Abstract
Aims To investigate the absorption, distribution and elimination of ethanol in women with abnormal gut as a result of gastric bypass surgery. Patients who undergo gastric bypass for morbid obesity complain of increased sensitivity to the effects of alcohol after the operation.
Methods Twelve healthy women operated for morbid obesity at least 3 years earlier were recruited. Twelve other women closely matched in terms of age and body mass index (BMI) served as the control group. After an overnight fast each subject drank 95% v/v ethanol (0.30 g kg−1 body weight) as a bolus dose. The ethanol was diluted with orange juice to 20% v/v and finished in 5 min. Specimens of venous blood were taken from an indwelling catheter before drinking started and every 10 min for up to 3.5 h post-dosing. The blood alcohol concentration (BAC) was determined by headspace gas chromatography.
Results The maximum blood-ethanol concentration (Cmax) was 0.741 ± 0.211 g l−1 (± s.d.) in the operated group compared with 0.577 ± 0.112 g l−1 in the controls (mean difference 0.164 g l−1, 95% confidence interval (CI) 0.021, 0.307). The median time to peak (tmax) was 10 min in the bypass patients compared with 30 min in controls (median difference −15 min (95% CI −10, −20 min). At 10 and 20 min post-dosing the BAC was higher in the bypass patients (P < 0.05) but not at 30 min and all later times (P > 0.05). Other pharmacokinetic parameters of ethanol were not significantly different between the two groups of women (P > 0.05).
Conclusions The higher sensitivity to ethanol after gastric bypass surgery probably reflects the more rapid absorption of ethanol leading to higher Cmax and earlier tmax. The marked reduction in body weight after the operation might also be a factor to consider if the same absolute quantity of ethanol is consumed.
Keywords: absorption, ethanol, gastric bypass, gut, obesity, pharmacokinetics, women
Introduction
Gastric bypass surgery is the most efficient means of treating morbid obesity in individuals with a body mass index (BMI) over 40 kg m−2[1–5]. This operation has become the most commonly performed procedure in bariatric surgery [3, 4]. A small pouch is formed by the upper part of the stomach and kept separate from the main part of the stomach. The pouch empties into the jejunum in a Roux-en-Y fashion [6, 7] and the resulting surgical anatomy resembles that of extended gastric resection for peptic ulcers and gastrectomy procedures for neoplastic diseases.
Many of those who undergo gastric bypass surgery complain of an increased sensitivity or reduced tolerance to the effects of alcohol compared with their experience before the operation. This might be explained in two ways. First, the dramatic reduction in body weight (20–40% within 1–2 years) means that for the same number of drinks consumed the person receives a higher ethanol dose expressed per kilogram of body weight. Second, the rapid absorption of ethanol after the surgery would produce a higher Cmaxand earlier tmaxand more pronounced feelings of inebriation during and shortly after drinking [8, 9]. A more rapid absorption of ethanol and higher Cmaxafter obesity surgery could have ramifications when skilled tasks are performed, such as driving a motor vehicle [10]. The risk of causing a crash increases as blood alcohol concentration (BAC) increases and punishable alcohol concentration limits exist in most countries [11].
The aim of this study was to investigate the absorption, distribution and elimination kinetics of ethanol in healthy women after gastric bypass surgery for morbid obesity. Another group of women, who had not been operated on, served as controls and received the same dose of ethanol under otherwise identical conditions.
Methods
Subjects
Twelve women who had undergone gastric bypass surgery with mean age 40 years and mean body weight 75 kg volunteered for this study and 12 other healthy non-operated women mean age 40 years, mean body weight 78 kg served as controls. Their demographic details are presented and compared in Table 1. All women in the treatment group had reached a stable body weight 3 years or more after surgery. Both groups were confirmed to be in good health by questioning and laboratory blood screening tests. They were all non-smokers and considered themselves social drinkers and none took any medication at the time of the study. The study protocol was approved by the ethics review board at the University Hospital in Örebro, Sweden.
Table 1.
