Abstract
Aims
It is widely held that tolerance develops to the effects of sustained caffeine consumption. This study was designed to investigate the effects of chronic, staggered caffeine ingestion on the responses of an acute caffeine challenge, during euglycaemia.
Methods
Twelve healthy volunteers were randomized using a double-blind, cross-over design to take either 200 mg caffeine (C-replete) or placebo (C-naïve) twice daily for 1 week. Following baseline measurements being made, the responses to 200 mg caffeine (blood-pressure, middle cerebral artery velocity, mood and cognitive performance) were examined over the subsequent 120 min. Blood glucose was not allowed to fall below 4.0 mmol l−1.
Results
After the caffeine challenge, middle cerebral artery blood velocity decreased in both conditions but was greater in the C-naïve condition (−8.0 [-10.0, −6.1] cm s−1 vs −4.9 [-6.8, −2.9] cm s−1 C-replete, P < 0.02). Systolic blood pressure rise was not significantly different in C-naïve, although this rise was more sustained over time (P < 0.04). Mood was adversely affected by regular caffeine consumption with tense aspect of mood significantly higher at baseline in C-replete 11.6 ± 0.6 C-naïve vs 16.3 ± 1.6 C-replete, P < 0.01). Cognitive performance was not affected by previous caffeine exposure.
Conclusions
Overall these results suggest that tolerance is incomplete with respect to both peripheral or central effects of caffeine.
Keywords: caffeine, caffeine-tolerance, euglycaemia
Introduction
Due to the enormous consumption of caffeine world-wide, its effects on performance and physiology have been extensively investigated. However the debate about the benefits and drawbacks of caffeine ingestion remains unresolved [1, 2]. Numerous placebo-controlled studies have been published showing improvement in a variety of cognitive function tests including simple reaction time [3], digit symbol substitution [4] and logical reasoning [5]. Although a smaller number of studies have shown no effect of caffeine stimulus recognition and memory [6, 7], overall the evidence to date is in favour of a psychostimulant action of caffeine. In addition caffeine consumption has been associated with positive effects on mood.
The relevance of these studies to everyday life is questionable because in the majority, subjects were required to abstain from caffeine overnight and the amount of caffeine administered was relatively high, up to 600 mg in a single dose. The problem with caffeine deprivation prior to testing, means that symptoms of caffeine withdrawal can be invoked [8]. Thus it is not possible to describe the net effects of caffeine with these studies as caffeine withdrawal has been shown to decrease performance in both humans (e.g. finger tapping [9]) and animals [10]).
There is evidence that acute ingestion of caffeine has a pressor effect. It is widely held that tolerance to this develops with sustained use [11] but this is disputed [12]. Furthermore other circulatory effects can be measured, e.g. decrease in cerebral [13] and forearm blood flow [14], which are potentially better markers of the action of caffeine on the cardiovascular system. Debrah et al. demonstrated a dissociation between central (cerebral blood flow) and peripheral (blood pressure) effects of caffeine with tolerance developing in the latter only [15].
Earlier studies which claimed to show tolerance to the pressor effects of caffeine [11, 16–18] have not mirrored ‘normal’ staggered caffeine consumption but are characterized by a prior period of prolonged abstinence followed by a high dose of caffeine administered once or twice daily. Subsequent studies, which have considered more usual patterns of consumption, have reported the pressor effects of further doses of caffeine during the morning albeit with a smaller response than the first cup of coffee [19, 20]. Shi et al. demonstrated that the tolerance phenomenon depended on the amount of caffeine consumed, schedule of consumption and elimination half-life [21]. They estimated, using a parametric pharmacokinetic-pharmacodynamic model for caffeine that it would take about 20 h or five drug half-lives for the effects of caffeine tolerance to wear off. Given that caffeine consumption is associated with 10–12 h of overnight abstinence with more caffeine consumed in the morning than afternoon hours, it can be demonstrated that overnight abstinence is enough for sensitivity to be recovered and that at best tolerance is partial.
The aim of this study was to investigate further the tolerance phenomenon in both central (cerebral blood flow, mood, cognitive function) and peripheral (blood pressure, heart rate) effects of caffeine consumption.
