Introduction
Many cardiovascular drugs act predominantly on the peripheral circulation and their effects on resistance vessels can be relatively easily assessed by relating changes in pressure to changes in flow [1–3]. The venous circulation however, serves a capacitance function and is better described in terms of pressure/volume (P/V) relations [4, 5].
Importance of venous physiology
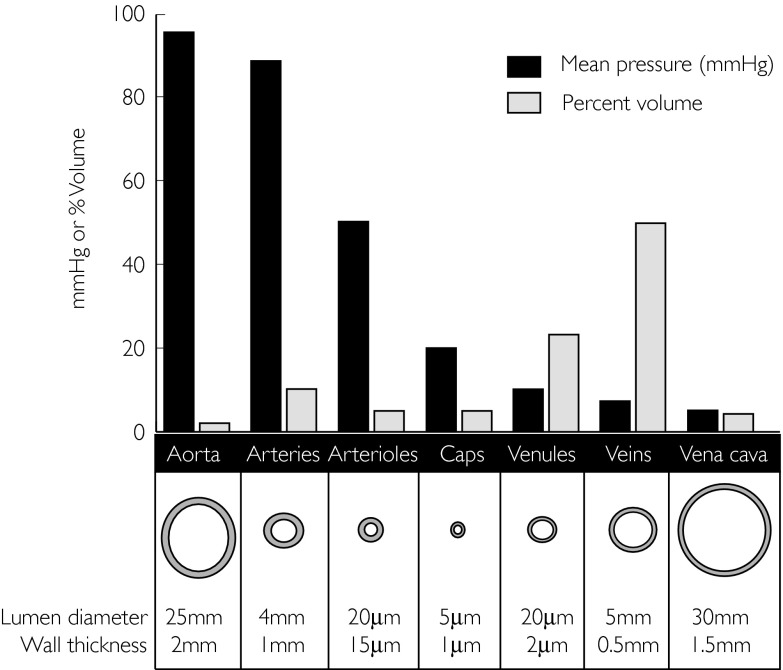
The veins and venules return the blood from the microcirculation to the heart. However they are far more than simple conduits. Indeed by regulating central blood volume and therefore preload, changes in venous tone regulate stroke volume via the Frank–Starling mechanism. Almost 80% of blood volume lies in these vessels, representing a large capacitance reservoir [5] (see Figure 1).
Figure 1.
The blood volume distribution, pressures within each vascular compartment, corresponding lumen diameters and wall thickness. Modified from Vascular anatomy. In: The Cardiovascular System at a Glance, eds Aarson PI & Ward JPT. Oxford: Blackwell Science Ltd, 1999: Page 10. With kind permission.
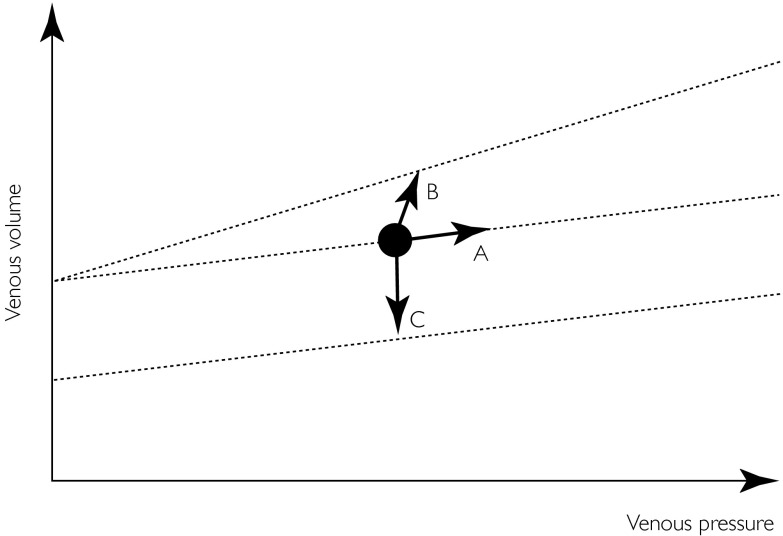
Changes in regional venous volume can be mediated via three mechanisms. First, passively along the P/V axis (as illustrated by ‘A’ in Figure 2), for example if venous outflow is obstructed temporarily by pressures between 10 and 40 mmHg. Secondly, by a change in compliance (at the extremes of the flat part of the P/V curve – see Figure 3), in which case the slope of the P/V relation is changed, as illustrated by ‘B’. Thirdly, actively due to primary changes in venous tone, as illustrated by ‘C’.
