 048560). Dr David Newby was a British Heart Foundation Junior Research Fellow (FS/95009). Professor David Webb was the recipient of a Research Leave Fellowship from the Wellcome Trust (WT 0526330).
References
- 1.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Clarke JG, Benjamin N, Larkin SW, Webb DJ, Davies GJ, Maseri A. Endothelin is a potent long-lasting vasoconstrictor in men. Am J Physiol. 1989;257:H2033–H2035. doi: 10.1152/ajpheart.1989.257.6.H2033. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai T, Yanagisawa M, Takuwa Y, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 5.Davenport AP, O'Reilly G, Molenaar P, et al. Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: evidence for expression of ETB receptors in human vascular smooth muscle. J Cardiovasc Pharmacol. 1993;22:S22–S25. doi: 10.1097/00005344-199322008-00008. [DOI] [PubMed] [Google Scholar]
- 6.Clozel M, Gray GA, Breu W, Loffler B, Osterwalder R. The endothelin ETB receptor mediates both vasodilatation and vasoconstriction in vivo. Biochem Biophys Res Commun. 1992;186:867–873. doi: 10.1016/0006-291x(92)90826-7. [DOI] [PubMed] [Google Scholar]
- 7.Haynes WG, Strachan FE, Webb DJ. Endothelin ETA and ETB receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation. 1995;92:357–363. doi: 10.1161/01.cir.92.3.357. [DOI] [PubMed] [Google Scholar]
- 8.Tsukahara H, Ende H, Magazine HI, Bahou WF, Goligorsky MS. Molecular and functional characterization of the non-isopeptide-selective ETB receptor in endothelial cells: receptor coupling to nitric oxide synthase. J Biol Chem. 1994;269:21778–21785. [PubMed] [Google Scholar]
- 9.DeNucci G, Thomas R, D’Orleans-Juste P, et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- 11.Haynes WG, Ferro CF, O'Kane KPJ, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93:1860–1870. doi: 10.1161/01.cir.93.10.1860. [DOI] [PubMed] [Google Scholar]
- 12.Verhaar MC, Strachan FE, Newby DE, et al. Endothelin-A receptor antagonist mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–756. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 13.Spratt JCS, Goddard J, Labinjoh C, et al. The haemodynamic effects of systemic endothelin A receptor antagonism in healthy humans in vivo. Br J Clin Pharmacol. 1999;47:576P. [Google Scholar]
- 14.Battistini B, Chailler P, D’Orleans Juste P, Briere N, Sirois P. Growth regulatory properties of endothelins. Peptides. 1993;14:385–399. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- 15.Haynes WG, Webb DJ. The endothelin family of peptides: local hormones with diverse roles in health and disease? Clin Sci. 1993;84:485–500. doi: 10.1042/cs0840485. [DOI] [PubMed] [Google Scholar]
- 16.Strachan FE, Webb DJ. The endothelin system: a novel therapeutic target in cardiovascular disease. Emerging Drugs. 1998;3:95–112. [Google Scholar]
- 17.Freed MI, Wilson DE, Thompson KA, Harris RZ, Ilson BE, Jorkasky DK. Pharmacokinetics and pharmacodynamics of SB 209670, an endothelin receptor antagonist: effects on the regulation of renal vascular tone. Clin Pharmacol Ther. 1999;65:473–482. doi: 10.1016/S0009-9236(99)70066-4. [DOI] [PubMed] [Google Scholar]
- 18.Weber C, Schmitt R, Birnboeck H, et al. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996;60:124–137. doi: 10.1016/S0009-9236(96)90127-7. [DOI] [PubMed] [Google Scholar]
- 19.Webb DJ. The pharmacology of human blood vessels in vivo. J Vasc Res. 1995;32:2–15. doi: 10.1159/000159072. [DOI] [PubMed] [Google Scholar]
