Abstract
Aims
HMG-CoA reductase inhibitors (statins) have been demonstrated to have in vitro vascular effects. The aim of this study was to determine whether statins actually have in vivo vascular effects independent of their cholesterol-lowering effect.
Methods
We investigated the effect of a single dose of cerivastatin on vascular endothelial function by measuring flow-mediated dilatation of the brachial artery on ultrasound in 30 healthy volunteers with normal serum cholesterol concentrations. They were randomized to either placebo group (n = 15) or cerivastatin group (n = 15), and flow-mediated dilatation and endothelium-dependent dilatation were evaluated at before and 1 h, 3 h, 6 h, and 12 h after administration of placebo or cerivastatin.
Results
There were no differences in total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, malondialdehyde-LDL, and high-sensitivity C-reactive protein before and after administration of placebo or cerivastatin. Cerivastatin significantly increased flow-mediated dilatation at 3 h (P < 0.001), and this increase rapidly returned to the baseline level 6 h after administration. Endothelium-independent dilatation of brachial artery was not altered.
Conclusions
A single dose of cerivastatin increased vascular endothelial responsiveness. Our data suggest that cerivastatin has a direct effect on the blood vessels that is independent of its lipid-lowering effect, and thus can be considered as a vascular statin.
Keywords: endothelial function, lipid-lowering therapy, pleiotropic effect, statin, ultrasound
Introduction
HMG-CoA reductase inhibitors (statins) decrease the conversion of HMG-CoA into mevalonate, a precursor molecule of endogenous cholesterol. Lipid-lowering therapy using statins has been shown to assist in the prevention of cardiovascular events by many clinical trials [1-3]. Recent studies have demonstrated that statins not only have a lipid-lowering effect, but also have direct vascular effects and can modulate the expression of various molecules in the vascular wall [4, 5]. Several authors have reported that cerivastatin alters the in vitro expression of matrix metalloproteinases [6, 7], tissue factor [6, 8], and nitric oxide synthase [9, 10] by vascular cells. Such effects of statins might contribute to the clinical benefit of these drugs in addition to their lipid-lowering effect [4, 5].
However, it is still unclear whether statins actually have direct vascular effects in vivo. The present study focuses on the direct in vivo vascular effect of statins that were independent of a lipid-lowering effect in humans.
Methods
Subjects
Thirty healthy male volunteers aged 26–38 years (mean: 30.0 years) were enrolled. They were randomized to either the placebo group (n = 15) or the cerivastatin group (n = 15). Randomization was double-blind and the placebo preparation was identical with cerivastatin. All subjects had normal cholesterol concentrations, no other cardiovascular risk factors, and no cardiovascular disease.
Ultrasound studies
Endothelial function was evaluated by measuring the flow-mediated dilatation (FMD) of the brachial artery [10]. It has been shown previously that FMD is a nitric oxide (NO)-dependent response and that its measurement is an accurate and reproducible method for evaluating arterial endothelial function [11].
The brachial artery diameter was measured on B-mode ultrasound images using a 7.0 MHz linear-array transducer and a standard Acuson 128XP/10 system (Mountain View, CA). The brachial artery was scanned longitudinally and the centre of the artery was identified at the point where the clearest view of the anterior and posterior intimal layers was obtained. After a resting scan (baseline) was obtained, a pneumatic tourniquet placed around the forearm was inflated to a pressure of 250 mmHg for 5 min and then was released to create an increase of blood flow. A second scan was performed continuously from 30 s before until 90 s after deflation of the cuff. Subsequently, 10–15 min were allowed for recovery of the vessel and then an additional scan was performed. Finally, sublingual nitroglycerin (NTG) spray (300 µg) was administered and scanning was repeated 3–4 min later. The ultrasonographer was blinded to the treatment given to each subject. Vessel diameters were measured by two independent observers, with FMD and NTG-induced dilatation being calculated as the percent change from the baseline diameter and their results being averaged.