Methods
Subjects
Fourteen healthy, left-hemisphere dominant, regular (daily) caffeine consumers (180–500 mg per day, seven males and aged 23–38 years) gave written, informed consent for the study, after approval was obtained from the local hospital ethics committee. None of the subjects had any relevant previous medical history, nor were they taking any regular medication or smoking. Each subject was informed that they would be required to attend the department on three separate occasions. The first session served to familiarize the subjects with the study protocol, which helped to minimize practise effects on the cognition tests. The results from this session were discarded. The following two studies were identical except for the dietary preparation. They were performed at least 2 weeks apart to avoid any carry-over effect.
Experimental procedure
Subjects were studied at the Metabolism Research Unit at Bournemouth Diabetes and Endocrine Centre. Prior to the second and third visits, subjects consumed a caffeine-free diet for 7 days. This was supplemented, in a double-blinded, randomized, cross-over design with either 200 mg caffeine capsules or matched placebo twice daily. The order was counter-balanced across participants. The final capsule was taken on the morning of the study, 1 h before attending the research unit. Thus, the subjects were either caffeine-replete (C-replete) or caffeine-naive (C-naïve) at the start of each study.
On the morning of a study, subjects were admitted at 09.00 h, having fasted overnight. At this time, subjects completed a questionnaire scoring the strength of 32 symptoms relating to caffeine-withdrawal rated on a scale of 0–3 [9]. A retrograde cannula was inserted into the dorsum of the nondominant hand and was kept patent by an infusion of 154 mmol l−1 sodium chloride. The hand was placed in a ‘hot-box’ (60 °C) to ‘arterialise’ venous blood. Potential distractions such as conversation and other background noise were minimized. After insertion of the cannula, subjects rested supine for 20 min before the experimental protocol began. Baseline measurements were taken of:
Heart rate and blood pressure using an automated device (Dinamap, Critikon, UK).
Brain blood flow using a transcranial Doppler technique (SciMed, Bristol, UK) to assess middle cerebral artery blood velocity (VMCA). The measured parameter was the mean value of the maximum velocity envelope. Although measurement of VMCA assumes that calibre changes in the vessel are small, alterations in VMCA reflect changes in cerebral blood flow during euglycaemia and hypoglycaemia [22].
Mood using the UWIST mood score [23] consisting of 24 adjectives divided into three equal groups describing hedonic (pleasurable), tense and energetic mood: maximum score for each aspect is 32 each item is scored 1–4.
Four-choice reaction time (4CRT) [24]. Calculations were made of total and number of correct reactions over a timed 5 min period. Prior to the start of each study, all subjects familiarized themselves with the timer to minimize any practice effect.
Visual information processing tests. Visual change detection (VCD) [25] assesses the speed of early visual processing by measuring the brain's ability to identify the locus of change in a stimulus array. The stimulus display consists of an array of 49 rectangles on a computer monitor screen to which, after a variable interval, a single identical rectangle is added. The subject's task is to identify this addition. The different time intervals between the presentation of the array and the onset of the change are 14, 28, 42, 56, 70 and 84 ms. The whole test involves 10 trials of the six different stimulus duration. A total accuracy score is obtained.
Visual movement detection (VMD) [25] resembles the VCD test in all respects, except that the target rectangle, rather than appearing after the rest of the array, appears with the array. After a variable interval it moves horizontally by a distance identical to its width (3 mm). This creates the subjective sensation of sudden movement. The test is generated in the same format as the VCD test. The interval between the onset of the array and the target rectangle appearing to move are also identical (i.e. 14–84 ms). A random block of 60 presentations (10 trials of 6 different stimulus duration) is also employed in this test and the total number of correct responses is obtained.
Plasma caffeine concentrations
Blood was taken from a vein draining the heated hand for subsequent measurement of caffeine by an enzyme immunoassay technique (EMIT® Behring Diagnostics, Milton Keynes, UK) on an Olympus AU560 autoanalyser (Olympus Optical, Eastleigh, UK). The intra- and interprecision of this assay is 4.0% and 3.9%; the lower limit of quantification 0.10 mmol l−1.
