Abstract
Aims
To investigate the influence of the CYP1A2*1F mutation on CYP1A2 activity in smoking and nonsmoking pregnant women.
Methods
Pregnant women (n = 904) who served as control subjects in a case-control study of early fetal loss were investigated. They were phenotyped for CYP1A2 using dietary caffeine and the urinary ratio AFMU + 1X + 1 U/1,7 U. An assay for CYP1A2*1F using 5′-nuclease assay (Taqman) was developed to genotype the population.
Results
The frequencies of *1 A and *1F alleles among Swedish women were 0.29 and 0.71, respectively. There was no statistically significant difference in CYP1A2 activity between the genotypes, although a trend towards enhanced activity was observed in *1F/*1F (log MRc 0.77) and *1F/*1 A (log MRc 0.82) genotypes compared with the *1 A/*1 A genotype (log MRc 0.71) (anovaP = 0.07). The mean difference between the *1 A homozygotes and the heterozygotes was 0.11 [95% confidence interval of the difference: (−0.21, −0.01)] and that between the *1 A and *1F homozygotes was 0.05 [95% confidence interval of the difference: (−0.13, 0.03)]. No significant effect (P = 0.22) of the *1F on CYP1A2 activity was observed in smokers, tested using an interaction term (smoking * genotype) in the anova model (*1F/*1F log MRc 0.79, *1F/*1 A log MRc 0.86, and *1 A/*1 A log MRc 0.73). In smokers, there was no difference in ratio between homozygotes for the *1 A and *1F alleles [mean difference −0.06; 95% confidence interval of the difference: −0.22, 0.11] or between *1 A/*1 A and *1 A/*1F genotypes [mean difference −0.13; 95% confidence interval of the difference: −0.29, 0.04].
Conclusions
The effect of the CYP1A2*1F mutation on CYP1A2 activity in smoking pregnant women could not be confirmed.
Keywords: CYP1A2 activity, CYP1A2*1F, polymorphism, pregnancy, smoking
Introduction
Cytochrome P450 (CYP) 1A2 is one of the major phase I enzymes in the liver, accounting for about 8–15% of the total liver P450 content ([1] and references therein). The CYP1A2 gene is located on chromosome 15 and contains 6 introns and 7 exons [2]. This enzyme is important in the metabolism of widely used drugs such as theophylline [3] and clozapine [4]. CYP1A2 also has a role in chemical carcinogenesis due to its activation of procarcinogens, such as heterocyclic amines and arylamines [5].
CYP1A2 is inducible by cigarette smoke [6] and by dietary components such as cruciferous vegetables and charcoal-broiled meat [7]. In contrast, CYP1A2 activity is reduced in women taking oral contraceptives [8] and during pregnancy [9].
In vivo, caffeine has been extensively used as a probe drug for assessment of CYP1A2 activity [6, 10]. The main route of elimination of caffeine is 3-demethylation to paraxanthine catalysed by CYP1A2 [3].
In human liver samples up to 40-fold variation in CYP1A2 mRNA expression has been found [11, 12]. Similarly, pronounced interindividual differences in activity have been demonstrated in vivo with the use of caffeine as a marker of CYP1A2 activity [7, 13, 14].
Recently a study by Ou-Yang et al. showed a phenotypic polymorphism of CYP1A2 and the percentage of poor metabolizers in a Chinese population sample was 5.2%[15]. Genetic variation in the CYP1A2 gene has been looked for extensively to explain the large variation in enzyme activity. Several mutations in the CYP1A2 gene have been described [16–19] but the influence on the activity of these SNPs has yet to be shown. However, one mutation in the intron 1 (C/A) at position 734 downstream from the transcriptional initiation site denoted as CYP1A2*1F (http://www.imm.ki.se/CYPalleles/) was reported to affect the inducibility of CYP1A2 activity in smokers [18]. In the latter a 36% higher caffeine metabolic ratio was observed in the *1F/*1F genotype compared with *1F/*1 A individuals [18]. This suggests that CYP1A2*1F represents a highly inducible genotype associated with increased CYP1A2 activity upon exposure to certain inducing agents.