Study design
FMD and NTG-induced dilatation of the brachial artery were examined at before and 1 h, 3 h, 6 h, and 12 h after administration of placebo or 0.3 mg cerivastatin. Various biochemical parameters (including total cholesterol (TC), low-density lipoprotein cholesterol (LDLC), high-density lipoprotein cholesterol (HDLC), triglyceride(TG), malondialdehyde-modified low-density lipoprotein (MDA-LDL), and high-sensitivity C-reactive protein (hsCRP)) were determined at before and 3 h after administartion of placebo or 0.3 mg of cerivastatin. This study protocol was approved by our institutional Ethics Committee and all subjects gave written informed consent.
Statistical analysis
Analyses were performed with SAS System 8e software (SAS Institute Inc., Cary, North Carolina, USA). Results are presented as the mean ± s.d. The normality of the distribution of data was evaluated by the Shapiro-Wilks one-sample test and the F-test was used to assess the homogeneity of variance. Student's t-test was used for the comparison of continuous data. A simple linear regression model was used to perform the trend test. One-way analysis of variance (anova) was used to test for significant differences between the groups, and Dunnett's multiple comparison method was applied when appropriate. A two-tailed P value of less than 0.05 was considered to indicate statistical significance.
Results
Flow-mediated dilatation
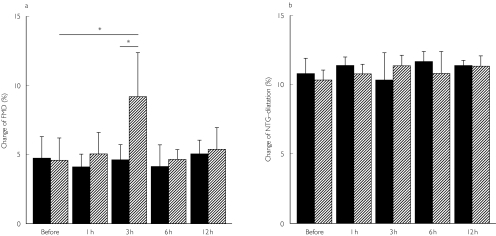
There was no difference in baseline diameter of brachial artery at before and 1 h, 3 h, 6 h, or 12 h after administration of placebo or cerivastatin (Figure 1). Although there was no difference in percentage FMD at before and 1 h, 3 h, 6 h, or 12 h after placebo administration (Figure 2a), administration of cerivastatin led to a significant increase in the percentage FMD of the brachial artery at 3 h after administration (P < 0.001, Figure 2a), which coincides with the time of maximum serum concentration of cerivastatin after a single administration. Then, the increased responsiveness returned to the baseline level at 6 h. In contrast, there was no difference in endothelium-independent vasodilatation mediated by NTG between the placebo and cerivastatin group (Figure 2b).
Figure 1.
Baseline diameter of brachial artery before and 1 h, 3 h, 6 h, or 12 h after administration of placebo (▪) or cerivastatin ( ).
).
Figure 2.
a) Endothelial function assessed by flow-mediated dilatation (FMD) of brachial artery. Cerivastatin significantly increased the change of FMD of the brachial artery at 3 h after administration. * P < 0.001 b) Endothelium-independent dilatation of brachial dilatation in response to nitroglycerin (NTG) treatment. There was no difference in NTG-induced vasodilatation after administration of placebo (▪) or cerivastatin ( ).
).
Biochemical markers
The subjects were all healthy men with no risk factors for atherosclerosis and no cardiovascular disease. Their lipid parameters, including TC, LDLC, HDLC, and TG, were not changed by a single dose of either placebo or cerivastatin. MDL-LDL, one of the major oxidatively modified LDL, and hsCRP, a marker of inflammation, were also unchanged (Table 1).
Table 1.