Thereafter, subjects consumed a cup of decaffeinated coffee containing 200 mg caffeine. All measurements were repeated in the same position, at 30 min intervals for the next 2 h with blood glucose concentrations measured every 15 min (YSI 2300 Stat Plus glucose analyser, Yellow Springs, Ohio, USA). Throughout, blood sugar was maintained above 4.0 mmol l−1 by ingestion 3–6 g of glucose when blood glucose <3.9 mmol l−1; thus avoiding the confounding effect of hypoglycaemia.
Statistical analysis
Overall differences between serial measurements were examined by summary measures [26]. Area under the curve (AUC) by the trapezoidal method and maximum response after the caffeine challenge were calculated for the responses of each individual. Group means were compared by paired Student's t-tests. Results are ex-pressed as individual means with point estimate of differences between means and 95%CI. Otherwise data are shown as mean ± s.e. mean.
Results
Baseline and maximum caffeine concentrations for the two experimental conditions are shown in Table 1 with average blood glucose readings for the duration of the studies. Caffeine withdrawal questionnaires showed no difference in total scores after caffeine abstinence or supplementation for 7 days (26.9 ± 2.5 C-naïve vs 27.8 ± 2.3 C-replete, P = 0.34).
Table 1.
Mean plasma caffeine and blood glucose concentrations during C-replete and C-naïve studies.
| Condition | Baseline caffeine (mg l−1) | Maximum caffeine (mg l−1) | Average blood glucose (mmol l−1) |
|---|---|---|---|
| C-replete | 2.33 ± 0.62** | 5.41 ± 1.45* | 4.50 ± 0.1 |
| C-naive | 0.17 ± 0.05** | 4.08 ± 1.18* | 4.09 ± 0.08 |
P < 0.001 C-naïve vs C-replete
P < 0.02 C-naïve vs C-replete.
Haemodynamics
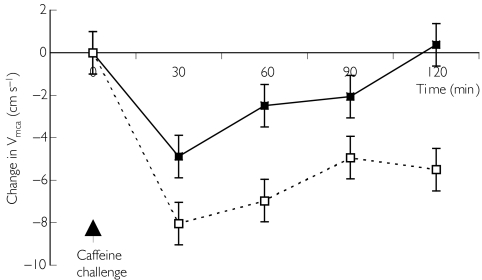
Baseline VMCA, heart rate and blood pressure were similar in both studies. After the caffeine challenge, VMCA decreased significantly in the both conditions, although this was more marked during the C-naïve condition (−8.0 [-10.0, −6.1] cm/s C-naïve vs 4.9 [-6.8, −2.9] cm s−1 C-replete, P < 0.02) (mean difference [95%CI]). The decrease in VMCA was sustained for the duration of the study (Figure 1).
Figure 1.
Change in middle cerebral artery blood velocity in C-replete (▪) and C-naïve (□) conditions following a caffeine challenge (mean ±s.e. mean). P < 0.01 for AUC C-naïve vs C-replete.
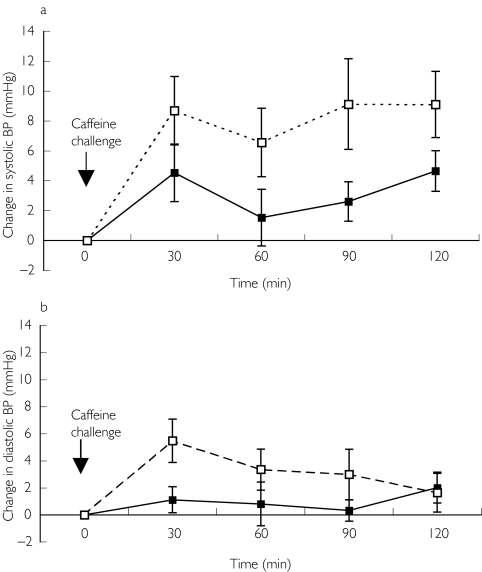
In the C-replete state, the caffeine challenge caused a significant rise in systolic blood pressure at 30 min but this was not significantly different from the rise in C-naïve study (+8.7 [2.1, 13.4] mmHg C-naïve vs +4.5 [0.8, 8.3] mmHg C-replete, P = 0.13). Although there were no differences in baseline blood pressures (systolic and diastolic) AUCs for change in systolic blood pressure against time were significantly different (P < 0.04) (Figure 2a). In contrast the initial rise in diastolic blood pressure was greater in the C-naïve condition (+5.5 [3.2, 8.6] mmHg C-naïve vs +1.1 [-0.8, 3.0] mmHg C-replete, P < 0.005) but this difference was not statistically sustained for the study duration (AUC C-naïve vs C-replete P = 0.055) (Figure 2b). Heart rate was unaffected by prevailing caffeine status.