Figure 2.
A venous pressure/volume (P/V) relation is shown. Venous volume, depicted by the black dot can change in 3 ways. ‘A’ illustrates an increase in venous volume along the slope of the P/V relation (dotted line), e.g. when venous outflow is obstructed temporarily over a physiological pressure range. ‘B’ illustrates an increase in venous volume following a change (here increase) in venous compliance, demonstrated by a changing slope of the P/V relation. ‘C’ illustrates a decrease in venous volume, in absence of a compliance change. Parallel-shifts reflect changes in venous tone (upward shift = decrease in tone, downward shift = increase in tone).
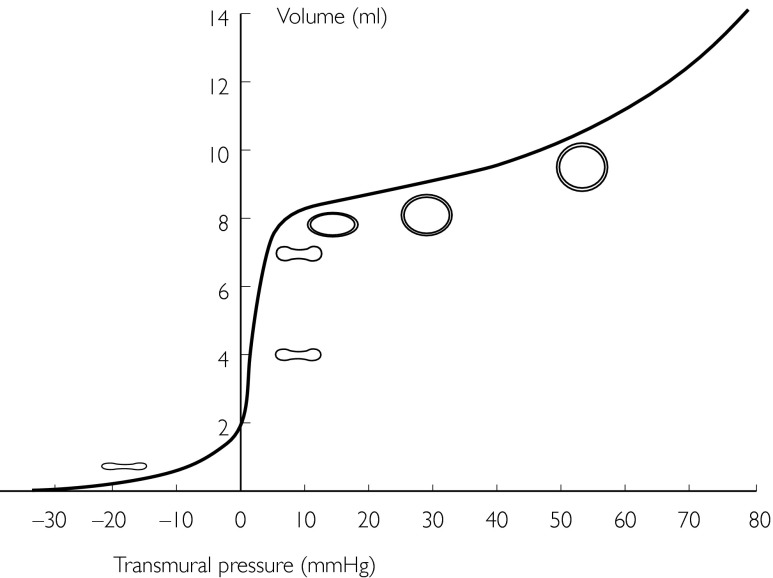
Figure 3.
The venous transmural pressure/volume relationship over a wide range of pressures (note venous transmural pressure may be negative). The slope of the curve is referred to as compliance. Importantly, the compliance curve is near linear between 10 and 30 mmHg. Modified from The venous system. In: The Cardiovascular System at a Glance, eds Aarson PI & Ward JPT. Oxford: Blackwell Science Ltd, 1999: Page 46. With kind permission.
Veins are thinner-walled than arteries (see Figure 1) and can therefore expand greatly. This explains why veins are much more compliant than arteries at low pressures. Despite this, there is sufficient smooth muscle in the walls of all but the smallest venules to actively modulate venous tone. It therefore follows that even small changes in venous tone are capable of translocating large amounts of blood to and from the central compartment. Cardiac performance is exquisitely sensitive to the state of cardiac filling such that stroke volume may change as much as 50% in response to a change in filling pressure of as little as 1 cm H2O if buffering reflexes are blocked or exhausted [4].
The importance of active control of the capacitance beds is especially relevant during exercise and in disease states when cardiac reserve is limited. For example, in healthy subjects upright exercise is associated with a 23% reduction in abdominal blood volume (liver 18%, kidney 24%, spleen 46%) and a 30% reduction of leg blood volume. This translocation of blood volume to the central compartment is closely correlated to oxygen consumption, contributing (via the Frank–Starling mechanism) to the increase in cardiac output during exercise [6]. Whilst impaired venoconstriction contributes to exercise hypotension in some patients with vasovagal syncope [7] and hypertrophic cardiomyopathy [8], an exaggerated response may potentially contribute to increased left ventricular and diastolic pressure on exercise in heart failure.
