Abstract
Aims
To evaluate the effect of the CYP1A2*1C and CYP1A2*1F polymorphisms on the inducibility of CYP1A2 by omeprazole in healthy subjects.
Methods
Mutations of CYP2C19 and CYP1A2 were identified by PCR-RFLP. Omeprazole, 120 mg day−1, was given to 12 extensive metabolizers (EM) with respect to CYP2C19 (six CYP1A2*1F/CYP1A2*1F and six CYP1A2*1C/CYP1A2*1F of CYP1A2) for 7 days. CYP1A2 activity was determined on three occasions, namely on day 1, day 9 and day 16 using the caffeine plasma index (the ratio of the concentrations of paraxanthine to caffeine), 6 h after oral administration of 200 mg caffeine.
Results
There was a significant difference (P = 0.002) between the caffeine ratios for CYP1A2*1F/CYP1A2*1F and CYP1A2*1C/CYP1A2*1F genotypes on day 9, but not on day 1 or day 16 (P > 0.05). The changes in the ratios from day 9 to day 1 (48% ± 20%vs 19% ± 20%) and from day 9 to day 16 (50% ± 31% vs 15% ± 22%) were significantly different (P < 0.05) between the CYP1A2*1F/CYP1A2*1F and CYP1A2*1C/CYP1A2*1F genotypes.
Conclusion
The CYP1A2*1C and CYP1A2*1F genetic polymorphisms influenced the induction of CYP1A2 activity in vivo by omeprazole.
Keywords: caffeine, CYP1A2, genetic polymorphism, induction
Introduction
CYP1A2, a member of cytochrome P450 (CYP) superfamily, plays a key role in the metabolism of a number of drugs and also participates in the activation of some procarcinogens [1]. It has also been suggested that high CYP1A2 activity is a susceptibility factor for hepatacelluar carcinoma and colon cancer [2]. Thus, characterization of factors that induce CYP1A2 gene expression may be of importance to therapeutics and to the aetiology of some cancers.
Studies show up to 70-fold variation in CYP1A2 activity [3]. At least four single nucleotide polymorphisms (SNPs) of the CYP1A2 gene have been identified, among which CYP1A2*1C and CYP1A2*1F are suggested to be associated with the induction of CYP1A2 activity in smokers [4–6]. Our previous study showed that the CYP1A2*1F/CYP1A2*1F and CYP1A2*1C/CYP1A2*1F genotypes were associated with the higher and lower inducibility of CYP1A2, respectively, in smokers and non-smokers from the Qidong area [8].
Two important alleles of CYP2C19, designated as CYP2C19*2 and CYP2C19*3, account for most poor metabolizers (PMs) with respect to CYP2C19 in Chinese populations. CYP2C19*2 accounts for approximately 80% of defective alleles, and CYP2C19*3 for the remainder [9]. CYP2C19*1/CYP2C19*1, CYP2C19*1/CYP2C19*2 or CYP2C19*1/CYP2C19*3 genotypes are normally extensive metabolizers (EMs), while CYP2C19*2/CYP2C19*2, CYP2C19*2/CYP2C19*3 or CYP2C19*3/CYP2C19*3 are PMs.
As a potent inhibitor of H+/K+-stimulated adenosine triphosphatase (a proton pump) in parietal cells of the gastric mucosa, omeprazole is widely used in the treatment of peptic ulceration and reflux esophagitis. Omeprazole is known to induce selectively human liver CYP1A2 activity in vitro and in vivo. Because the in vivo induction of CYP1A2 by omeprazole depends on the plasma concentration of the drug, and CYP2C19 participates in the oxidation of omeprazole [10], CYP1A2 activity is affected differentially by omeprazole in EMs compared with PMs with respect to CYP2C19 [11, 12].
The objective of this study was to evaluate the association between the CYP1A2*1C and CYP1A2*1F genetic polymorphisms and CYP1A2 inducibility in healthy subjects by using omeprazole as a substrate for the latter.
Methods
Subjects
This study was conducted according to the Declaration of Helsinki and with the approval of Ethics Committee of the Xiang-Ya School of Medicine, Central South University, Changsha, China. Seventy-nine Chinese healthy male volunteers aged 18–20 years were selected randomly in this open study after providing informed consent. All subjects were non-smokers and healthy as determined by medical history, physical examination and laboratory screening. They were required to refrain from taking any medication or alcohol for at least 2 weeks prior to the start of the study and during the course of the investigation.