- 20.Ferro CJ, Haynes WG, Johnston NR, Lomax CC, Newby DE, Webb DJ. The peptide endothelin receptor antagonist, TAK-044, produces sustained inhibition of endothelin-1 mediated arteriolar vasoconstriction. Br J Clin Pharmacol. 1997;44:377–383. doi: 10.1046/j.1365-2125.1997.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newby DE, Sciberras DG, Mendel CM, Gertz BJ, Boon NJ, Webb DJ. Intra-arterial substance P mediated vasodilatation in the human forearm: pharmacology, reproducibility and tolerability. Br J Clin Pharmacol. 1997;43:493–499. doi: 10.1046/j.1365-2125.1997.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrie JR, Ueda S, Morris AD, Murray LS, Elliott HL, Connell JMC. How reproducible is bilateral forearm plethysmography? Br J Clin Pharmacol. 1998;45:131–139. doi: 10.1046/j.1365-2125.1998.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts DH, Tsao Y, Breckenridge AM. The reproducibility of limb blood flow measurements in human volunteers at rest and after exercise by using mercury-in-silastic strain gauge plethysmography under standardized conditions. Clin Sci. 1986;70:635–638. doi: 10.1042/cs0700635. [DOI] [PubMed] [Google Scholar]
- 24.Walker H, Jackson G, Ritter JM, Chowienczyk PJ. Reproducibility of bilateral forearm plethysmography to endothelial-dependent vasodilators. Br J Clin Pharmacol. 1999 doi: 10.1046/j.1365-2125.2001.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiinberg N, Walter-Larson S, Eriksen C, Nielsen PE. An evaluation of semi-automatic blood pressure manometers against intra-arterial blood pressure. J Ambulatory Monitoring. 1988;1:303–309. [Google Scholar]
- 26.Kerslake DM. The effect of the application of an arterial occlusion cuff to the wrist on the blood flow in the human forearm. J Physiol. 1949;108:451–457. doi: 10.1113/jphysiol.1949.sp004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two measurements of clinical measurement. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- 28.Chin-Dusting JPF, Cameron JD, Dart AM, Jennings GLR. Human forearm venous occlusion plethysmography: methodology, presentation and analysis. Clin Sci. 1999;96:439–440. [PubMed] [Google Scholar]
- 29.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 30.Panza JA, Quyyumi AA, Brush JEJ, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 31.Dahlof B, Gustafsson D, Hedner T, Jern S, Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reactions. J Hypertens. 1990;8:811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kiowski W, Luscher TF, Linder L, Buhler FR. Endothelin-1 induced vasoconstriction in humans: reversal by calcium-channel blockade but not by nitrovasodilators or endothelium-derived relaxing factor. Circulation. 1991;83:469–475. doi: 10.1161/01.cir.83.2.469. [DOI] [PubMed] [Google Scholar]
- 33.Motwani JG, Struthers AD. Dose-response study of the redistribution of intravascular volume by angiotensin II in man. Clin Sci. 1992;82:397–405. doi: 10.1042/cs0820397. [DOI] [PubMed] [Google Scholar]
- 34.Love MP, Haynes WG, Gray GA, Webb DJ, McMurray JJ. Vasodilator effects of endothelin-converting enzyme inhibition and endothelin ETA receptor blockade in chronic heart failure patients treated with ACE inhibitors. Circulation. 1996;94:2131–2137. doi: 10.1161/01.cir.94.9.2131. [DOI] [PubMed] [Google Scholar]
- 35.Hand MF, Haynes WG, Webb DJ. Reduced endogenous endothelin-1-mediated vascular tone in chronic renal failure. Kidney Int. 1999;55:613–620. doi: 10.1046/j.1523-1755.1999.00291.x. [DOI] [PubMed] [Google Scholar]
- 36.Newby DE, Flint LL, Fox KAA, Boon NA, Webb DJ. Reduced responsiveness to endothelin-1 in peripheral resistance vessels of patients with syndrome X. J Am Coll Cardiol. 1998;31:1585–1590. doi: 10.1016/s0735-1097(98)00143-0. [DOI] [PubMed] [Google Scholar]
- 37.Hosada K, Hammer RE, Richardson JA, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce mega colon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 38.Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]