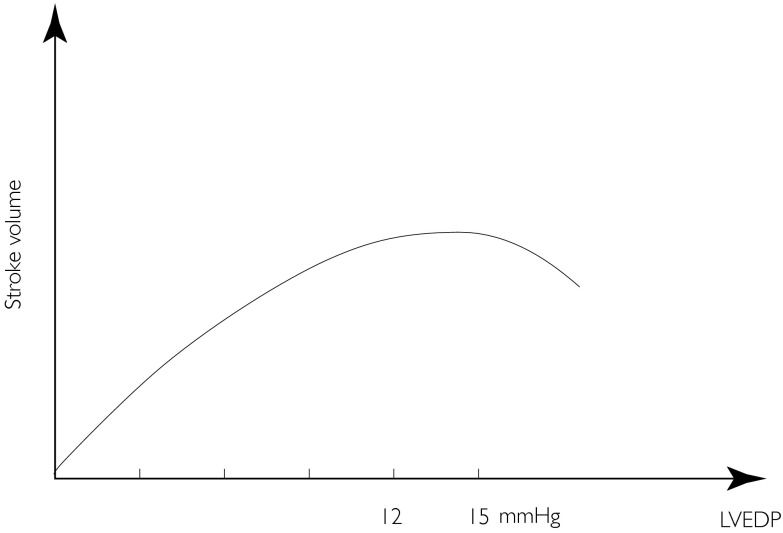
When acute heart failure is induced in a canine model, there is profound baroreflex mediated venoconstriction (due to hypotension) accounting for roughly 80% of the increase in left ventricular end-diastolic pressure (LVEDP), the left ventricular dysfunction itself only accounting for 20% of this increase in LVEDP [9, 10]. In CHF a rise in LVEDP (due to venoconstriction) may result in a fall rather than a rise in stroke volume [11]. This is due to the phenomenon of diastolic ventricular interaction (DVI) [11–14] in which filling of the LV is impeded by external constraint from the right ventricle (RV) and pericardium. The implication is that whilst in most physiological situations, venoconstriction may be a compensatory mechanism to maintain stroke volume, in pathophysiological situations in which RVEDP and LVEDP are elevated, venoconstriction could be deleterious due to a reduction in stroke volume. In general, this phenomenon appears to be apparent when LVEDP is greater than approximately 15–20 mmHg. This implies not only that ventricular interaction and venous capacitance modulate left ventricular preload [14] but also that there is an optimal LVEDP to maximize use of the Frank–Starling mechanism (Figure 4).
Figure 4.
Frank–Starling or ventricular function curve with descending limb, implying that there may be an optimal left ventricular end-diastolic pressure (between 12 and 15 mmHg) to maximize use of the Frank–Starling mechanism.
Varying responses of individual venous beds
Much of the work on the venous system has been undertaken on conduit veins (e.g. dorsal hand veins or saphenous veins). Yet these large veins contribute modestly to the total blood volume compared with the small veins and venules. Furthermore, it is generally unwise to extrapolate findings from one vascular bed to another. Different stimuli (neural, humoral, or paracrine) may elicit varying responses in various beds. Quantitatively the two most important venous beds are the muscular beds and the abdominal compartment (including intestinal, splenic, renal and hepatic beds). Whilst the spleen and the splanchnic veins appear to react qualitatively and quantitatively similar to reflex stimuli [15], the hepatic circulation, comprising of two systems, behaves different and assessment requires an invasive approach [16, 17]. Capacitance vessels in skeletal muscle may also behave differently. For example, it is believed that the capacitance vessels in skeletal muscle have more active involvement in the carotid sinus reflex than those of the intestinal beds [18]. Nevertheless part of the constricting effect on small muscular veins may be a humoral effect, due to circulating catecholamines, following intense excitation of the vasomotor centre [18, 19]. In contrast, in larger veins such as the femoral veins and the vena cava, active changes can be elicited easily by the carotid sinus mechanism [20–22]. From the response of the vessels to electric stimulation of the sympathetic nerves as an index of changes in sympathetic outflow, it appears that a decrease in carotid sinus pressure causes the same increase in nerve traffic to the resistance and capacitance vessels of the splanchnic region but a greater increase in traffic to the limb resistance vessels [18, 22–24]. The density of adrenergic innervation of individual blood vessels varies widely, partly reflecting their degree of participation in centrally controlled responses.