Figure 2.
Change in a) systolic and b) diastolic blood pressure in C-replete (▪) and C-naïve (□) conditions following a 200 mg caffeine challenge. P < 0.04 for AUC for systolic BP C-naïve vs C-replete, P < 0.055 for AUC for diastolic BP C-naïve vs C-replete.
Symptom score
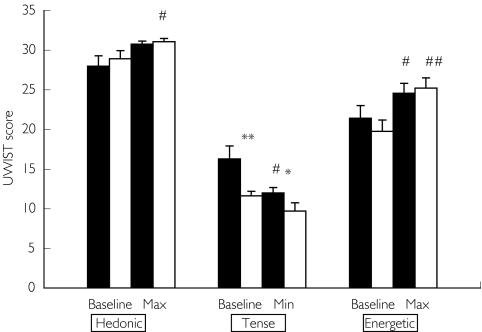
Tense mood was most affected by caffeine status (Figure 3). Baseline measurements were significantly different (11.6 ± 0.6 C-naïve vs 16.3 ± 1.6 C-replete, P < 0.01). Caffeine consumption in the C-replete state was associated with a greater decrease in tense mood (−1.1 [−2.6, −0.34] C-naïve vs −4.7 [−7.5, −1.9] C-replete, P < 0.02). Whilst caffeine consumption improved energetic mood in both conditions this effect was not significantly different between conditions (P = 0.24) (Figure 3). Hedonic mood increased significantly from baseline during C-naïve only (increase in hedonic mood score +2.9 ± 1.2 above baseline, P < 0.05), although the increase observed was not significantly different between conditions (P = 0.51) (Figure 3).
Figure 3.
Mood scores in C-replete (▪) and C-naïve (□) conditions at baseline and the most marked response following the caffeine challenge (mean ± s.e. mean). *= C-naive vs C-replete P < 0.02, ** = C-replete vs C-naïve P < 0.01, # = Max/Min vs Baseline P < 0.05, ## = Max/Min vs Baseline P < 0.005.
Psychometric tests
VCD and VMD did not improve with caffeine consumption in either condition (Table 2). Four choice reaction time improved by 0.02 ± 0.01 s, following caffeine ingestion in both the C-naïve and C-replete conditions.
Table 2.
Total scores of VCD and VMD tests during C-replete and C-naïve studies (mean ±s.e. mean).
| Baseline | 60 min | 120 min | |
|---|---|---|---|
| VCD | |||
| C-replete | 42.7 ± 1.6 | 43.6 ± 1.3 | 43.6 ± 1.0 |
| C-naive | 42.5 ± 1.7 | 44.9 ± 1.2 | 44.8 ± 1.4 |
| VMD | |||
| C-replete | 54.8 ± 1.2 | 54.9 ± 0.9 | 55.1 ± 0.6 |
| C-naive | 54.4 ± 1.4 | 53.2 ± 1.6 | 55.6 ± 1.1 |
Discussion
Whilst the development of tolerance to some effects of caffeine has been previously demonstrated [27], it remains a controversial area. In this study, during euglycaemia, VMCA and blood pressure (systolic and diastolic) responses to acute caffeine ingestion were attenuated with sustained caffeine use compared with a caffeine-naïve state. However tolerance was incomplete as significant changes from baseline were still recorded in VMCA and systolic blood pressure. Thus vascular responses were demonstrated with a subsequent dose of caffeine in the fully C-replete state. (Average British caffeine consumption is 359 mg day−1 [28]). In the course of the C-replete study 200 mg caffeine on rising is taken, followed by a further 200 mg caffeine challenge.) Interestingly daily caffeine consumption was associated with an increase in tense mood, which resolved after further caffeine consumption. It was only this aspect of mood that was associated with a significantly different response to the caffeine challenge between the two conditions. Development of (partial) tolerance to the haemodynamic changes is in contrast to the effect on psychomotor function. Here prior caffeine ingestion was not associated with different performance in all the tests, compared with the C-naïve state. Finally the differences recorded are not explained by the treatment of caffeine withdrawal symptoms as these were at a similar level at the beginning of both C-replete and C-naïve studies.