Chemicals
Omeprazole was purchased from Dinuo Pharmaceuticals Inc. (Changsha, China). Caffeine (1,3,7X), paraxanthine (1,7X) and β-hydroxyethyl-theophylline (HT) (used as an internal standard) were all products of the Sigma Chemical Co. (St Louis, MO USA). The caffeine administered (200 mg per capsule) was kindly provided by the Hunan Pharmaceutical Company (Changsha, China). All of the restriction endonucleases, primers and reagents used in the genotyping analysis were supplied by Sangon Inc. (Shanghai, China).
Genotyping analysis
All volunteers took part in this test. Genomic DNA was extracted from white cells from peripheral venous blood. PCR-RFLP was used to identify the genotypes of CYP2C19 [9] and CYP1A2 [7] as described previously. Twelve subjects with particular genotypes were enrolled in the study; they were six CYP2C19*1 homozygotes with the CYP1A2*1F/CYP1A2*1F genotype and six CYP2C19*1 homozygotes with the CYP1A2*1C/CYP1A2*1F genotype.
Study protocol
Omeprazole, 120 mg day−1 (three 20 mg capsules twice daily, at 08.00 h and 20.00 h), was given to the subjects from day 2 to day 8 [11, 12]. On days 1, 9 and 16 blood was collected at 6 h after oral administration of caffeine for the analysis of drug (1,3,7X) and its metabolite paraxanthine (1,7X).
Analysis of 1,7X and 1,3,7X
Plasma samples were extracted with chloroform and isopropanol (9 : 1), following the addition of internal standard HT, and the concentrations of 1,7X and 1,3,7X were determined using a Hewlett-Packard 1050 series h.p.l.c. with a reversed-phase column (Spherisorb ODS-2, 250 mm × 4 mm i.d., 5 µm particle size); a mobile phase of methanol : acetonitrile: 0.05% acetic acid (12 : 8 : 80) at a flow rate of 1 ml min−1, a column temperature of 40 °C and a detection wavelength of 282 nm [3]. The retention times of 1,7X, HT and 1,3,7X were 5.5, 6.8 and 9.8 min, respectively. No endogenous interfering peaks were found in the blank samples. The 1,7X and 1,3,7X standard curves were linear over the range of 1–100 µm, with correlation coefficients of 0.999 and 0.997, respectively. Recovery of 1,7X and 1,3,7X ranged from 94% to 104%. The coefficients of variation for the intraday and interday reproducibility for 1,7X and 1,3,7X were less than 10%. The method had a limit of detection of 0.1 µm both for 1,7X and 1,3,7X.
Data analysis
The ratio of 1,7X/1,3,7X was used as an index of CYP1A2 activity in vivo. The degree of induction of CYP1A2 by omeprazole was expressed as the change in the ratios of 1,7X/1,3,7X from day 1 to day 9 (day 9/day 1) or from day 9 to day 16 (day 9/day 16). The Mann–Whitney U-test and Wilcoxon non-parametric test, were used with the level of significance set at P < 0.05. Values were expressed as mean ± s.d.
Results
Frequencies of CYP1A2 and CYP2C19 alleles
The frequencies of the CYP2C19*1, CYP2C19*2 and CYP2C19*3 alleles in the 79 subjects were 0.60, 0.37 and 0.03, respectively. The frequencies of the CYP1A2*1C and CYP1A2*1F alleles were 0.22 and 0.66, respectively. Six genotypes were identified with respect to the CYP1A2*1C and CYP1A2*1F genetic polymorphisms. No CYP2C19*3 homozygotes were detected.
Induction of CYP1A2 activity by omeprazole
The ratio of 1,7X/1,3,7X of six subjects with the CYP1A2*1F/CYP1A2*1F genotype increased significantly (P = 0.004) after 7 days omeprazole treatment (day 9), and returned to the baseline value at the end of the 1 week washout period (day 16) (Figure 1). However, the ratio did not change significantly in the six CYP1A2*1C/CYP1A2*1F genotypes.
Figure 1.