The cutaneous veins are densely innervated [25] and respond to a variety of stimuli (e.g. cold pressor, limb exercise, or deep breathing, which can be inhibited with α-adrenoceptor blockade, consistent with a neurally mediated mechanism). However, cutaneous veins show not only no consistent immediate response to changes in arterial baroreceptor activity but changes in sympathetic outflow to cutaneous veins are often opposite to those of other capacitance vessels. Stimulation of carotid chemoreceptors and muscle metaboreceptors for example causes reflex constriction in the splanchnic circulation whilst dilating cutaneous veins [26]. Furthermore, whilst thermoregulatory mechanisms are believed to predominate over other inputs in the control of venomotor tone at ambient temperatures, during prolonged exercise in the heat a cutaneous vasoconstrictor drive superimposes its effect upon a high vasodilator drive and thus limits the absolute volume contained in the skin [27–30]. The total amount of blood contained at a given time within the skin circulation at ambient temperatures is believed to be relatively small (3%). However, Fortney et al.[31] could show that the degree of cutaneous vasoconstriction during exercise in the heat is greater after an acute reduction in blood volume than in normovolaemic conditions, implying that skin veins indeed are a functioning efferent arm of the blood pressure regulating mechanism. Tripathi and colleagues [32] provided information that this venoconstriction could be due to low pressure baroreceptor unloading.
With a constant input from peripheral receptors of vascular beds with varying demands, metabolic states and function, the interaction between the pressor and depressor areas is modulated both by the metabolic conditions in the brain and by the activity of higher centres such as the cortex, hypothalamus, and limbic system, thus fulfilling the role of a central integration of all information in order to optimize the overall performance of the whole body.
It is clear from the foregoing that an understanding of the regulatory mechanisms involved in the control of venous tone is an important aspect of cardiovascular physiology. Yet our understanding of normal venous physiology in man is poor. Difficulties in developing valid and reliable techniques for assessing venous tone and compliance in the human capacitance bed have hampered attempts to gain a greater understanding of its precise role in human health and disease. Established techniques are available [33–37], but as discussed below, all have limitations when applied to the assessment of the capacitance bed. The introduction of ‘radionuclide plethysmography’ into the research arena more than 20 years ago [38, 39] was able to overcome some of the limitations of conventional plethysmographic techniques and has contributed to a better understanding of human venous physiology [40–42] and exercise physiology in health [6] and disease [7, 43–45].
Terminology
Because confusion can arise over the terminology used to describe the physical characteristics of veins, we include (Table 1) a short description of the terms used in this review. In the remainder of this review we will focus on the methodology, validity, applicability, and detailed description of radionuclide plethysmography, highlighting differences compared with conventional plethysmography and pointing out advantages and disadvantages. A brief outline of a typical protocol, study design and data analysis should facilitate the setting up of the technique.
Table 1.
Description of terms used in this review.
| Capacitance | The term capacitance remains poorly defined and is not synonymous with venous volume as a smaller amount of volume resides within the heart and the arterial tree (Figure 1). In general terms it relates to the total contained volume of the vasculature to a given transmural pressure over at least the physiological range of transmural pressures. In this review we will use, for simplicity, the term venous capacitance synonymous with venous volume. |
| Compliance | A general term describing the change in dimension following a change in stress. Translated into venous physiology this means that compliance is the ratio of the change in volume (ΔV) resulting from a change in transmural distending pressure (ΔP), or ΔV/ΔP. It is the slope of the pressure/volume relationship (PVR) at a given point of the ‘curve’ (Figure 2). Because venous compliance is very high at low pressures, the slope of the venous PVR over the physiological pressure range (10–40 mmHg) is nearly linear. |
| Unstressed volume | The volume of blood in a vessel at zero transmural pressure is defined as unstressed volume. It is a virtual volume established by extrapolating a linear portion of the PVR to zero transmural pressure. |
History, applicability and basic principles
Imaging of the peripheral blood pool, using labelled albumin [46] or red blood cells [47], was first used to assess changes in venous volume and tissue fluid accumulation in animal experiments in the early 60 s by Ablad et al. and Baker et al. In 1981 Rutlen [38] and Clements [39] described independently the application of this technique, termed radionuclide plethysmography, to study the human peripheral circulation. The technique, which despite its name actually does not involve a plethysmographic element, combines equilibrium blood pool scintigraphy (EBPS) with a standard occlusive technique. In brief, red cells are labelled using a modified in vivo labelling technique, based on the method described by Callahan [48]. Using this technique (details described below) at least 95% of injected isotope is bound to red cells, and therefore confined to the intravascular space. It follows that radioactive counts are proportional to intravascular volume. Since the vast majority of blood in the peripheral circulation is contained within the veins [5] changes in counts largely reflect changes in venous volume. Following radiolabelling P/V relations are constructed by obstructing venous outflow in a stepwise manner for 1 min. During each 1 min interval a dynamic image (split into six intervals of 10 s) of the region of interest (ROI) is continuously acquired. Combined with either local or systemic drug infusion, or physiological stimuli such as lower body negative pressure, carotid stimulation or exercise, construction of P/V relations allow assessment of changes in venous tone. A parallel shift of the P/V relation implies a change in venous tone. A change in slope indicates altered compliance.