Mood is influenced by different patterns of caffeine consumption. It has been demonstrated with free-living and laboratory studies that chronic caffeine consumption is associated with increased anxiety, tension, restlessness, nervousness and anger [29–31]. The UWIST mood score has not previously been used in caffeine studies but the results here would concur with other scores used. The marked reduction in tense mood following caffeine ingestion during C-replete could represent the treatment of caffeine withdrawal symptoms. These can develop over a short period of time [32, 33]. It may seem somewhat illogical that people chose to consume a substance, which increases negative feelings. Green & Suls [31] have suggested that consumption continues because of a perceived benefit such as increased productivity. Whilst hedonic mood did not improve significantly from baseline during C-replete unlike energetic mood, the size of these in-creases were not significantly different between the two caffeine conditions. This would suggest that tolerance to caffeine's more arousing effects did not develop although this is in contrast to Green & Suls’ study [31].
Increases in positive feelings are the usual feature of studies involving acute caffeine ingestion in naïve subjects [34, 35], although no dose–response relationship has been demonstrated [36]. Older people show increased positive effects of caffeine on mood compared to younger subjects with younger people expressing more anger with caffeine consumption [37]. With respect to mood, tolerance has been largely overlooked by investigators in this field, although Zwyghuizen-Doorenbos et al. suggested that instead of tolerance developing Pavlovian conditioning to the alerting effects of caffeine may also occur [38].
This study adds to the body of previous work that tolerance to the haemodynamic effects of caffeine consumption is present but partial. The pressor response may be reinstated by a brief period of abstinence [19], which may be as short as 3 h [39]. In the conditions of this experiment partial tolerance was demonstrated to the response in VMCA with regular caffeine ingestion. Earlier, Mathew et al. demonstrated that cerebral blood flow decreases with caffeine ingestion [13, 40]. In the latter study subjects withheld from caffeine consumption for a minimum of 2 h only, with an 18% decrease in CBF associated in caffeine consumption still demonstrated [40]. This effect was still measurable 90 min after the caffeine challenge.
Four-choice reaction time improved in both conditions to the same degree but no improvement was seen in either condition with the visual perception threshold tests. The Health and Lifestyle Survey provided an opportunity to examine the issue of caffeine consumption on cognition and tolerance [41]. In this study 9003 British adults completed a number of performance tests (including choice reaction), as well as information on caffeine intake. Overall caffeine consumption showed a dose–response relationship with improved cognitive performance with no evidence for the phenomenon of tolerance to caffeine. In contrast, whilst caffeine has been shown to narrow attention and thus increase selectivity of information processing [42, 43], it has subsequently been demonstrated that the stimulatory visual effects of caffeine are limited to situations with only a few visual inputs [44].
Caffeine consumption is so widespread that a small effect can potentially influence a population's health [45]. More specifically, caffeine has been shown to influence the physiological (decreased VMCA [46, 47], increased counterregulatory hormone responses [46, 47]) and psychological (increased symptoms [46, 47], decreased cognitive changes [46]) responses to hypoglycaemia). The question as to whether tolerance develops to these effects was partially addressed in a subsequent study whereby caffeine consumption was associated with increased awareness of hypoglycaemic events in free-living type 1 diabetic patients [48]. The present study further suggests that similar parameters continue to be affected despite sustained caffeine use.
