Abstract
Aims
To study the effects of electrostatics in a plastic spacer on the lung deposition of salbutamol in asthmatic children.
Methods
Twenty-five children (5–12 years) with mild asthma were given salbutamol hydrofluoroalkane pressurized metered dose inhaler 400 µg via a 750 ml plastic spacer on separate days. Blood samples were taken for plasma salbutamol at 5, 10, 15 and 20 min after inhalation to measure lung bioavailability as a surrogate for relative lung dose. With immediate inhalation following actuation, a new rinsed spacer (NewRinsed) was compared with a used spacer after repeated daily use (Used), a spacer rinsed after repeated use (UsedRinsed) and a spacer primed with benzalkonium chloride to avoid electrostatics (Primed1). In addition, spacers were evaluated using a 15 s inhalation delay following actuation with primed (PrimedDelay) and rinsed (RinsedDelay) spacers. Data were log transformed and expressed as geometric mean fold difference for the average plasma salbutamol concentration (Cav) over 20 min.
Results
There were significant differences (P < 0.05) in Cav (as geometric mean fold difference and 95% CI) between Primed1 vs NewRinsed 1.92 fold (95% CI 1.15, 3.20) and between Used vs NewRinsed 1.75 fold (1.11, 2.76). There were no significant differences comparing Primed1, Used or UsedRinsed. There were also significant differences (P < 0.05) between Primed1 vs PrimedDelay 2.34 fold (1.31, 4.19), or vs RinsedDelay 3.59 fold (2.15, 5.99); and for Used vs PrimedDelay 2.14 fold (1.24, 3.69), or vs RinsedDelay 3.28 fold (2.13, 5.04).
Conclusions
The relative lung dose of salbutamol from a plastic spacer may differ considerably depending on spacer handling suggesting that nonelectrostatic spacers may be the best way forward.
Keywords: asthma, children, electrostatics, lung dose, plastics, salbutamol, spacer
Introduction
The pressurized metered dose inhaler (pMDI) in combination with a spacer device is commonly used in paediatrics for aerosol drug treatment. This combination of devices facilitates a high lung dose and a low extra-pulmonary dose as compared with pMDI alone or other devices such as dry powder inhalers and nebulisers [1]. The spacer eliminates the need for co-ordination between actuation of the pMDI and inhalation and may therefore together with a facemask be adapted for young children, toddlers and even infants [1]. However, a range of devices and patient related confounders influences the relationship between prescribed dose and that delivered to the lung from a pMDI and spacer. Device related factors comprise the pMDI characteristics as well as spacer design, including spacer volume, size, valve system, dead space and material [1, 2].
Electrostatic charges present in plastic spacers have been shown to reduce drug delivery by several fold in vitro [3] and in vivo [4, 5]. Most importantly this electrostatic effect varies depending on how the spacer is handled. It was the primary objective of the present study to estimate the variability in dose reaching the lung in asthmatic children that is attributable to the day-to-day handling technique of a plastic spacer.
Methods
Patients
Subjects with stable mild asthma and unchanged prophylactic asthma therapy for 1 month prior to inclusion were sought among 5 to 12 year-old children from our outpatient clinic. All patients selected were familiar with spacer inhalations. Written informed consent to participate in the study was obtained from a parent or legal guardian. The child gave a verbal assent. The Danish National Board of Health and the Ethics Committee of Copenhagen approved the study.
Study design
The study was of a single (laboratory) blind, 7-way crossover design. The patients inhaled 4 × 100 µg salbutamol from pMDI via a large volume plastic spacer on 7 study days. Except for the benzalkonium primed spacer, which was shared by all patients, each patient had his or her own spacer. Pre-treatment of the spacer differed on the 7 study days. A new spacer was rinsed in tap water and allowed to air dry before use (NewRinsed). After first use the new spacer was used for another 5 days with 2 puffs each morning and evening (Used). The used spacer was then rinsed in lukewarm water and allowed to air dry before next use (UsedRinsed). A new spacer was primed by immersing three times in a solution of 0.05% benzalkonium chloride and allowed to air dry (Primed1). Inhalation with the primed spacer was repeated on another study day (Primed2). Immediate inhalation following actuation was performed with all the above spacers. In addition, spacers were evaluated using a 15 s inhalation delay following actuation with primed (PrimedDelay) and rinsed (RinsedDelay) spacers.
Methodology
HFA salbutamol (Ventolin Evohaler, GlaxoSmithKline, Uxbridge, UK) was administered as four inhalations each of 100 µg via a 750 ml plastic spacer with a single one-way valve (Volumatic®, GlaxoSmithKline, Uxbridge, UK). The patients were instructed to inhale through the mouthpiece breathing normally while using a nose clip.