The present study aimed to confirm this observation in a larger population. For this purpose we developed a 5′nuclease allele-specific assay (Taqman®). The allele frequency of the CYP1A2*1F was determined in a sample of pregnant women, and the effect of the *1F mutation on CYP1A2 activity in smokers and nonsmokers in this population was studied.
Methods
Participants consisted of pregnant women in gestational weeks 6–12 who had served as control subjects in a case-control study of early foetal loss in Uppsala County, Sweden between 1996 and 1998 [20]. In that study, controls were primarily recruited from antenatal care and were matched to cases by week of gestation (n = 953), but were also recruited from women requesting induced abortion (n = 273) [20]. Phenotype data on the pregnant women included in this study has been used in a previous publication describing the relationship between caffeine metabolism and risk of spontaneous abortion [21]. The mean (± s.d.) age of all women was 28.4 ± 4.9 years. Forty-six of 1226 women were excluded because no samples were taken. Phenotype measurements were missing for 23% of the women. CYP1A2 allele frequency was calculated in 1170 women. Statistical analysis was performed on data from 904 women. Informed consent was obtained from all subjects, and the study was approved by the Ethics Committee of the Medical Faculty at Uppsala University.
The women were subjected to an extensive face-to face interview performed by specially trained midwives using a structured questionnaire. The interview included questions about drug treatment, intake of caffeine containing beverages, and smoking habits. Caffeine intake was estimated as described previously [20, 21]. Average daily caffeine intake during the last completed gestational week before the interview was calculated (weekly total caffeine intake in mg/7 days (for more details see Cnattingius et al. [20]).
Laboratory methods
All chemicals were purchased from Sigma (St Louis, MO., USA) or BDH (Poole, England) and were of at least analytical grade. Reagents and optical plates for the allelic discrimination assay were obtained from Applied Biosystems (Stockholm, Sweden).
Subjects were asked to provide both blood and urine samples at the time of the interview. Urine samples were collected in 14 ml tubes containing 500 µl of 1 m HCl and were analysed by an h.p.l.c. method, as described previously [22]. The urinary ratio (AFMU + 1 U + 1X)/1,7 U (MRc) was used as a marker of CYP1A2 activity [23]. Between-day coefficient of variation (%) for all metabolites were less than 18% at 12.5 µm (the lowest control concentration) and less than 10% at 400 µm. The limit of quantification was 10 µm for AFMU and 1X and 5 µm for 1 U and 1,7 U. Blood samples were collected in EDTA tubes. Genomic DNA was extracted from whole blood samples as described previously [24]. The samples were stored at −20 °C until analysis.
Analysis of cotinine in plasma was performed by gas chromatography using N-ethylnorcotinine as internal standard [25]. Smokers were defined as subjects with cotinine concentrations above 15 ng ml−1[26].
Allelic discrimination using 5′nuclease assay
The design of primers and fluorescently labelled probes was performed using the PrimerExpress 1.0 software (Applied Biosystems) and the sequences are depicted in Table 1. Primers and probes were synthesized by CyberGene AB (Novum, Stockholm, Sweden). Probes specific for each allele were included in the PCR assay. PCR was performed in 25 µl TaqMan Universal PCR Master Mix containing 0.3 µm forward and reverse primers, 65 pm of TET - probe (6 - carboxy - 4,7,2′,7′ - tetrachloro-fluorescein), 50 pm of FAM - probe (6 - carboxy - fluorescein) and 5 ng of genomic DNA as template. Amplification was either performed using a ABI 7700 sequence detection system (Taqman®) or using a GeneAmp® PCR System 9700 with the following cycle profile: 1 cycle at 50 °C for 2 min, 1 cycle at 95 °C for 10 min, 35 cycles at 95 °C for 15 s, and 60 °C for 1 min. Detection was performed using an ABI 7700 sequence detection system (Taqman®). In each plate samples with known genotype were included. The results of the control samples were consistent in all runs. The output from the ABI 7700 sequence detection system was plotted on a graph where the outcome and the quality of the allelic discrimination were assessed (Figure 1). Nontemplate controls were used for detection of DNA contamination (for more details see Livak [27]).