Biochemical parameters.
| Before | Placebo 3 h | P value | Before | Cerivastatin 3 h | P value | |
|---|---|---|---|---|---|---|
| TC, (mg dl−1) | 189.2 ± 22.8 | 184.2 ± 31.3 | NS | 182.4 ± 22.4 | 179.8 ± 26.3 | NS |
| LDLC, (mg dl−1) | 122.9 ± 30.0 | 118.7 ± 26.0 | NS | 114.4 ± 22.0 | 123.2 ± 35.6 | NS |
| HDLC, (mg dl−1) | 61.0 ± 12.9 | 60.4 ± 14.9 | NS | 59.0 ± 14.0 | 59.0 ± 14.2 | NS |
| TG, (mg dl−1) | 98.8 ± 80.1 | 90.0 ± 54.2 | NS | 106.2 ± 126.3 | 74.9 ± 45.4 | NS |
| MDA-LDL, (Ul−1) | 86.7 ± 29.5 | 85.4 ± 33.9 | NS | 81.6 ± 33.7 | 88.9 ± 31.4 | NS |
| hsCRP, (ng ml−1) | 456.2 ± 389.1 | 437.2 ± 248.0 | NS | 456.6 ± 390.0 | 466.4 ± 315.1 | NS |
Values are mean ± s.d. TC indicates total cholesterol; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; TG, triglyceride; MDA-LDL, malondialdehyde-modified low-density lipoprotein; hsCRP, high sensitivity C-reactive protein.
Discussion
In the present study, we demonstrated in healthy humans that a single dose of cerivastatin rapidly increased endothelial responsiveness. Several statins have already been reported in vivo to decrease the serum cholesterol concentration and improve endothelial function in 2 weeks [13, 14]. Because even 2 weeks of statin therapy will reduce the serum cholesterol concentration, it was unclear from these studies whether the vascular effect of statins was independent of their lipid-lowering effect. It seems likely that the vascular effect of statins shown in these previous studies is actually dependent on its lipid-lowering effect, unlike that of cerivastatin revealed in the present study. A very recent report by Tsunekawa et al. [15] also demonstrated that cerivastatin improves endothelial function within 3 days independent of its lipid-lowering effect in elderly diabetic patients. Surprisingly, we detected and reported here an acute (3 h) transient effect of a single dose of a statin on endothelial responsiveness in humans. The time course of this effect coincides with that of blood concentration after a single administration of cerivastatin [16]. Taking our finding together with previous reports that cerivastatin has a high bioavailability in vivo [17], this statin seems to have a direct in vivo effect on the vascular wall independent of its lipid-lowering effect, suggesting that it can be considered as a vascular statin.
There have been two mechanisms proposed with respect to the beneficial effect of statins. One is that the cholesterol-lowering effect of these drugs inhibits the progression of atherosclerosis and the occurrence of cardiovascular events, as has been established by many clinical trials [1-3]. The second proposed mechanism is that modulation of gene expression including NO synthase in the vascular wall leads to the stabilization of atherosclerotic plaque, although such effects have mainly been assessed by in vitro experiments using cultured cells [6-8]. The effect within 3 days shown in the previous study may be caused by NO produced by up-regulated NO synthase mRNA. However, the acute transient response in 3 h cannot be explained by either of the above mechanisms, because a single administration of cerivastatin neither reduces the serum cholesterol nor up-regulates protein synthesis in the vascular wall within only 3 h. Therefore, a third novel mechanism of action that regulates the NO production system such as stabilization of NO synthase mRNA [9, 10], stabilization of NO synthase protein, or a direct influence on NO synthase activity seems to be involved in the 3 h response. Laufs et al. [9] have demonstrated that cerivastatin improves stabilization of NO synthase mRNA within hours. Tsunekawa et al. [15] have hypothesized that rapid improvement of endothelial function by cerivastatin was caused by antioxidant action. MDL-LDL, one of the major oxidatively modified LDL, was not altered by a single dose of cerivastatin in the present study. Recent studies have demonstrated that signalling through Akt, which is a downstream kinase of phosphatidylinositol 3′-kinase, plays an important role in the functional regulation of NO synthase by modulating its phosphorylation level in endothelial cells within hours [18]. Although there is a possibility that such a rapid regulation system may be involved in the 3 h effect of cerivastatin, the real mechanism remains unclear so far. Accordingly, further investigation of this issue is necessary.