The technique was further validated and refined by Manyari and coworkers who modified its use and expanded its application to assess splanchnic capacitance in animal experiments [9, 10, 49] and humans [41] in health and disease. This group also demonstrated the suitability and applicability of the technique to investigate venous reflex control in health and disease states associated with abnormal vasomotor response [50]. Our group later expanded this application to investigate venous reflex responses in hypertrophic cardiomyopathy [8], post-myocardial infarction [44], and during dynamic leg exercise [43–45]. We have also adapted the technique to assess venous endothelial function in health [42] and CHF [51] and more recently improved the technique by expressing changes in one arm to changes in the contralateral arm, as is convention for plethysmographic techniques, to take account of potential systemic changes during pharmacological intervention and prolonged experiments.
Whilst some of the earlier studies assessed peripheral counts by means of simple scintillation probes, mobile small field of view (SFOV) gamma cameras permit the simultaneous imaging of the region of interest (see below).
Practical methodology
Red cell labelling
Red cells are labelled with 99m technetium (99mTc) using a modified in vivo labelling technique [48]. The content of one vial of Amerscan (stannous agent, Amersham, UK) is dissolved in 6 ml of normal saline to form stannous medronate complex. Immediately after solution, 0.03 ml kg−1 body weight−1 is withdrawn from the vial and injected directly into a vein. In the presence of stannous ion 99mTc is reduced in the cells and becomes bound to the β chains of the globin. Twenty minutes after injection of the stannous solution 3 ml of blood are withdrawn from an antecubital vein into a 10 ml syringe containing 750 MBq of 99mTc pertechnetate diluted in 2. 5 ml normal saline. The syringe is placed into a lead cylinder and gently agitated for 10 min to prevent clotting and facilitate binding. The now (ex vivo) labelled blood is re-injected 10 min later via a separate butterfly. To minimize further the amount of free circulating 99mTc and to allow complete in vivo binding to occur, scintigrams are not recorded for a further 15 min. Using this technique at least 95% of the injected radionuclide is bound to red cells, and therefore confined to the intravascular space.
Forearm studies
In studies using intra-arterial drug infusion the antecubital vein of both arms are cannulated with an 18 gauge indwelling cannula for baseline blood sampling. After red cell labelling (see above), a 27-gauge unmounted steel needle sealed with dental wax to an epidural catheter is inserted into the brachial artery of the nondominant arm under sterile conditions. The infused arm is then positioned on the face of a SFOV camera equipped with an integrated computer system. During intra-arterial infusion studies the noninfused arm may be studied using a second camera to take account of systemic vascular changes over time (Figure 5). Twenty to 30 min after infusion of saline is commenced, two baseline venous P/V relations are recorded (see below). This can be combined with assessment of forearm blood flow and forearm vascular resistance. Thereafter the infusion of the study drug commences at the chosen concentration and an infusion speed of ≤1 ml min−1. Venous dose–response curves can than be created by repeating P/V relations at incremental drug doses. Venous-effluent blood samples from both arms can be taken for assessment of plasma concentration of the drug under investigation and for calculation of, e.g. second messenger ‘spillover’ at each dose.
Figure 5.