Comparison of subject demographics and pharmacokinetic parameters of ethanol in gastric bypass patients (operated group) and a control group of women. Values are mean ± s.d. except for time to peak (tmax) where medians and range are given. For definition of the parameters see Methods section.
| Parameter | Operated group (n = 12) | Control group (n = 12) | Mean difference (95% confidence interval) |
|---|---|---|---|
| Age (years) | 39.6 ± 7.6 | 40.3 ± 9.0 | −0.7 (−6.35, 7.75) |
| Body weight (kg) | 74.9 ± 8.48 | 78.1 ± 10.0 | −3.2 (−4.65, 11.05) |
| Height (m) | 1.67 ± 0.065 | 1.66 ± 0.032 | 0.01 (−0.033, 0.053) |
| BMI (kg m−2) | 26.9 ± 4.0 | 28.2 ± 3.76 | −1.3 (−1.98, 4.58) |
| BAC (g l−1) | |||
| 10 min | 0.713 ± 0.252 | 0.171 ± 0.138 | 0.542 (0.371, 0.715)1 |
| 20 min | 0.611 ± 0.120 | 0.471 ± 0.180 | 0.140 (0.010, 0.270)1 |
| 30 min | 0.508 ± 0.075 | 0.529 ± 0.150 | −0.022 (−0.124, 0.081) |
| Cmax (g l−1) | 0.741 ± 0.211 | 0.577 ± 0.112 | 0.164 (0.021, 0.307)1 |
| tmax (min) | 10 (10–20) | 30 (20–50) | −15 (−10, –20)1 |
| Co (g l−1) | 0.497 ± 0.073 | 0.516 ± 0.073 | −0.019 (−0.043, 0.081) |
| Estimated time to zero BAC (mino) | 211 ± 33.7 | 227 ± 9.8 | −16 (5, −37) |
| Rate of BAC decline (g l−1 h−1) | 0.144 ± 0.029 | 0.139 ± 0.029 | 0.005 (−0.019, 0.030) |
| Area under the curve (g l−1 min) | 56.1 ± 12.8 | 54.6 ± 10.2 | 1.5 (−8.3, 11.3) |
| Vd (l kg−1) | 0.616 ± 0.100 | 0.592 ± 0.087 | 0.024 (−0.055, 0.103) |
| Rate of elimination from body (g kg−1 h−1) | 0.087 ± 0.0136 | 0.081 ± 0.0127 | 0.006 (−0.004, 0.018) |
Statistically significant mean difference.
After an overnight fast the subjects received a standard dose of ethanol equivalent to 0.30 g kg−1 body weight. Pure ethanol (95% v/v) was mixed with orange juice to give a concentration of about 20% v/v, and was drunk in 5 min. This amount of ethanol corresponds to 220 ml table wine (12 vol%) for a person with body weight of 70 kg. Both groups received the drink in a supine position. Intake of food or other drink was not allowed during the experiment, which lasted for about 4 h.
Blood sampling and analysis of ethanol
Venous blood was obtained through an indwelling catheter before ethanol ingestion and every 10 min for 3.5 h after the start of drinking. Specimens were drawn into 5 ml Vacutainer tubes containing sodium fluoride (20 mg) and heparin (120 units). Aliquots of blood (100 µl) were removed from each tube and immediately diluted 1 in 10 with an aqueous solution of n-propanol (0.08 g l−1) as internal standard. The diluted blood specimens were transferred into 22 ml glass vials, which were made airtight with crimped-on aluminium caps in preparation for analysis by headspace gas chromatography as described in detail elsewhere [12]. The analytical precision of this method of analysis, expressed as coefficient of variation, was < 1% at a mean BAC of 0.80 g l−1.