Although our data strongly suggest the existence of at least one vascular statin, this study was aimed neither to show the clinical usefulness of cerivastatin nor the clinical implication of statins in healthy subjects with normal cholesterol concentrations or normal endothelial function. We cannot conclude from this study that cerivastatin has a long-term clinical benefit in patients with impaired endothelial function even though a single dose of cerivastatin has a transient effect on endothelial responsiveness. Also, cerivastatin has been withdrawn from the market due to its side-effects.
Conclusions
A single dose of cerivastatin rapidly and transiently increased vascular endothelial responsiveness as evaluated by FMD, indicating that cerivastatin has a direct effect in humans on the vasculature independent of its cholesterol-lowering effect, and supporting the concept of the existence of a vascular statin.
Acknowledgments
This study was supported by a Grant-in-Aid for Encouragement of Young Scientists (to H.N). We wish to thank Katsunori Shimada (Department of Biostatics, Statz Co.) for the statistical analysis. We also thank Yoshihiro Ando and Yasushi Mineoka for their generous support and encouragement during this study.
References
- 1.Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease. The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 3.The Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 4.Bellosta S, Bernini F, Ferri N, et al. Direct vascular effects of HMG-CoA reductase inhibitors. Atherosclerosis. 1998;137(Suppl):S101–S109. doi: 10.1016/s0021-9150(97)00319-5. [DOI] [PubMed] [Google Scholar]
- 5.Corsini A, Pazzucconi F, Arnaboldi L, et al. Direct effects of statins on the vascular wall. J Cardiovasc Pharmacol. 1998;31:773–778. doi: 10.1097/00005344-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 7.Bellosta S, Via D, Canavesi M, et al. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol. 1998;18:1671–1678. doi: 10.1161/01.atv.18.11.1671. [DOI] [PubMed] [Google Scholar]
- 8.Colli S, Eligini S, Lalli M, et al. Vastatins inhibits tissue factor in cultured human macrophages: a novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol. 1997;17:265–272. doi: 10.1161/01.atv.17.2.265. [DOI] [PubMed] [Google Scholar]
- 9.Laufs U, Endres M, Stagliano N, et al. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hausding M, Witteck A, Rodriguez-Pascual F, et al. Inhibition of small G proteins of the rho family by statins or clostridium difficile toxin B enhances cytokine-mediated induction of NO synthase II. Br J Pharmacol. 2000;131:553–561. doi: 10.1038/sj.bjp.0703607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen KE, Celermanjer DS, Spiegelhalter DJ, et al. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J. 1995;74:247–253. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John S, Delles C, Jacobi J, et al. Rapid improvement of nitric oxide bioavailability after lipid-lowering therapy with cerivastatin within two weeks. J Am Coll Cardiol. 2001;37:1351–1358. doi: 10.1016/s0735-1097(01)01128-7. [DOI] [PubMed] [Google Scholar]
- 14.Marchesi S, Lupattelli G, Siepi D, et al. Short-term atorvastatin treatment improves endothelial function in hypercholesterolemic women. J Cardiovasc Pharmacol. 2000;36:617–621. doi: 10.1097/00005344-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Tsunekawa T, Hayashi T, Kano H, et al. Cerivastatin, a hydroxymethylglutaryl coenzyme A reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days. Circulation. 2001;104:376–379. doi: 10.1161/hc2901.094094. [DOI] [PubMed] [Google Scholar]
- 16.Muck W. Clinical pharmacokinetics of cerivastatin. Clin Pharmacokinet. 2000;39:99–116. doi: 10.2165/00003088-200039020-00002. [DOI] [PubMed] [Google Scholar]
- 17.Muck W, Ritter W, Ochmann K, et al. Absolute and relative bioavailability of the HMG-CoA reductase inhibitor cerivastatin. Int J Clin Pharmacol Ther. 1997;35:255–260. [PubMed] [Google Scholar]
- 18.Brouet A, Sonveaux P, Dessy C, et al. Hsp 90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]