In conclusion, whilst the history of exposure to caffeine will influence the effect of a dose, tolerance to this drug's vascular effects is incomplete both centrally and peripherally. In contrast tolerance was not demonstrated in performance (4 CRT) nor improvements in positive aspects of mood. Identifying these effects on a larger population's health requires careful, further investigation but remains an important question as caffeine consumption is ubiquitous.
Acknowledgments
Joanne Watson held a Novo Nordisk (UK) Fellowship whilst carrying out this research.
We would like to acknowledge the help from Melanie Weiss, Julia Ingleby, Mary Cavan with help in subject recruitment and data collection. Dr Michael Lunt helped with middle cerebral artery blood velocity recording and analysis and Dr Joseph Begley carried out the serum caffeine analysis.
References
- 1.James JE. Understanding CaffeineA Biobehavioural Analysis. Thousand Oaks: CA. Sage; 1997. [Google Scholar]
- 2.Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehavioral Rev. 1998;23:563–576. doi: 10.1016/s0149-7634(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 3.Azcona O, Barbanoj MJ, Torrent J, Jane F. Evaluation of the central effects of alcohol and caffeine interaction. Br J Clin Pharmacol. 1995;40:393–400. doi: 10.1111/j.1365-2125.1995.tb04562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.File SE, Bond AJ, Lister RG. Interaction between effects of caffeine and lorazepam in performance tests and self-ratings. J Clin Psychopharmacol. 1982;2:102–106. [PubMed] [Google Scholar]
- 5.Suenaga N, Inamitsu T, Hatakenaka M, Yanaga F, Shiraishi F. Effect of caffeine on the mental calculation. Fukuoka Acta Med. 1997;88:27–29. [PubMed] [Google Scholar]
- 6.Rush C, Higgins S, Bickel W, Hughes J. Acute behavioral effects of lorazepam and caffeine, alone and in combination, in humans. Behav Pharmacol. 1994;5:245–254. doi: 10.1097/00008877-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Rush C, Higgins S, Hughes J, Bickel W. Acute behavioral effects of trizolam and caffeine, alone and in combination, in humans. Exp Clin Pharmacol. 1994;2:211–222. doi: 10.1097/00008877-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 8.James JE. Does caffeine enhance or merely restore degraded psychomotor performance? Neuropsychobiology. 1994;30:124–125. doi: 10.1159/000119151. [DOI] [PubMed] [Google Scholar]
- 9.Silverman K, Evans SM, Strain EC, Griffiths RR. Withdrawal syndrome after the double blind cessation of caffeine consumption. New Engl J Med. 1992;327:1109–1114. doi: 10.1056/NEJM199210153271601. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths R, Woodson P. Caffeine physical dependence: a review of human and laboratory animal studies. Psychopharmacology. 1988;94:437–451. doi: 10.1007/BF00212836. [DOI] [PubMed] [Google Scholar]
- 11.Robertson D, Wade D, Workman R, Woosley RI, Oates JA. Tolerance to the humoral and hemodynamic effects of caffeine in man. J Clin Invest. 1981;67:1111–1117. doi: 10.1172/JCI110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James JE. Chronic effects of habitual caffeine consumption on laboratory and ambulatory blood pressure levels. J Cardiovascular Res. 1994;1:159–164. doi: 10.1177/174182679400100210. [DOI] [PubMed] [Google Scholar]
- 13.Mathew RJ, Wilson WH. Caffeine induced changes in cerebral circulation. Stroke. 1985;16:814–817. doi: 10.1161/01.str.16.5.814. [DOI] [PubMed] [Google Scholar]