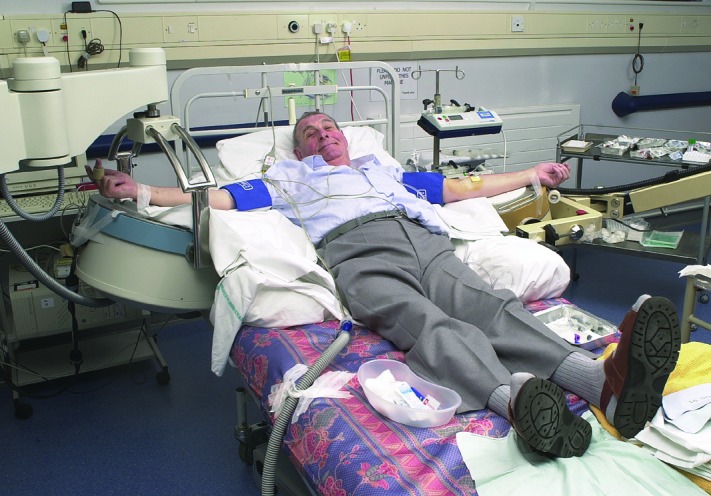
A typical setting for a forearm study. Both forearms rest on the surface of a gamma camera collimator.
Splanchnic/intestinal studies
A dorsal hand vein or antecubital vein is cannulated with an 18 gauge indwelling cannula and adequate baseline blood sampling is performed. The subject is then comfortably positioned on a bed in supine position. After red cell labelling both gamma cameras are positioned as follows: One gamma camera is positioned above the subjects abdomen to record (changes of) venous volume in the intestinal vascular bed (Figure 6). The bladder and the iliac bifurcation are used as landmarks, and lead-strips applied to the skin facilitate monitoring of a constant region. The liver, spleen and kidneys are excluded from the region of interest. A second camera may be positioned to monitor the count rate derived from the spleen. Subjects are first taught to relax while breathing with various levels of continuous airway pressure (CPAP). This procedure can then be combined with pharmacological interventions. Subjects are instructed not to move, and care is taken to maintain a constant subject – camera position throughout the experiment. Changing the level of CPAP requires between 5 and 10 s.
Figure 6.
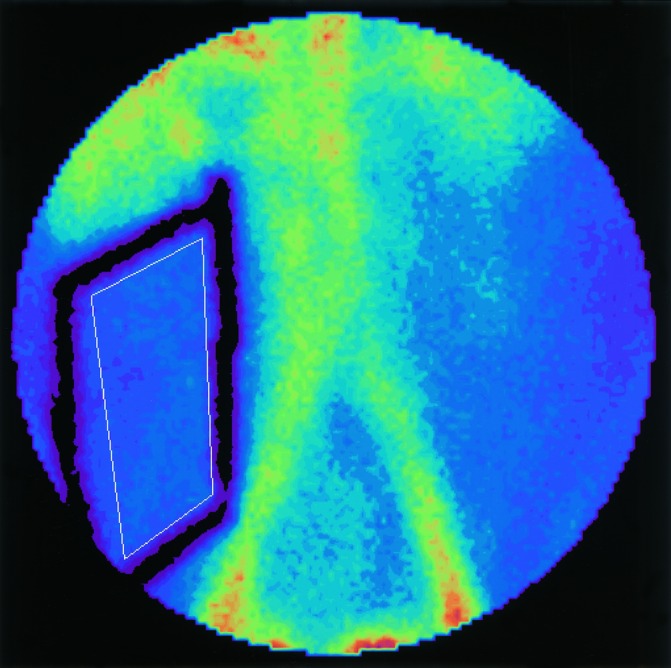
An abdominal scintigram. Lead markers (thick black lines) attached to the skin facilitate the monitoring of a constant ROI (thin white line). The ROI is drawn clear of large conduit vessels.