Before and at 5, 10, 15 and 20 min after the end of drug administration, blood samples of 5 ml for determination of salbutamol in plasma were obtained. Plasma salbutamol was assessed by high performance liquid chromatography (h.p.l.c.). The extraction process used silica absorption with chromatography followed by reverse phase ion pair h.p.l.c. and electrochemical detection. The lower limit of quantification (LOQ) was 0.2 ng ml−1. The analytical imprecision (at 5 ng ml−1) was 7.8% (intra-assay) and 6.7% (interassay).
Statistical analysis
The average concentration (Cav) was calculated for each patient and spacer. If all four samples after inhalation were below the LOQ, Cav was set to this value (0.2 ng ml−1). Results were log transformed before statistical analysis by anova and pairwise Bonferroni comparisons between spacers. Geometric mean fold differences with 95% confidence intervals (95% CI) were calculated for each pair.
Results
Twenty-five patients were included, and a total of 126 out of 175 planned visits were completed. The missing visits were due to difficulties with repeated venepuncture. Sixteen patients completed all seven visits. No adverse events were reported. The plasma concentration of salbutamol was below the LOQ in all samples taken before drug administration.
The mean time profiles over 20 min for each spacer and the scatterplot for Cav are shown in Figures 1 and 2.
Figure 1.

Geometric mean plasma salbutamol concentration-time profile after inhalation of 400 µg of HFA salbutamol pMDI from a 750 ml plastic spacer on 7 different days (see text for details on spacer handling characteristics). •Primed 1; ○PrimedDelay; ▾Used; ▿UsedRinsed; ▪RinsedDelay; □Primed 2; ♦NewRinsed.
Figure 2.
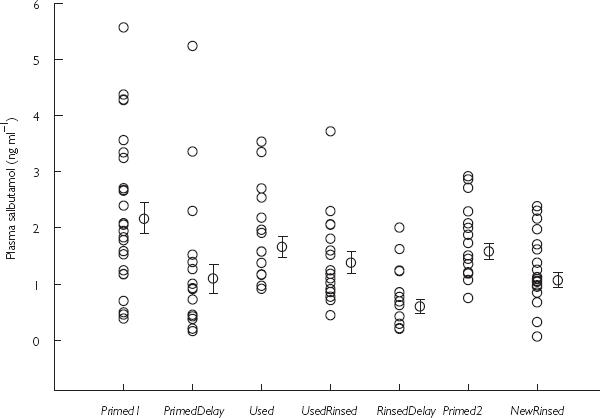
Scatterplot with geometric mean and s.e mean showing individual average plasma salbutamol concentration over the first 20 min after inhalation of 400 µg HFA salbutamol pMDI from a plastic spacer on 7 different days (see text for details).
Comparison between Primed1 and Primed2 was not statistically significant with a 1.12 fold (95% CI 0.72, 1.75) difference. Primed1 gave a significantly higher Cav (P < 0.05) than NewRinsed, amounting to a 1.92 fold (1.15, 3.20) difference. Comparisons between Used and NewRinsed showed a statistically significant difference (P < 0.05) in Cav of 1.75 fold (1.11, 2.76).
There were no significant differences in Cav between Primed1 vs Used: 1.10 fold (0.71, 1.69), Primed1 vs UsedRinsed: 1.42 fold (0.89, 2.25), or Used vs UsedRinsed: 1.29 fold (0.91, 1.85).
Primed1 produced significantly greater Cav (P < 0.05) vs PrimedDelay: 2.34 fold (1.31, 4.19), or vs RinsedDelay: 3.59 fold (2.15, 5.99); and for Used vs PrimedDelay: 2.14 fold (1.24, 3.69), or vs RinsedDelay: 3.28 fold (2.13, 5.04). Cav was significantly greater (P < 0.05) for NewRinsed vs RinsedDelay: 1.87 fold (1.07, 3.26), and for UsedRinsed vs RinsedDelay: 2.53 fold (1.57, 4.08).
Discussion
This study shows that the dose of salbutamol delivered to the lung in asthmatic school children may differ considerably depending on day-to-day handling of a large volume plastic spacer. For example, the largest difference amounted to a 3.59 fold difference (i.e. a 72% difference) when comparing a benzalkonium primed spacer with immediate inhalation and a rinsed spacer with delayed inhalation. Lung deposition differed by 2.34 fold (i.e. a 57% difference) when the aerosol was inhaled from a benzalkonium primed spacer comparing immediate vs delayed inhalation. This might conceivably reflect real life situations in young children with low tidal volumes or poor compliance. We did not observe any significant differences between spacers which were primed with benzalkonium chloride or rinsed with water after repeated use, suggesting that ordinary rinsing may be adequate for reducing electrostatic charge.
The effect of a delay between actuation and inhalation illustrates the influence of electrostatics on the passive fall-out of aerosol in the spacer. Electrostatic attraction causes a continuous and rapid disappearance of the aerosol [1]. In plastic spacers, the aerosol half-life is typically 10 s, compared with 30 s if the static charge is abolished [3, 6, 7]. The aerosol is entrained during emptying of the spacer and the inspiratory volume required to obtain the full dose is several fold the spacer volume. If the aerosol has a short half-life, the respiratory pattern of the patient including tidal volume and respiratory frequency affects the inhaled dose. The present study extends our previous in vivo study showing a 50% difference in lung deposition after inhalation from a primed vs an unprimed plastic spacer in schoolchildren [4] and reduced lung deposition after delayed inhalation in adults [8].