- 14.Daniels J, Mole PA, Shaffrath J, Stebbins C. Effects of caffeine on blood pressure, heart rate, and forearm blood flow during dynamic leg exercise. J Appl Physiol. 1998;85:154–159. doi: 10.1152/jappl.1998.85.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Debrah K, Haigh R, Sherwin R, Murphy J, Kerr D. Effect of acute and chronic caffeine use on the cerebrovascular, cardiovascular and hormonal responses to orthostasis in healthy volunteers. Clin Sci. 1995;89:475–480. doi: 10.1042/cs0890475. [DOI] [PubMed] [Google Scholar]
- 16.Ammon H, Bieck P, Mansalaz D, Verspohl E. Adaptation of blood pressure to continuous heavy coffee drinking in young volunteers. Br J Clin Pharmacol. 1983;15:701–706. doi: 10.1111/j.1365-2125.1983.tb01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izzo J, Ghosal A, Kwong T, Freeman R, Jaenike J. Age and prior caffeine use alter the cardiovascular and adrenomedullary responses to oral caffeine. Am J Cardiol. 1983;52:769–773. doi: 10.1016/0002-9149(83)90413-7. [DOI] [PubMed] [Google Scholar]
- 18.Höfer I, Battig K. Cardiovascular behavioral and subjective effects of caffeine under field conditions. Pharmacol Biochem Behav. 1994;48:899–908. doi: 10.1016/0091-3057(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 19.Lane J, Manus D. Persistent cardiovascular effects with repeated caffeine administration. Psychosomatic Med. 1989;51:373–380. doi: 10.1097/00006842-198907000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein I, Shapiro D, Hui K, Yu J. Blood pressure response to the ‘second cup of coffee’. Psychosomatic Med. 1990;52:337–345. doi: 10.1097/00006842-199005000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, Benowitz NL, Denaro CP, Sheiner LB. Pharmacokinetic-pharmacodynamic modelling of caffeine: Tolerance to pressor effects. Clin Pharmacol Ther. 1993;53:6–14. doi: 10.1038/clpt.1993.3. [DOI] [PubMed] [Google Scholar]
- 22.Sorteberg W. Cerebral artery blood velocity and cerebral blood flow. In: Aaslid R, Newall DW, editors. Transcranial Doppler. New York: Raven Press; 1992. pp. 57–66. [Google Scholar]
- 23.Matthews G, Jones D, Chamberlain A. Redefining the measurements of mood: the UWIST Mood Adjective Checklist. Br J Psychol. 1990;81:17–42. [Google Scholar]
- 24.Wilkinson RT, Houghton D. Portable four choice reaction time test with magnetic tape memory. Behav Res Meth Instrumentation. 1975;7:441–446. [Google Scholar]
- 25.McCrimmon RJ, Deary IJ, Huntly BJP, MacLeod KJ, Frier BM. Visual information processing during controlled hypoglycaemia in humans. Brain. 1996;119:1277–1287. doi: 10.1093/brain/119.4.1277. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JNS, Altman DG, Campbell MJ, Roystan P. Analysis of serial measurements in medical research. Br Med J. 1993;300:230–236. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colton T, Gosselin R, Smith R. The tolerance of coffee drinkers to caffeine. Clin Pharmacol. 1967;9:31–39. doi: 10.1002/cpt19689131. [DOI] [PubMed] [Google Scholar]
- 28.Scott N, Chakraborty J, Marks V. Caffeine consumption in the United Kingdom: a retrospective survey. Food Sci Nutrit. 1989;42F:183–191. [Google Scholar]
- 29.Lane J, Williams R. Cardiovascular effects of caffeine and stress in regular coffee drinkers. Psychophysiology. 1987;24:157–164. doi: 10.1111/j.1469-8986.1987.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 30.France C, Ditto B. Cardiovascular response to occupational stress and caffeine in telemarketing employees. Psychosom Med. 1989;51:145–151. doi: 10.1097/00006842-198903000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Green P, Suls J. The effects of caffeine on ambulatory blood pressure, heart rate and mood on coffee drinkers. J Behav Med. 1996;19:111–128. doi: 10.1007/BF01857602. [DOI] [PubMed] [Google Scholar]