Data analysis
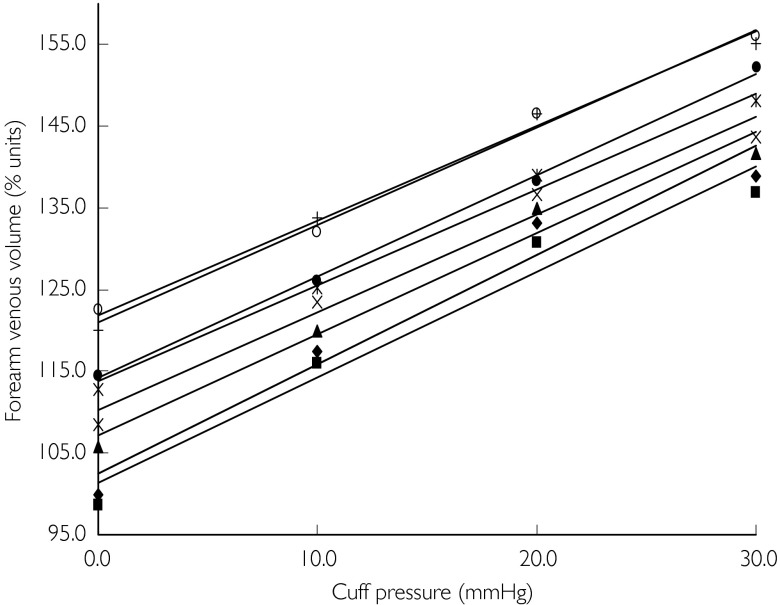
Following red cell labelling images are required as a dynamic study of 10 s frames, either continuously or as a series of 1 min studies. Regional P/V relations are constructed by increasing venous transmural pressure in a stepwise fashion, either by inflation of upper arm cuffs (arm-studies) or by incremental CPAP (splanchnic-studies). Following any intervention (e.g. cuff inflation or alteration of CPAP) the first 30 s data-set is ignored (upper panel in Figure 7), to allow regional volume to stabilize and only the next 30 s data-set (lower panel in Figure 7) is used for analysis. The images are summed and a region of interest is defined on the image (Figures 6 and 8), obtained during normal saline infusion (baseline). All images in the study are viewed with this ROI to confirm that no patient movement has occurred during the acquisition. The counts from this ROI for each of the appropriate 30 s intervals are corrected for physical decay and the count obtained with no intervention (baseline) is arbitrarily taken to reflect 100% volume. All subsequent readings are expressed as percentage change of this baseline value. These counts, or scintigraphic vascular volumes in percentage-units are plotted on the y-axis against cuff/CPAP pressure on the x-axis to form venous P/V plots (Figure 9). Linear regression is performed for each plot, and a linear model is accepted if the r2 value is ≥0.9. The slope of each plot is a measure of venous compliance. Therefore a parallel shift in P/V relations not only reflects a change in venous capacitance, but in absence of a change in compliance is simultaneously indicative of a change in venous tone. A parallel up-ward shift reflects a reduction in venous tone whilst a parallel downward shift reflects an increase in venous tone. This is exemplified in Figure 2. Relative capacitance changes following intervention can be grouped and are compared to baseline and expressed as ‘% units’ (Figure 9).
Figure 7.
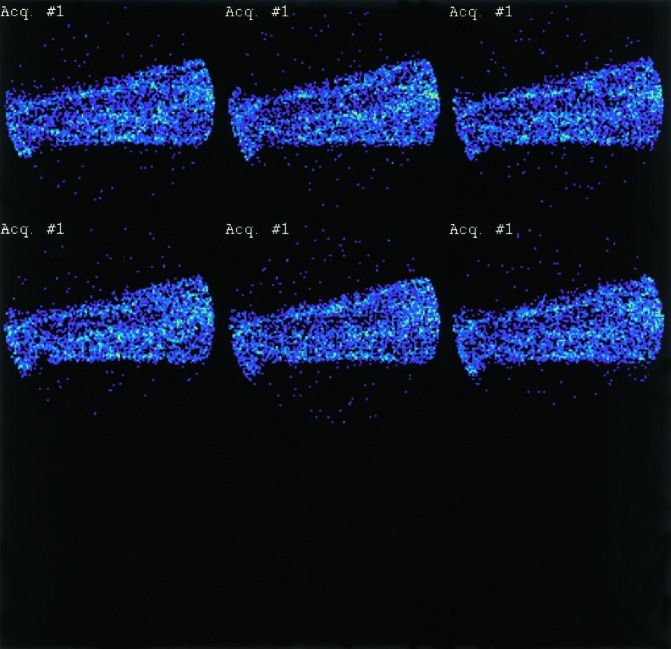
A 1 min dynamic recording split into six intervals of 10 s. The lower panel (the last 30 s after pressure has equalized) is used for analysis.