Pharmacokinetics of ethanol
Concentration-time profiles of ethanol were constructed from 21 venous blood samples and these curves were evaluated as described by Mathews et al. (13). Pharmacokinetic parameters of ethanol were calculated assuming a one-compartment model with zero-order elimination after reaching the post-absorptive phase [14, 15]. The peak blood-alcohol concentration (Cmax) and the time of its occurrence tmax were noted. The rate constant for blood-alcohol concentration decline (ko) was obtained by linear regression analysis using selected concentration-time points. Each profile was inspected and only those data points on the post-absorptive declining phase were used in the calculations. The apparent volume of distribution of ethanol (Vd) was found by dividing the dose of ethanol given (0.30 g kg−1) by the y-intercept oftheregression line (Cextrapolated). The areas under the concentration-time profiles (AUC) were calculated by the linear trapezoidal method. The rate of elimination of ethanol from the whole body was calculated by dividing the dose given by the estimated time to reach zero BAC (min), which was also obtained by extrapolation (16).
Results
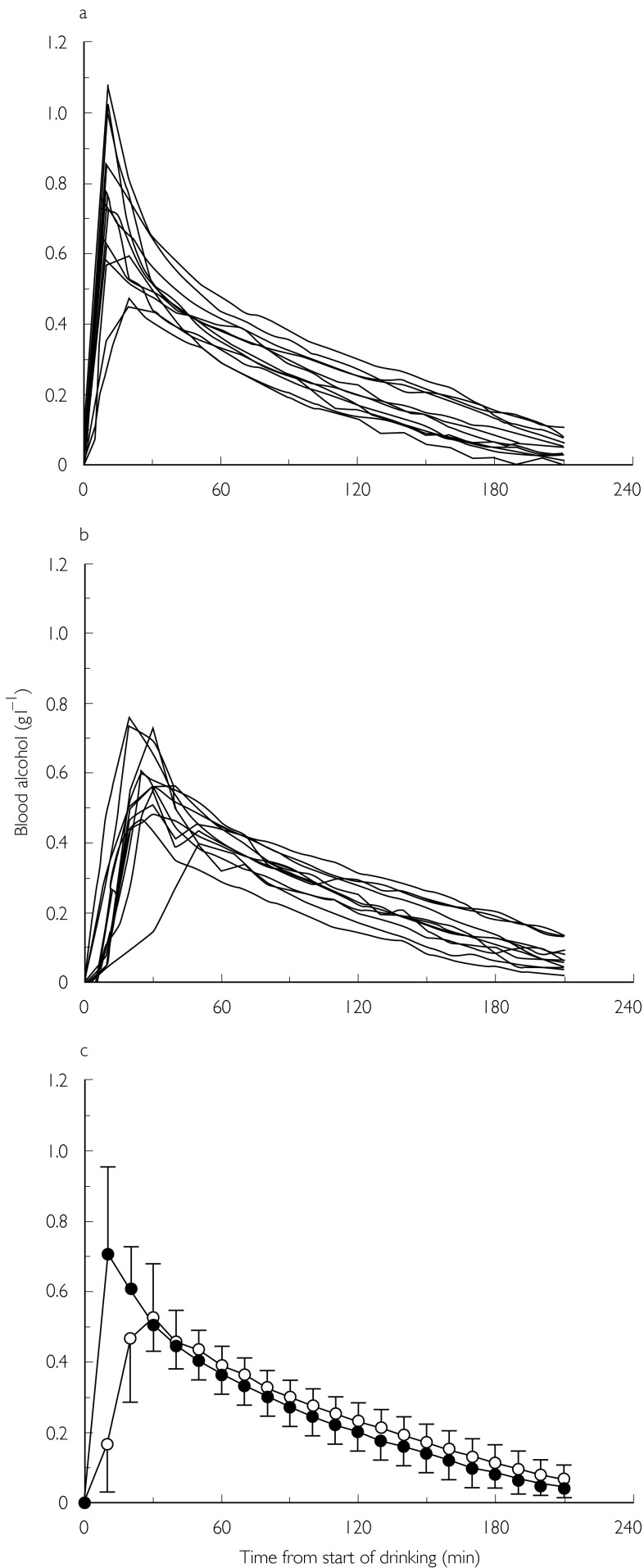
The individual BAC curves for the gastric bypass patients are shown in Figure 1a and the corresponding curves for the controls are shown in Figure 1b. Figure 1c compares the average BAC profiles for the two groups of women. A more rapid absorption of alcohol with higher Cmax and earliertmaxcan be seen for most of the bypass group.