Table 1.
PCR primers and hybridization probes for allelic discrimination and sequencing of CYP1A2*1F. The bold figures show the mutation site.
| Primer | Sequences |
|---|---|
| TaqMan probe – TET | 5′-CTC TGT GGG CCC AGG ACG CAT-3′ |
| TaqMan probe – FAM | 5′-TC TGT GGG CAC AGG ACG CAT GG-3′ |
| TaqMan Forward primer | 5′-TTT CCA GCT CTC AGA TTC TGT GAT-3′ |
| TaqMan Reverse primer | 5′-GGA TAC CAG AAA GAC TAA GCT CCA TC-3′ |
| Forward primer* | 5′-TTC CCC ATT TTG GAG TGG TC-3′ |
| Reverse primer* | 5′-CCG AGA AGG GAA CAG ACT GG-3′ |
Primers used for sequencing.
Figure 1.
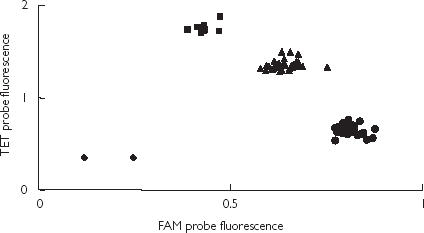
The allelic discrimination of 86 subjects using Taqman® with TET and FAM probes. The genotypes are represented as CYP1A2*1F (•), CYP1A2*1 A/*1F (▴) and CYP1A2*1 A (▪), and nontemplate controls (♦).
Sequencing of PCR-products
The identity of two samples of each genotype was confirmed by sequencing with primers shown in Table 1. QIAex II- (QIAGEN, Hilden Germany) purified PCR product (10 ng) was sequenced by CyberGene AB (Novum, Stockholm, Sweden) on an ABI 377 (Applied Biosystems).
Statistical analysis
The ratio (MRc) was log transformed before statistical analysis. A 3-way anova was used with log MRc as a dependent variable, and CYP1A2 genotype, smoking habits (smoker and nonsmoker) and caffeine intake above and below 150 mg day-1 as independent variables. Furthermore, the two–factor interaction between smoking habits and genotype was also included in the model to assess the potential effect of smoking on the influence of genotype. Statistical analysis was performed on a total of 904 individuals (Statistica 6.0, Statsoft Inc. OK, USA). To calculate 95% confidence interval on differences Fishers LSD post hoc test was used.
Results
The allelic discrimination method was designed to study the CYP1A2*1F polymorphism in Intron 1 of the CYP1A2 gene. The different genotypes were confirmed by sequencing. The plot from the allelic discrimination assay is shown in Figure 1. In Table 2 the frequency of the CYP1A2*1 A and *1F alleles are shown together with data from other published studies.
Table 2.
Allele frequency of the CYP1A2*1F mutation in Intron 1 from our study and from other published studies.
| Study | Number of subjects | *1 A (Wt) (95% C.I.) | *1F (95% C.I.) |
|---|---|---|---|
| Present study | 1170 | 0.29 (0.27, 0.31) | 0.71 (0.69, 0.73) |
| Sachse et al. [18] | 236 | 0.32 (0.28, 0.36) | 0.68 (0.64, 0.72) |
| Christiansen et al. [32] | 60* | 0.32 (0.23, 0.40) | 0.68 (0.60, 0.77) |
| Basile et al. [30] | 85 | 0.38 (0.31, 0.45) | 0.62 (0.55, 0.69) |
| Han et al. [28] | 168 | 0.33 (0.28, 0.38) | 0.67 (0.62, 0.72) |
Only the controls are shown.
A log normal distribution of the MRc was observed in the total population with a mean log MRc of 0.76 ± 0.32 (s.d.). An overall 160-fold variation of the MRc was observed (range 0.6–92).
One hundred and sixty-four women were smokers. These subjects had a mean log MRc of 0.81, which was statistically higher than the mean value of 0.72 observed in nonsmokers [mean difference = 0.06; 95% CI on the difference = 0.003, 0.11, P = 0.02].