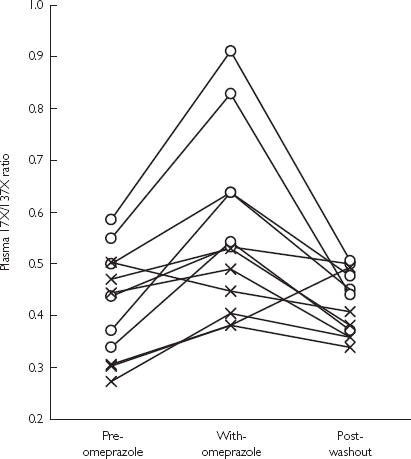
Influence of 7 days of omeprazole treatment on the plasma caffeine metabolic ratio (1,7X/1,3,7X). ○: CYP1A2*1F/CYP1A2*1F genotypes, n = 6; ×: CYP1A2*1C/CYP1A2*1F genotypes, n = 6.
There was a significant difference (P = 0.002) between the ratios for the CYP1A2*1F/CYP1A2*1F and CYP1A2*1C/CYP1A2*1F genotypes on day 9, but not on day 1or day 16 (P > 0.05) (Table 1).
Table 1.
Influence of 7 days of omeprazole treatment on the plasma caffeine metabolic ratio (1,7X/1,3,7X).
| % change | 95% CI on the difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | n | Dose of omeprazole | Day 1 | 1,7X/1,3,7X Day 9 | Day 16 | Day 9/Day 1 | Day 9/Day 16 | Day 16/Day 1 | Day 9/Day 1 | Day 9/Day 16 |
| CYP1A2*1F/CYP1A2*1F | 6 | 120 mg day−1 | 0.46 ± 0.10a | 0.68 ± 0.16 | 0.46 ± 0.05a | 48 ± 20b | 50 ± 31b | 2 ± 19 | 22, 71 | −1, 97 |
| CYP1A2*1C/CYP1A2*1F | 6 | 120 mg day−1 | 0.38 ± 0.10 | 0.44 ± 0.06c | 0.39 ± 0.06 | 19 ± 20d | 15 ± 22d | 8 ± 34 | −16, 37 | −51, 64 |
Data are mean ± s.d.
P < 0.05, compared with day 9
P < 0.05, compared with the % change between day 16/day 1
P < 0.05
P < 0.01 compared with that for CYP1A2*1F/CYP1A2*1F genotypes.
The differences in the ratios between day 9 and day 1 were 48% ± 20% and 19% ± 20% for the CYP1A2*1F/CYP1A2*1F and CYP1A2*1C/CYP1A2*1F genotypes, respectively. This difference between genotypes was statistically significant (P = 0.025), as was that for the changes between day 9 and day 16 (50% ± 31% vs 15% ± 22%, P = 0.048). There was no significant difference (P > 0.05) between the CYP1A2 genotypes with respect to the changes between day 16 and day 1 (2% ± 19% vs 8% ± 34%).
Discussion
The frequencies of the CYP1A2 and CYP2C19 alleles in our study were similar to those in previous reports [4–6, 9]. Since CYP1A2 activity increases in EMs with respect to CYP2C19 with high dose (120 mg day−1) omeprazole, but not with 40 mg day−1 [11, 12], CYP2C19*1 homozygotes, but not CYP2C19*1/CYP2C19*2 or CYP2C19*1/CYP2C19*3 heterozygotes, were selected for further investigation to minimize the influence of CYP2C19 gene dose on the plasma concentration of omeprazole. The results supported a role for the CYP1A2*1C and CYP1A2*1F genetic polymorphisms in the inducibility of CYP1A2 in EMs with respect to CYP2C19, who were administered omeprazole.
Transcriptionally mediated enzyme induction of omeprazole is believed to occur because of increased expression of hepatic CYP1A2 mRNA [13]. Our study also supported this hypothesis since induction by omeprazole differed between the CYP1A2 genotypes. Whether the mechanism involved in the induction of CYP1A2 activity by omeprazole is the same as that occurring in cigarette smokers remains to be elucidated. Since varia-tion was observed in the ratios for the CYP1A2*1F/CYP1A2*1F and CYP1A2*1C/CYP1A2*1F genotypes (see Table 1), the induction by omeprazole might be related to factors other than the CYP1A2*1C and CYP1A2*1F genetic polymorphisms.