Figure 1.
Concentration–time profiles of ethanol in women after gastric bypass surgery (a) and a control group (b). The bottom panel (c) shows mean curves (± s.d.) for the two groups of women (n = 12) after they drank ethanol (0.30 g kg−1 body weight) on an empty stomach (• operated, ○ control).
Table 1 compares demographic details of the subjects and pharmacokinetic parameters of ethanol between the groups. The mean BAC was higher at 10 and 20 min post-dosing in the bypass group (P < 0.05) but not at 30 min and all later times (P > 0.05). The mean Cmax was higher and tmax occurred earlier in the bypass patients (P < 0.05). Other parameters of ethanol kinetics were not significantly different in the bypass and control groups. The rate constant for blood-concentration decline (ko) was 0.144 ± 0.029 g l−1 h−1vs 0.139 ± 0.029 g l−1 h−1 and the apparent distribution volume (Vd) was 0.616 ± 0.100 l kg−1vs 0.592 ± 0.087 l kg−1, in the bypass and control groups, respectively.
Discussion
The absorption, distribution, and elimination kinetics of ethanol in humans have been studied extensively but to our knowledge controlled studies in subjects with abnormal gut after gastric bypass surgery have not been reported [16–18]. The present study was prompted by complaints from patients undergoing surgery for morbid obesity, who claimed they had become more sensitive to the effects of drinking alcohol. Because of the dramatic decrease in body weight 3 years after the operation, it was not practical to use subjects as their own controls. Instead we used a parallel group design with another group of women matched in terms of age and body mass index and who had not had this surgery.
The main finding from this study was a higher Cmax and earlier tmax in the bypass group of women. However, the BAC remained higher than controls only for 30 min post-dosing and thereafter concentrations in the control group were slightly higher than the bypass group. The small volume of the stomach after the operation, when the stomach pouch is only about 25 ml, and also the absence of normal stomach emptying probably accounts for the faster transfer of fluids into the jejunum and consequently an increased rate of alcohol absorption [8, 9]. Other pharmacokinetic parameters, such as the rate of blood-alcohol concentration decline, apparent volume of distribution and AUC were not significantly different between the two groups of women.
The higher Cmax and earlier tmax probably explains the enhanced feelings of inebriation after gastric bypass surgery [19]. Gastric emptying rate is a major determinant of alcohol absorption owing to the larger surface area available in the jejunum [16–18]. As a consequence of bypass surgery there is a higher Cmax and earlier tmax[15]. In some of the bypass subjects, the BAC-time profiles during the first 60 min post-drinking resembled those seen when ethanol was given intravenously [20]. After reaching Cmax the BAC drops precipitously, a phenomenon called a diffusion plunge, when alcohol distributes from the central (blood) compartment into the peripheral tissues, where most of the body water is present [9]. Even in some of the control group who have intact stomachs, absorption of ethanol occurred rapidly after drinking on an empty stomach after an overnight fast, which also facilitates rapid gastric emptying [8, 9, 15, 19].
The higher Cmax in bypass patients after drinking small amounts of alcohol could have negative consequences when skilled tasks are performed, such as driving a motor vehicle. The effects of alcohol on performance and behaviour are more pronounced when the BAC is rising and near Cmax[21]. Not infrequently BAC is back-calculated from the concentration at the time of blood sampling to an earlier time, such as that of a traffic accident [22, 23]. In retrograde calculations of this kind, factors influencing the rate and extent of absorption of alcohol are important to consider including an abnormal gut after gastric bypass or other surgery.