There were no significant differences in the mean log MRc between the different gestational weeks (anovaP = 0.21). Therefore, gestational week was not considered as a possible covariate in further analysis.
We also investigated the effect of caffeine intake the week before interview on log MRc. Women were stratified into two groups based on low and high caffeine intake. Out of 904 women 394 had a daily caffeine intake above 150 mg. There was a significantly decreased metabolic ratio in the high caffeine intake group compared with the low intake group (mean difference = 0.09; 95% confidence interval on the difference = 0.04, 0.13, anovaP = 0.002).
Overall genotype did not have a statistically significant influence on log MRc, although there was a tendency towards higher MRc in *1 A/*1F and *1F/*1F individuals compared with homozygous wild types (anovaP = 0.07). The mean difference between the *1 A genotype and the heterozygous was 0.11 (95% confidence interval on the difference = −0.21, −0.01) and between the *1 A genotype and *1F genotype was 0.05 (95% confidence interval on the difference = −0.13, 0.03). To test whether the effect of genotype on the MRc differs in smokers and nonsmokers we introduced an interaction term (smoking * genotype) into the model. This analysis showed that no statistically significant modifying effect of smoking was present (P = 0.22). In smokers, there was no difference between homozygous for the *1 A-allele and *1F-allele (mean difference = 0.06; 95% confidence interval on the difference = −0.22, 0.11) or between *1 A/*1 A and *1 A/*1F (mean difference = 0.13; 95% confidence interval on the difference = −0.29, 0.04) (Table 3). In Table 4, the mean log MRc, S.D. and 95% confidence interval data are shown, stratified according to genotype, smoking status and caffeine intake.
Table 3.
The mean log urinary caffeine metabolic ratio (AFMU + 1 U + 1X)/1,7 U (MRc), s.d. and 95% confidence interval (CI) in different CYP1A2 genotype groups divided according to smoking status (n = number of subjects).
| Genotype | Smoking status | Mean log MR c ± s.d. | 95% CI | n |
|---|---|---|---|---|
| *1 A/*1 A | Non-smokers | 0.71 ± 0.31 | (0.63, 0.79) | 58 |
| Smokers | 0.73 ± 0.31 | (0.59, 0.87) | 18 | |
| *1 A/*1F | Non-smokers | 0.75 ± 0.33 | (0.71, 0.79) | 307 |
| Smokers | 0.86 ± 0.34 | (0.78, 0.94) | 67 | |
| *1F/*1F | Non-smokers | 0.76 ± 0.32 | (0.73, 0.79) | 375 |
| Smokers | 0.79 ± 0.29 | (0.73, 0.85) | 79 |
Table 4.
The mean log urinary caffeine metabolic ratio (AFMU + 1 U + 1X)/1,7 U (MRc), s.d. and 95% confidence interval (CI) are shown stratified according to CYP1A2 genotype, smoking status and caffeine intake (n = number of subjects).
