Bacterial brain infections represent life threatening events, often unresponsive to standard antibiotic treatment. Whereas therapeutic failure might be caused by infections with multiresistant bacteria, the central nervous system (CNS) differs from other infectious loci, since the infection site, i.e. brain parenchyma, is secluded from the rest of the body by the blood brain barrier (BBB), which renders the brain impermeable to many exogenous and endogenous compounds. Therefore, success of antibiotic therapy is also dependent on the ability of antibiotics to cross the BBB and penetrate the CNS.
Unfortunately, our knowledge about antibiotic CNS penetration in patients is rudimentary and has so far been restricted to single point measurements in brain tumours, intracranial abscesses, or the cerebrospinal fluid (CSF) [1]. Recently, clinical microdialysis (MD) was suggested as a novel method to measure directly antibiotic brain concentrations humans [2]. MD has evolved as an important instrument for the measurement of effect site concentrations of various analytes in different clinical settings, including neurometabolic monitoring in intensive care units [3]. However, the CNS penetration and intracerebral pharmacokinetics of drugs have not yet been routinely monitored [4]. For antibiotics in particular, MD would allow definition of the relationships between effect site pharmacokinetics, namely, those concentrations to which pathogens are directly exposed and clinical re-sponse, with possible consequences for current dosage recommendations.
To describe plasma to brain distribution of fosfomycin, a broad spectrum antibiotic with reported favourable penetration characteristics to peripheral tissues [5] and the CSF [6], MD experiments were conducted in three patients. These were a 28 year-old woman with left frontal intracerebral haematoma, a 22 year-old man after severe head injury and cerebral contusions and a 65-year-old woman with subarachnoid bleeding (SAB) and intracerebral haemorrhage. They all needed neurosurgical intensive care treatment and received prophylactic antibiotic medication. The study was approved by the local ethics committee and was performed in accordance with the Declaration of Helsinki and the GCP Guideline of the EC. The study met the criteria set by the ethics committee for patients who are unable to give consent because of incapacity.
Fosfomycin concentrations in brain interstitium were measured employing commercially available microdialysis brain catheters (CMA 70, CMA, Sweden) after implantation into brain parenchyma of the noninjured frontal lobe in combination with a routinely used ventricular catheter (NMT Neurosciences Implants S.A. Sophia Antipolis cedex, France). The MD catheter was placed with its tip in the white matter, and the system was perfused with Ringer's solution at a flow rate of 1.5 µl min−1. After equilibration and baseline sampling, 4 g fosfomycin (Fosfomycin®, Biochemie GmbH, Vienna, Austria) were administered as an i.v. bolus via a central venous catheter. Fosfomycin from dialysates and arterial blood samples, collected in defined intervals up to 6 h, was analysed by gas chromatography [7]. Intracerebral concentrations were calculated from dialysate concentrations as described previously [5]. The ratio AUCplasma/AUCbrain was calculated as a measure of CNS penetration.
In patient 3 intracerebral fosfomycin concentrations could not be quantified. At first sight no definite explanation for this finding could be identified, as the MD catheter was inserted into the noninjured hemisphere. However, transcranial Doppler ultrasound recordings of both middle cerebral arteries indicated severe vasospasm, known to occur frequently after SAB, and a CT scan performed 2 weeks later showed multiple infarctions of both hemispheres in the middle and anterior cerebral artery territory. The patient died 4 weeks after admission with signs of ventriculitis and after having developed refractory intracranial hypertension and cerebral oedema.
Figure 1 depicts fosfomycin concentration vs time profiles for plasma and brain interstitium for the other two patients. AUCplasma/AUCbrain ratios were 4.8 and 13.3 for patient 1 and 2, respectively. Plasma and brain Cmax values were 606 and 42 µg ml−1 (patient 1) and 244 and 12 µg ml−1 (patient 2). Patient 1 died from ARDS and septicaemia 10 days after admission, without signs of CSF or cerebral infection. Follow-up of patient 2 was unremarkable.
Figure 1.