We evaluated concentration-time profiles of ethanol using a one-compartment model with zero-order elimination, the so called Widmark method [14, 15]. This model is intuitive and has proven itself over many years, especially when alcohol is taken as a bolus dose on an empty stomach [15–17]. When comparing two groups of subjects or two treatments, we feel that the Widmark method is appropriate to compare and contrast the concentration-time profiles for individual subjects [14]. Although more sophisticated multicompartment models involving Michaelis-Menten kinetics have been advocated for ethanol and are useful after intravenous infusion [20], these methods are less practical when alcohol is taken by mouth. Indeed, support for the one-compartment model comes from using ethanol as a tracer to estimate total body water (TBW) by the dilution principle [24]. A recent study in women concluded that the zero-order model was valid from 0.5 h after Cmax until BAC reached 0.2 g l−1 because at lower concentrations the main alcohol metabolizing enzymes are no longer saturated [25].
Much attention has focused on the notion of first-pass metabolism of ethanol occurring in the stomach by class IV isoforms of alcohol dehydrogenase (ADH) located in gastric mucus [26–29]. In the present study, one might expect a greater contribution from gastric ADH in the control group of subjects with intact gut compared with the bypass group with a much smaller absorption surface and presumably less ADH enzyme. Assuming equal rates of alcohol clearance in the two groups of women, the lack of any differences in AUC speaks against any differences in pre-systemic metabolism of ethanol whether gastric or hepatic.
In conclusion, we suggest that after gastric bypass surgery patients should be warned about drinking alcohol too quickly because even relatively small amounts of alcohol, such as two small glasses of wine (0.3 g kg−1) might produce unexpectedly high BAC shortly after the end of drinking. Also when other surgical procedures are performed on the gut such as gastric resection and gastrectomy, a more rapid absorption of ethanol can be expected. The present experiment with alcohol as a model substance might have implications for the absorption and pharmacological effects of other drugs or when prescription drugs are taken together with alcohol [30].
The costs of this study were supported in part by the Swedish National Board of Forensic Medicine (Rättsmedicinalverket).
References
- 1.Mokdad AH, Serdula MA, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991–98. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 2.Dickerman RM. Gastric exclusion surgery in the management of morbid obesity. Ann Rev Med. 1982;33:263–270. doi: 10.1146/annurev.me.33.020182.001403. [DOI] [PubMed] [Google Scholar]
- 3.Baxter J. Obesity surgery – another unmet need. Br Med J. 2000;321:523–524. doi: 10.1136/bmj.321.7260.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanovski JA, Yanovski SZ. Recent advances in basic obesity research. JAMA. 1999;282:1504–1506. doi: 10.1001/jama.282.16.1504. [DOI] [PubMed] [Google Scholar]
- 5.Brolin RE. Gastric bypass. Surg Clin North Am. 2001;81:1077–1095. doi: 10.1016/s0039-6109(05)70185-7. [DOI] [PubMed] [Google Scholar]
- 6.Mason EE. Modified VBG vs gastric bypass. Obes Surg. 2000;10:68–69. doi: 10.1381/09608920060674166. [DOI] [PubMed] [Google Scholar]
- 7.Jones KB. Experience with the Roux-en-Y gastric bypass and commentary on current trends. Obes Surg. 2000;10:233–239. doi: 10.1381/096089200321668659. [DOI] [PubMed] [Google Scholar]
- 8.Jones AW, Jönsson KÅ, Neri A. Peak blood-ethanol concentration and the time of its occurrence after rapid drinking on an empty stomach. J Forens Sci. 1991;36:376–385. [PubMed] [Google Scholar]
- 9.Hahn RG, Norberg Å, Jones AW. ‘Overshoot’ of ethanol in the blood after drinking on an empty stomach. Alc Alcohol. 1997;32:501–505. doi: 10.1093/oxfordjournals.alcalc.a008285. [DOI] [PubMed] [Google Scholar]