| Genotype | Smoking status | Caffeine intake | Mean log MRc ± s.d. | 95%CI | n |
|---|---|---|---|---|---|
| *1 A/*1 A | Non-smokers | Low | 0.76 ± 0.32 | (0.66, 0.86) | 39 |
| Smokers | Low | 0.81 ± 0.39 | (0.54, 1.08) | 8 | |
| Non-smokers | High | 0.61 ± 0.25 | (0.50, 0.72) | 19 | |
| Smokers | High | 0.66 ± 0.30 | (0.47, 0.85) | 10 | |
| *1 A/*1F | Non-smokers | Low | 0.79 ± 0.34 | (0.74, 0.84) | 188 |
| Smokers | Low | 0.98 ± 0.33 | (0.82, 1.14) | 16 | |
| Non-smokers | High | 0.69 ± 0.30 | (0.64, 0.74) | 119 | |
| Smokers | High | 0.82 ± 0.34 | (0.73, 0.91) | 51 | |
| *1F/*1F | Non-smokers | Low | 0.81 ± 0.34 | (0.77, 0.85) | 237 |
| Smokers | Low | 0.80 ± 0.27 | (0.69, 0.91) | 22 | |
| Non-smokers | High | 0.68 ± 0.38 | (0.62, 0.74) | 138 | |
| Smokers | High | 0.78 ± 0.30 | (0.70, 0.86) | 57 |
Discussion
The frequency of the CYP1A2*1F allele in our population of Swedish women was similar to that reported for other Caucasian populations [18, 32]. We were unable to demonstrate an effect of the CYP1A2*1F allele on CYP1A2 inducibility in smokers, as observed in healthy volunteers by Sachse et al. [18], despite studying three times more smokers than these authors. However, in agreement with Sachse et al. [18] we did not see differences in MRc between genotypes in the nonsmoking group. Nevertheless there was a tendency for an overall enhancing effect of CYP1A2*1F on CYP1A2 activity in the combined population of smokers and nonsmokers (P = 0.07). If this difference is real, it may be concealed by the large variation in CYP1A2 activity observed in this study. The use of random sampling and dietary caffeine as a phenotyping procedure might require a study of larger numbers. In a recent study an association between the CYP1A2 phenotype and CYP1A2 genotype has been shown [28]. The authors investigated the CYP1A2*1F mutation and also one located in the 5′-flanking region. They concluded that a combination of these mutations was associated with a high inducibility of CYP1A2 in smokers as well as in nonsmokers, but they could not distinguish which allele was responsible because of the small number of subjects [28].
Caffeine clearance has been shown to be decreased during pregnancy [9]. Tsutsumi et al. [29] studied the effect of pregnancy on CYP1A2 activity using the caffeine urinary metabolic ratio (MRc). This was decreased by 35% in early pregnancy (week 8–12) compared with after delivery [29]. Since our study population consisted of pregnant women in gestational weeks 6–12, the effect of the *1F mutation on MRc might be abolished during pregnancy due to that the phenotype-genotype relationship was altered due to pregnancy related effects on CYP1A2 activity.
The CYP1A2*1F mutation is located in intron 1 and, therefore, does not lead to a change in the protein structure. The presence of conserved regions in intron 1 suggests that regulatory elements are located in this region. Different studies have reported an association between this polymorphism and certain disease states. For example, there has been a report of an association between severity of tardive dyskinesia in schizophrenia and this mutation [30]. It was concluded that patients homozygous for the *1 A allele were at increased risk to develop more severe tardive dyskinesia, compared with individuals who are heterozygous or homozygous for the *1F-allele [30]. However, a more recent study could not confirm this finding [31]. Another report demonstrated an increased frequency of the CYP1A2*1F allele in patients with porphyria cutanea tarda [32].
We assessed the CYP1A2 activity by using the urinary metabolic ratio (AFMU + 1X + 1 U)/1,7 U [23]. Our protocol involved the use of dietary caffeine and randomly collected urine samples. It has previously been shown that data using this approach correlates with those from the standardized caffeine intake and sampling time methodology [22]. We found an almost 160-fold variation in CYP1A2 activity in our total population. This is larger than that reported previously (10–15 fold) in healthy volunteers using standardized caffeine testing [13, 33]. The larger variation may be partly due to random sampling, but a contributing effect of pregnancy cannot be excluded. Sachse et al. used the plasma ratio 1,7X/1,3,7 X [18] but in the present study we used the urinary metabolic ratio (AFMU + 1 U + 1X)/1,7 U as an index of CYP1A2 activity. Some reports have suggested that the plasma ratio 1,7X/1,3,7 X gives a better estimate of CYP1A2 activity and is less biased than the urinary ratio (AFMU + 1 U + 1X)/1,7 U used by us [34–36].
In agreement with earlier findings [6] we observed that smoking increased the caffeine metabolic ratio, despite the large variation in our CYP1A2 activities. An increase in the amount of CYP1A2 protein in human liver from smoking individuals [37] also supports the in vivo finding that CYP1A2 is inducible by smoking.