- 32.Lane JD. Effects of brief caffeinated-beverage deprivation on mood, symptoms, and psychomotor performance. Pharmacol Biochem Behav. 1997;58:203–208. doi: 10.1016/s0091-3057(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 33.Lane J, Phillips-Bute B. Caffeine deprivation affects vigilance performance and mood. Physiol Behav. 1998;65:171–175. doi: 10.1016/s0031-9384(98)00163-2. [DOI] [PubMed] [Google Scholar]
- 34.Griffiths R, Biglow G, Liebson I. Reinforcing effects of caffeine in coffee and capsules. J Exp Anal Behav. 1989;52:127–140. doi: 10.1901/jeab.1989.52-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverman K, Griffiths R. Low-dose discrimination and self-reported mood effects in normal volunteers. J Exp Anal Behav. 1992;57:91–107. doi: 10.1901/jeab.1992.57-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinlan P, Lane J, Moore K, Aspen J, Rycroft J, O'Brien D. The acute physiological and mood effects of tea and coffee: the role of caffeine level. Pharmacol Biochem Behav. 2000;66:19–28. doi: 10.1016/s0091-3057(00)00192-1. [DOI] [PubMed] [Google Scholar]
- 37.Arciero P, Gardner A, Benowitz N, Poehlman E. Relationship of blood pressure, heart rate and behavioral mood state to norepinephrine kinetics in younger and older men following caffeine ingestion. Eur J Clin Nutrit. 1998;52:805–812. doi: 10.1038/sj.ejcn.1600651. [DOI] [PubMed] [Google Scholar]
- 38.Zwyghuizen-Doorenbos A, Roehrs T, Lipschutz T, Timms V, Roth T. Effects of caffeine on alertness. Psychopharmacol. 1990;100:36–39. doi: 10.1007/BF02245786. [DOI] [PubMed] [Google Scholar]
- 39.Sung B, Whitsett T, Lovallo W, al’Absi M, Pincomb G, Wilson M. Prolonged increase in blood pressure by a single oral dose of caffeine in mild hypertensive men. Am J Hyperten. 1994;7:755–758. doi: 10.1093/ajh/7.8.755. [DOI] [PubMed] [Google Scholar]
- 40.Mathew R, Wilson W. Caffeine consumption, withdrawal and cerebral blood flow. Headache. 1985;25:305–309. doi: 10.1111/j.1526-4610.1985.hed2506305.x. [DOI] [PubMed] [Google Scholar]
- 41.Jarvis M. Does caffeine intake enhance absolute levels of cognitive performance. Psychopharmacol. 1993;110:45–52. doi: 10.1007/BF02246949. [DOI] [PubMed] [Google Scholar]
- 42.Anderson K, Revelle W. Impulsivity caffeine and proof–reading: A test of the Easterbrook hypothesis. J Exp Psychol Hum Percept Perform. 1982;8:614–624. doi: 10.1037//0096-1523.8.4.614. [DOI] [PubMed] [Google Scholar]
- 43.Lorist M, Snel J, Kok A. Influence of caffeine on information processing stages in well rested and fatigued subjects. Psychopharmacol. 1994;113:411–421. doi: 10.1007/BF02245217. [DOI] [PubMed] [Google Scholar]
- 44.Kenemans J, Verbaten M. Caffeine and visuo-spatial attention. Psychopharmacol. 1998;135:353–360. doi: 10.1007/s002130050522. [DOI] [PubMed] [Google Scholar]
- 45.James J. Is habitual caffeine use a preventable cardiovascular risk factor? Lancet. 1997;349:279–281. doi: 10.1016/S0140-6736(96)04253-5. [DOI] [PubMed] [Google Scholar]
- 46.Kerr D, Sherwin RS, Pavalkis F, et al. Effect of caffeine on the recognition of responses to hypoglycemia in humans. Ann Intern Med. 1993;119:799–804. doi: 10.7326/0003-4819-119-8-199310150-00005. [DOI] [PubMed] [Google Scholar]
- 47.Debrah K, Sherwin RS, Murphy J, Kerr D. Effect of caffeine on the recognition of and physiological responses to hypoglycaemia in insulin-dependent diabetes. Lancet. 1996;347:19–24. doi: 10.1016/s0140-6736(96)91557-3. [DOI] [PubMed] [Google Scholar]
- 48.Watson JE, Jenkins P, Hamilton M, Lunt D, Kerr D. Influence of caffeine on the frequency and perception of hypoglycemia in free-living patients with type 1 diabetes mellitus. Diabetes Care. 2000;23:455–459. doi: 10.2337/diacare.23.4.455. [DOI] [PubMed] [Google Scholar]