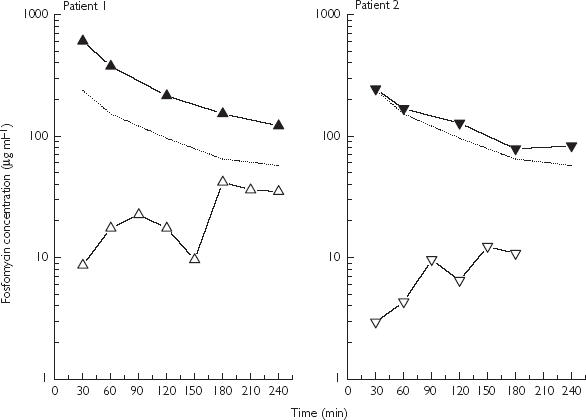
Fosfomycin concentration vs time profiles for plasma (▴) and brain tissue (▵) following i.v. bolus administration of 4 g fosfomycin to two neurosurgical patients. In the third patient fosfomycin could not be quantified. (The dotted line indicates estimated fosfomycin concentrations in a peripheral tissue based on data from Frossard et al. [5]).
In the present study BBB transport of fosfomycin was variable. Compared with the almost complete equilibration between plasma and peripheral tissues concentrations [5] (Figure 1, dotted line), interstitial brain concentrations did not equilibrate with corresponding plasma concentrations. However quantifiable cerebral Cmax values were above MIC values for relevant pathogens such as Streptococcus pneumoniae or Neisseria meningitidis. Variability in plasma/brain penetration ratios might be explained by the degree to which the integrity of the BBB is disrupted by the underlying disease [8]. Since BBB permeability is not predictable, current dosing is usually based on CSF sampling or empirical data. However, CSF concentrations might differ from actual brain tissue concentrations due to a ventricular-lumbar concentration gradient [1]. Therefore, direct access to the effect site, as provided by clincial MD, is mandatory to relate accurately target site concentrations to clinical effect. Only the patient with cerebral vasospasm, and thus a dramatically reduced capillary exchange surface developed an intracerebral infection. Thus it might be hypothesized that in addition to BBB transport or oedema formation associated with infraction, which would reverse the pressure gradient and the bulk flow of a molecule, the function of the capillary surface area and capillary density might profoundly influence drug pharmacokinetics within human brain parenchyma.
References
- 1.Nau R, Sörgel F, Prange HW. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin Pharmacokinet. 1998;35:223–246. doi: 10.2165/00003088-199835030-00005. [DOI] [PubMed] [Google Scholar]
- 2.Mindermann T, Zimmerl W, Gratzl O. Rifampin concentrations in various compartments of the human brain: a novel method for determining drug levels in the cerebral extracellular space. Antimicrob Agents Chemother. 1998;42:2626–2629. doi: 10.1128/aac.42.10.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson OG, Brandt L, Ungerstedt U, Saveland H. Bedside detection of brain ischemia using intracerebral microdialysis: subarachnoid hemorrhage and delayed ischemic deterioration. Neurosurgery. 1999;45:1176–1184. doi: 10.1097/00006123-199911000-00032. [DOI] [PubMed] [Google Scholar]
- 4.Hammarlund-Udenaes M. The use of microdialysis in CNS drug delivery studies. Pharmacokinetic perspectives and results with analgesics and antiepileptics. Adv Drug Deliv Rev. 2000;45:283–294. doi: 10.1016/s0169-409x(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 5.Frossard M, Joukhadar C, Erovic B, et al. Distribution and antimicrobial activity of fosfomycin in the interstitial space fluid of human soft tissues. Antimicrob Agents Chemother. 2000;44:2728–2732. doi: 10.1128/aac.44.10.2728-2732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeifer G, Frenkel C, Entzian W. Pharmacokinetic aspects of cerebrospinal fluid penetration of fosfomycin. Int J Clin Pharmacol Res. 1985;5:171–174. [PubMed] [Google Scholar]
- 7.Dios-Vieitez MC, Goñi MM, Renedo MJ, Fos D. Determination of fosfomycin in human urine by capillary gas chromatography: Application to clinical pharmacokinetic studies. Chromatographia. 1996;43:293–295. [Google Scholar]
- 8.Paradridge WM. Brain drug transport and blood–brain-barrier transport. Drug Delivery. 1993;1:83–101. [Google Scholar]