In our population we observed a decrease in CYP1A2 activity with an increased amount of ingested caffeine. Previous studies have provided evidence that caffeine may exhibit dose-dependent kinetics [38, 39]. The clearance of caffeine decreased both in single and repeated dose studies following increasing amounts of ingested caffeine. In contrast, a few reports have shown an induction of CYP1A2 activity at higher levels of caffeine intake [14, 40].
In the present study we could not confirm the effect of CYP1A2*1F on the inducibility of smoking on CYP1A2 activity as observed by Sachse et al. [18]. Further studies are needed to investigate the regulation of CYP1A2 during pregnancy and the effect of the *1F allele on CYP1A2 activity. We cannot exclude the possibility that differences in methodology may have concealed a real effect on inducibility. More research on SNPs or haplotypes in the CYP1A2 gene is warranted to explain further the large variation in CYP1A2 activity.
Acknowledgments
We gratefully acknowledge Dr Ümit Yasar and Dr Sven Cnattingius for valuable scientific discussions. We kindly acknowledge the work carried out by Dr F. Kuylenstierna and colleagues at Pharmacia Corporation, Helsingborg, Sweden, for conducting the cotinine laboratory analyses. This study was supported financially by the Swedish Medical Research Council (04496) and by the International Epidemiology Institute through a grant from the National Soft Drink Association.
References
- 1.Rodrigues AD. Integrated cytochrome P450 reaction phenotyping: attempting to bridge the gap between cDNA-expressed cytochromes P450 and native human liver microsomes. Biochem Pharmacol. 1999;57:465–480. doi: 10.1016/s0006-2952(98)00268-8. [DOI] [PubMed] [Google Scholar]
- 2.Ikeya K, Jaiswal AK, Owens RA, et al. Human CYP1A2: sequence, gene structure, comparison with the mouse and rat orthologous gene, and differences in liver 1A2 mRNA expression. Mol Endocrinol. 1989;3:1399–1408. doi: 10.1210/mend-3-9-1399. [DOI] [PubMed] [Google Scholar]
- 3.Campbell ME, Grant DM, Inaba T, Kalow W. Biotransformation of caffeine, paraxanthine, theophylline, and theobromine by polycyclic aromatic hydrocarbon-inducible cytochrome (s) in human liver microsomes. Drug Metab Dispos. 1987;15:237–249. [PubMed] [Google Scholar]
- 4.Bertilsson L, Carillo JA, Dahl M-L, et al. Clozapine disposition covaries with CYP1A2 activity determined by a caffeine test. Br J Clin Pharmacol. 1994;38:471–473. doi: 10.1111/j.1365-2125.1994.tb04385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eaton DL, Gallagher EP, Bammler TK, Kunze KL. Role of cytochrome P4501A2 in chemical carcinogenesis: implications for human variablilty and enzyme activity. Pharmacogenetics. 1995;5:259–274. doi: 10.1097/00008571-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Kalow W, Tang B-K. Caffeine as a metabolic probe: Exploration of the enzyme-inducing effect of cigarette smoking. Clin Pharmacol Ther. 1991;49:44–48. doi: 10.1038/clpt.1991.8. [DOI] [PubMed] [Google Scholar]
- 7.Vistisen K, Poulsen HE, Loft S. Foreign compound metabolism capacity in man measured from metabolites of dietary caffeine. Carcinogenesis. 1992;13:1561–1568. doi: 10.1093/carcin/13.9.1561. [DOI] [PubMed] [Google Scholar]
- 8.Abernethy DR, Todd EL. Impairment of caffeine clearance by chronic use of low-dose oestrogen- containing oral contraceptives. Eur J Clin Pharmacol. 1985;28:425–428. doi: 10.1007/BF00544361. [DOI] [PubMed] [Google Scholar]
- 9.Knutti R, Rothweiler H, Schlatter C. Effects of pregnancy on the pharmacokinetics of caffeine. Eur J Clin Pharmacol. 1981;21:121–126. doi: 10.1007/BF00637512. [DOI] [PubMed] [Google Scholar]