- 10.Zador PL, Krawchuk SA, Voas RB. Alcohol-related relative risk of driver fatalities and driver involvement in fatal crashes in relation to driver age and gender; an update using 1996 data. J Studies Alc. 2000;61:387–395. doi: 10.15288/jsa.2000.61.387. [DOI] [PubMed] [Google Scholar]
- 11.Jones AW. Blood and breath alcohol concentrations. Br Med J. 1992;305:955. doi: 10.1136/bmj.305.6859.955-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones AW, Schuberth J. Computer-aided headspace gas chromatography applied to blood-alcohol analysis; Importance of on-line process control. J Forens Sci. 1989;34:1116–1127. [PubMed] [Google Scholar]
- 13.Mathews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widmark EMP. In: Principles and Application of Medicolegal Alcohol Determinations. Baselt RC, editor. Davis: Biomedical Publications; 1981. [Google Scholar]
- 15.Jones AW. Inter-individual variations in the disposition and metabolism of ethanol in healthy men. Alcohol. 1984;1:385–391. doi: 10.1016/0741-8329(84)90008-9. [DOI] [PubMed] [Google Scholar]
- 16.Wartburg Von J-P. Pharmacokinetics of alcohol. In: Batt RC, Crow KE, editors. Human Metabolism of Alcohol. Vol. 1. Boca Raton: CRC Press; 1989. Pharmacokinetics, medicolegal and general interests. [Google Scholar]
- 17.Holford NH. Clinical pharmacokinetics of ethanol. Clin Pharmacokinet. 1987;13:237–292. doi: 10.2165/00003088-198713050-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kalant H. Pharmacokinetics of ethanol; absorption, distribution and elimination. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. New York and Oxford: Oxford University Press; 1996. pp. 15–58. [Google Scholar]
- 19.Jones AW, Neri A. Age-related differences in blood-ethanol concentration and subjective feelings of intoxication in healthy men. Alc Alcohol. 1985;20:45–52. [PubMed] [Google Scholar]
- 20.Norberg Å, Gabrielsson JL, Jones AW, Hahn RG. Intra-individual and inter-individual variation in pharmacokinetic parameters of ethanol in breath, venous blood and urine. Br J Clin Pharmacol. 2000;49:390–408. doi: 10.1046/j.1365-2125.2000.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin CS, Moss HB. Measurement of acute tolerance to alcohol in human subjects. Alcohol Clin Exp Res. 1993;17:211–216. doi: 10.1111/j.1530-0277.1993.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones AW, Pounder DJ. Measuring blood-alcohol concentration for clinical and forensic purposes. Chapter 5.2. In: Karch S, editor. Drug Abuse Handbook. Boca Raton: CRC Press; 1998. pp. 327–356. [Google Scholar]
- 23.Jackson PR, Tucker GT, Woods HF. Backtracking booze with Bayes – the retrospective interpretation of blood alcohol data. Br J Clin Pharmacol. 1991;31:55–63. doi: 10.1111/j.1365-2125.1991.tb03857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones AW, Hahn RG, Stalberg HP. Pharmacokinetics of ethanol in plasma and whole blood; Estimation of total body water by the dilution principle. Eur J Clin Pharmacol. 1992;42:445–448. doi: 10.1007/BF00280133. [DOI] [PubMed] [Google Scholar]
- 25.Mumenthaler MS, Taylor JL, Yesavage JA. Ethanol pharmacokinetics in white women; nonlinear model fitting versus zero-order elimination analysis. Alcohol Clin Exp Res. 2000;24:1353–1362. [PubMed] [Google Scholar]
- 26.Frezza M, Di Padova C, Pozzato G, et al. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 27.Gentry RT, Baraona E, Lieber CS. Agonist; Gastric first pass metabolism of alcohol. J Lab Clin Med. 1994;123:21–26. [PubMed] [Google Scholar]
- 28.Levitt MD. Antagonist; The case against first-pass metabolism of ethanol in the stomach. J Lab Clin Med. 1994;123:28–31. [PubMed] [Google Scholar]
- 29.Levitt MD, Levitt DG. Appropriate use and misuse of blood concentration measurements to quantitate first-pass metabolism. J Lab Clin Med. 2000;136:275–280. doi: 10.1067/mlc.2000.109100. [DOI] [PubMed] [Google Scholar]
- 30.Gubbins PO, Bertch KE. Drug absorption in gastrointestinal disease and surgery – Clinical pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1991;21:431–447. doi: 10.2165/00003088-199121060-00004. [DOI] [PubMed] [Google Scholar]