When a drug can induce a specific CYP gene, the metabolism of other coadministered compounds may be altered, resulting in a change in response. In this study, we studied induction caused by omeprazole in 12 EMs with respect to CYP2C19 with different genotypes for CYP1A2. The induction by omeprazole, 120 mg day−1, on CYP1A2 expression in EMs with respect to CYP2C19 is close to that observed with 40 mg day−1 in PMs with respect to CYP2C19, each phenotype show-ing similar plasma concentration of omeprazole in vivo [11, 12]. Using the plasma caffeine ratio as an index of CYP1A2 activity, the EMs exhibited an increase of between −11% and 60% which was similar to the results (4.2%∼48.7%) reported by Rost et al. who used the urinary caffeine index [12]. Thus, when omeprazole and substrates of CYP1A2 are coadministrated, we would not expect clinically relevant drug interactions in EMs with respect to CYP2C19, as long as usual therapeutic doses are used [14]. However, induction of CYP1A2 may be of importance in CYP2C19 PMs.
Acknowledgments
This project was supported by the National Natural Science Foundation of China, F30130210 and by China Medical Board of New York grants 99–697.
References
- 1.Xu ZH, Zhou HH. Cytochrome P450 1A2 in drug metabolism. J Clin Pharmacol. 1996;12:115–121. [Google Scholar]
- 2.Han XM, Zhou HH. Polymorphism of CYP450 and cancer susceptibility. Acta Pharmacol Sin. 2000;21:673–679. [PubMed] [Google Scholar]
- 3.Ouyang DS, Huang SL, Wang W, Xie HG, Xu ZH, Zhou HH. Phenotypic polymorphism and gender-related differences of CYP1A2 activity in a Chinese population. Br J Clin Pharmacol. 2000;49:145–151. doi: 10.1046/j.1365-2125.2000.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima M, Yokoi T, Mizutani M, Kinoshita M, Funayama M, Kamataki T. Genetic polymorphism in the 5′-flanking region of human CYP1A2 gene: Effect on the CYP1A2 inducibility in humans. J Biochem (Tokyo) 1999;125:803–808. doi: 10.1093/oxfordjournals.jbchem.a022352. [DOI] [PubMed] [Google Scholar]
- 5.Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C¡úA polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–449. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chida M, Yokoi T, Fukui T, Kinoshita M, Yokota J, Kamataki T. Detection of three genetic polymorphisms in the 5′-flanking region and intron 1 of human CYP1A2 in the Japanese population. Jpn J Cancer Res. 1999;90:899–902. doi: 10.1111/j.1349-7006.1999.tb00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han XM, Chen XP, Wu QN, Jiang CH, Zhou HH. G-2964A and C734A genetic polymorphisms of CYP1A2 in Chinese population. Acta Pharmacol Sin. 2000;21:1031–1034. [PubMed] [Google Scholar]
- 8.Han XM, OuYang DS, Lu PX, et al. Plasma caffeine metabolite ratio (17X/137X) in vivo associated with G-2964A and C734A polymorphisms of human CYP1A2. Pharmacogenetics. 2001 doi: 10.1097/00008571-200107000-00006. in press. [DOI] [PubMed] [Google Scholar]
- 9.Shu Y, Zhou HH. Individual and ethnic differences in CYP2C19 activity in Chinese populations. Acta Pharmacol Sin. 2000;21:193–199. [PubMed] [Google Scholar]
- 10.Andersson T, Regardh CG, Dahl-Puustinen ML, Bertilsson L. Slow omeprazole metabolizers are also poor S-mephentoin hydroxylators. Ther Drug Monit. 1990;12:415–416. doi: 10.1097/00007691-199007000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Rost KL, Brosicke H, Heinemeyer G, Roots I. Specific and dose-dependent enzyme induction in human beings. Hepatology. 1994;20:1204–1212. [PubMed] [Google Scholar]
- 12.Rost KL, Roots I. Accelerated caffeine metabolism after omeprazole treatment is indicated by urinary metabolite ratios: Coincidence with plasma clearance and breath test. Clin Pharmacol Ther. 1994;55:402–411. doi: 10.1038/clpt.1994.49. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson A, Hurwitz A. Omeprazole and the induction of human cytochrome P-450: a response to concerns about potential adverse effects. Gastroenterology. 1991;100:1157–1164. doi: 10.1016/0016-5085(91)90317-e. [DOI] [PubMed] [Google Scholar]
- 14.Sarich T, Kalhorn T, Magee S, et al. The effect of omeprazole pretreatment on acetaminophen metabolism in rapid and slow metabolizers of S-mephenytoin. Clin Pharmacol Ther. 1997;62:21–28. doi: 10.1016/S0009-9236(97)90148-X. [DOI] [PubMed] [Google Scholar]