- 10.Fuhr R. Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics. 1994;4:109–16. doi: 10.1097/00008571-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Andersen MR, Farin FM, Omiecinski CJ. Quantification of multiple human cytochrome P450 mRNA molecules using competitive reverse transcriptase-PCR. DNA Cell Biol. 1998;17:231–238. doi: 10.1089/dna.1998.17.231. [DOI] [PubMed] [Google Scholar]
- 12.Finnström N, Thorn M, Loof L, Rane A. Independent patterns of cytochrome P450 gene expression in liver and blood in patients with suspected liver disease. Eur J Clin Pharmacol. 2001;57:403–409. doi: 10.1007/s002280100318. [DOI] [PubMed] [Google Scholar]
- 13.Schrenk D, Brockmeier D, Mörike K, Bock KW, Eichelbaum M. A distribution study of CYP1A2 phenotypes among smokers and non-smokers in a cohort of healthy Caucasian volunteers. Eur J Clin Pharmacol. 1998;53:361–367. doi: 10.1007/s002280050394. [DOI] [PubMed] [Google Scholar]
- 14.Tantcheva-Poor I, Zaigler M, Rietbrock S, Fuhr U. Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999;9:131–144. published erratum appears in Pharmacogenetics 1999; 9: 781. [PubMed] [Google Scholar]
- 15.Ou-Yang DS, Huang SL, Wang W, et al. Phenotypic polymorphism and gender-related differences of CYP1A2 activity in a Chinese population. Br J Clin Pharmacol. 2000;49:145–151. doi: 10.1046/j.1365-2125.2000.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chida M, Yokoi T, Fukui T, et al. Detection of three genetic polymorphisms in the 5′-flanking region and intron 1 of human CYP1A2 in the Japanese population. Jpn J Cancer Res. 1999;90:899–902. doi: 10.1111/j.1349-7006.1999.tb00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang JD, Guo WC, Lai MD, Guo YL, Lambert GH. Detection of a novel cytochrome P-450 1A2 polymorphism (F21L) in Chinese. Drug Metab Dispos. 1999;27:98–101. [PubMed] [Google Scholar]
- 18.Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C → A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–449. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aitchison KJ, Gonzalez FJ, Quattrochi LC, et al. Identification of novel polymorphisms in the 5′ flanking region of CYP1A2, characterization of interethnic variability, and investigation of their functional significance. Pharmacogenetics. 2000;10:695–704. doi: 10.1097/00008571-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cnattingius S, Signorello LB, Anneren G, et al. Caffeine intake and the risk of first-trimester spontaneous abortion. N Engl J Med. 2000;343:1839–1845. doi: 10.1056/NEJM200012213432503. [DOI] [PubMed] [Google Scholar]
- 21.Signorello LB, Nordmark A, Granath F, et al. Caffeine metabolism and the risk of spontaneous abortion of normal karyotype fetuses. Obstet Gynecol. 2001;98:1059–1066. doi: 10.1016/s0029-7844(01)01575-7. [DOI] [PubMed] [Google Scholar]
- 22.Nordmark A, Lundgren S, Cnattingius S, Rane A. Dietary caffeine as a probe agent for assessment of cytochrome P4501A2 activity in random urine samples. Br J Clin Pharmacol. 1999;47:397–402. doi: 10.1046/j.1365-2125.1999.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell ME, Spielberg SP, Kalow W. A urinary metabolite ratio that reflects systemic caffeine clearance. Clin Pharmacol Ther. 1987;42:157–165. doi: 10.1038/clpt.1987.126. [DOI] [PubMed] [Google Scholar]
- 24.Smith CAD, Wadelius M, Gough AC, et al. A simplified assay for the arylamine N-acetyltransferase 2 polymorphism validated by phenotyoing with isoniazid. J Med Genet. 1997;34:758–760. doi: 10.1136/jmg.34.9.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsson P, Kylenstierna F, Johansson C-J, Gunnarsson P, Bende M. Pharmacokinetics of nicotine after intranasal administration. In: Aldkofler F, Thurau K, editors. Effects of Nicotine on Biological Systems Advances in Pharmacological Sciences. Basel: Birkhauser; 1991. pp. 57–61. [Google Scholar]