Scintigraphic measurements of lung deposition after inhalation of radio labelled aerosols are common in adult studies and have been used in some paediatric studies [5, 9]. However, repeated use of radioactive drugs for research purposes is not feasible. Conventional pharmacokinetic methods measuring the complete plasma profile give reliable estimates of absolute lung dose, provided the dose absorbed from the gut is accounted for using oral activated charcoal. The early plasma salbutamol profile is not confounded by systemic absorption from the gut, but only provides data on relative lung deposition [10].
The lack of relationship between the prescribed dose to children and that delivered to the lung is important since it partially obscures clinical outcome. This hampers the ability of the clinician to navigate and titrate the dose from clinical response. It seems obvious that manufacturers of spacer devices should use only nonelectrostatic materials in the future. Metal is an obvious, robust and safe choice for a nonelectrostatic spacer [3, 11] since it carries no charge, no matter how it is handled, and requires no chemical treatment. For example, in one study in adults evaluating the early plasma salbutamol profile, there was a 44% difference in lung dose (measured as Cav) between a 250 ml metal spacer compared with a reservoir dry powder inhaler [12].
In conclusion, the dose received by the lung from plastic spacers may vary considerably by up to 72% depending on day to day handling, especially when inhalation is delayed. This suggests that nonelectrostatic spacers should supercede plastic devices.
Acknowledgments
This study was supported by a joint departmental research grant from Universities of Dundee and Copenhagen and received no support from the pharmaceutical industry. None of the authors has any conflicts of interest.
References
- 1.Bisgaard H. Delivery of inhaled medication to children. J Asthma. 1997;34:443–467. doi: 10.3109/02770909709055389. [DOI] [PubMed] [Google Scholar]
- 2.Zak M, Madsen J, Berg E, Bulow J, Bisgaard H. A mathematical model of aerosol holding chambers. J Aerosol Med. 1999;12:187–196. doi: 10.1089/jam.1999.12.187. [DOI] [PubMed] [Google Scholar]
- 3.Bisgaard H, Anhøj J, Klug B, Berg E. A non-electrostatic spacer for aerosol delivery. Arch Dis Child. 1995;73:226–230. doi: 10.1136/adc.73.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anhøj J, Bisgaard H, Lipworth BJ. Effect of electrostatic charge in plastic spacers on the lung delivery of HFA-salbutamol in children. Br J Clin Pharmacol. 1999;47:333–336. doi: 10.1046/j.1365-2125.1999.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildhaber JH, Janssens HM, Pierart F, Dore ND, Devadason SG, LeSouef PN. High-percentage lung delivery in children from detergent-treated spacers. Pediatr Pulmonol. 2000;29:389–393. doi: 10.1002/(sici)1099-0496(200005)29:5<389::aid-ppul8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Wildhaber JH, Devadason SG, Hayden MJ, et al. Electrostatic charge on a plastic spacer device influences the delivery of salbutamol. Eur Respir J. 1996;9:1943–6. doi: 10.1183/09031936.96.09091943. [DOI] [PubMed] [Google Scholar]
- 7.Berg E, Madsen J, Bisgaard H. In vitro performance of three combinations of spacers and pressurized metered dose inhalers for treatment in children. Eur Respir J. 1998;12:472–476. doi: 10.1183/09031936.98.12020472. [DOI] [PubMed] [Google Scholar]
- 8.Clark DJ, Lipworth BJ. Effect of multiple actuations, delayed inhalation and antistatic treatment on the lung bioavailability of salbutamol via a spacer device. Thorax. 1996;51:981–984. doi: 10.1136/thx.51.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zar HJ, Weinberg EG, Binns HJ, Gallie F, Mann MD. Lung deposition of aerosol – a comparison of different spacers. Arch Dis Child. 2000;82:495–498. doi: 10.1136/adc.82.6.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindle M, Chrystyn H. Relative bioavailability of salbutamol to the lung following inhalation using metered dose inhalation methods and spacer devices. Thorax. 1994;49:549–553. doi: 10.1136/thx.49.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisgaard H. A metal aerosol holding chamber devised for young children with asthma. Eur Respir J. 1995;8:856–860. [PubMed] [Google Scholar]
- 12.Lipworth BJ, Clark DJ. Lung delivery of salbutamol by dry powder inhaler (Turbuhaler) and small volume antistatic metal spacer (Airomir CFC-free MDI plus NebuChamber) Eur Respir J. 1997;10:1820–1823. doi: 10.1183/09031936.97.10081820. [DOI] [PubMed] [Google Scholar]