- 26.Peacock JL, Cook DG, Carey IM, et al. Maternal cotinine level during pregnancy and birthweight for gestational age. Int J Epidemiol. 1998;27:647–656. doi: 10.1093/ije/27.4.647. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 28.Han XM, Ou-Yang DS, Lu PX, et al. Plasma caffeine metabolite ratio (17X/137X) in vivo associated with G- 2964A and C734A polymorphisms of human CYP1A2. Pharmacogenetics. 2001;11:429–435. doi: 10.1097/00008571-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsumi K, Kotegawa T, Matsuki S, et al. The effect of pregnancy on cytochrome P4501A2, xanthine oxidase, and N- acetyltransferase activities in humans. Clin Pharmacol Ther. 2001;70:121–125. doi: 10.1067/mcp.2001.116495. [DOI] [PubMed] [Google Scholar]
- 30.Basile VS, Ozdemir V, Masellis M, et al. A functional polymorphism of the cytochrome P450 1A2 (CYP1A2) gene: association with tardive dyskinesia in schizophrenia. Mol Psychiatry. 2000;5:410–417. doi: 10.1038/sj.mp.4000736. [DOI] [PubMed] [Google Scholar]
- 31.Schulze TG, Schumacher J, Muller DJ, et al. Lack of association between a functional polymorphism of the cytochrome P450 1A2 (CYP1A2) gene and tardive dyskinesia in schizophrenia. Am J Med Genet. 2001;105:498–501. doi: 10.1002/ajmg.1472. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen L, Bygum A, Jensen A, et al. Association between CYP1A2 polymorphism and susceptibility to porphyria cutanea tarda. Hum Genet. 2000;107:612–614. doi: 10.1007/s004390000415. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen BB, Brösen K. Determination of urinary metabolites of caffeine for the assessment of cytochrome P4501A2, xanthine oxidase and N-acetyltransferase activity in humans. Ther Drug Monit. 1996;18:254–262. doi: 10.1097/00007691-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Fuhr U, Rost KL, Engelhardt R, et al. Evaluation of caffeine as a test drug for CYP1A2, NAT2 and CYP2E1 phenotyping in man by in vivo versus in vitro correlations. Pharmacogenetics. 1996;6:159–176. doi: 10.1097/00008571-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Carrillo JA, Christensen M, Ramos SI, et al. Evaluation of caffeine as an in vivo probe for CYP1A2 using measurements in plasma, saliva, and urine. Ther Drug Monit. 2000;22:409–417. doi: 10.1097/00007691-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Rostami-Hodjegan A, Nurminen S, Jackson PR, Tucker GT. Caffeine urinary metabolite ratios as markers of enzyme activity: a theoretical assessment. Pharmacogenetics. 1996;6:121–149. doi: 10.1097/00008571-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Sesardic D, Boobis AR, Edwards RJ, Davies DS. A form of cytochrome P450 in man, orthologous to form d in the rat, catalyses the O-de-ethylation of phenacetin and is inducible by cigarette smoking. Br J Clin Pharmacol. 1988;26:363–372. doi: 10.1111/j.1365-2125.1988.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng WS, Murhy TL, Smith MT, et al. Dose-dependent pharmacokinetics of caffeine in humans: relevance as a test of quantitative liver function. Clin Pharmacol Ther. 1990;47:516–524. doi: 10.1038/clpt.1990.66. [DOI] [PubMed] [Google Scholar]
- 39.Denaro CP, Brown CR, Wilson M, Jacob P, Benowitz NL. Dose-dependency of caffeine metabolism with repeated dosing. Clin Pharmacol Ther. 1990;48:277–285. doi: 10.1038/clpt.1990.150. [DOI] [PubMed] [Google Scholar]
- 40.Horn EP, Tucker MA, Lambert G, et al. A study of gender-based cytochrome P4501A2 variability: a possible mechanism for the male excess of bladder cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:529–533. [PubMed] [Google Scholar]