Figure 8.
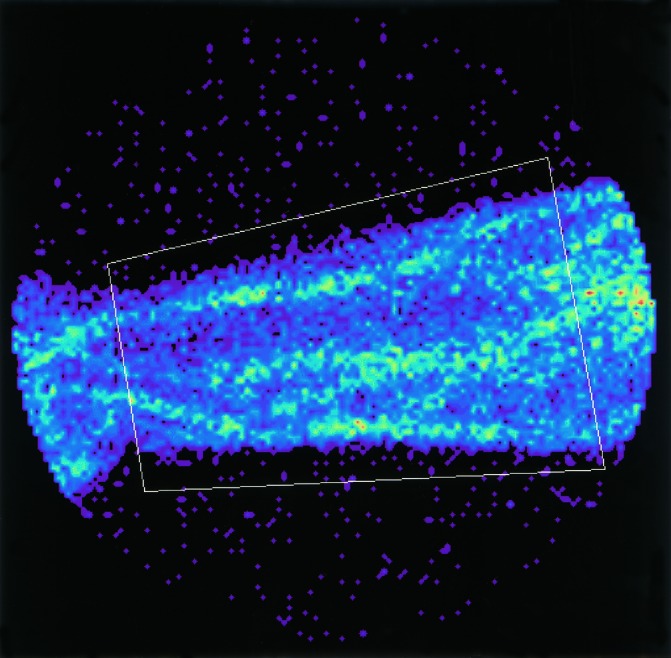
This shows a region of interest (ROI) drawn over a forearm. It is suggested that the region should be drawn just clear of the edge of the arm (to allow for small expansion and movements) but inside the edge of the camera field.
Figure 9.
Grouped pressure/volume relations of a typical dose–response study. A dose dependent parallel up-ward shift, indicating increase in venous volume and decrease in venous tone following intra-arterial ANP administration, is shown. ♦, Saline 1; squf;, saline 2; ▴, ANP 0.05 µg min−1; ×, 0.1 µg min−1; ✶, 0.5 µg min−1; • 1.10 µg min−1; + 2.75 µg min−1; ○, 5.50 µg min−1.
Validation
Variability
In his original description of radionuclide plethysmography in 1981, Rutlen [38] obtained absolute counts in the calf, under resting conditions, over a 30 min period (n= 14). Count rate varied very little under resting conditions. Manyari evaluated variability between two control measurements of forearm venous volume using the square root of the residual mean square as applied to consecutive quantitative radionuclide studies. Variability was 3.11%, which compared favourably with variability using strain gauge plethysmography of 3.24% [52].
Validation against fluid displacement plethysmography
To determine if changes in intravascular volume obtained by imaging the radiolabelled blood pool correlated with changes recorded with a standard plethysmographic technique, Rutlen also recorded simultaneously changes following cuff occlusion (15 and 30 mmHg), before and after GTN, in nine healthy volunteers. Changes obtained in one arm as assessed by radionuclide plethysmography were correlated in a linear fashion (r= 0.71 to r= 0.98) with simultaneously obtained changes in the contralateral arm, as assessed by water displacement plethysmography before and after these interventions [38].
Validation against strain gauge plethysmography
Manyari assessed changes in regional forearm volume in response to sublingual GTN and oral nifedipine in 16 patients with a history of recurrent chest pains who had otherwise a low probability of coronary artery disease and no history or evidence of heart failure, hypertension, diabetes mellitus or peripheral vascular disease [52]. He constructed P/V relations and assessed changes in one arm using radionuclide plethysmography and strain gauge plethysmography in the contralateral arm. Results using the different methods were closely correlated (r= 0.91 to r= 0.99). Clements correlated percentage changes in count rate and changes in forearm volume simultaneously in the same arm in eight subjects. He also demonstrated a close correlation with r values between 0.95 and 0.99 [39].
Issues regarding red cell binding
Radionuclide plethysmography uses radioactive counts within a ROI to represent venous volume. As binding to red cells is not 100% it is likely that a small amount of activity derives from tissue, i.e. is extravascular. Poor labelling efficiency will therefore impact on the accuracy of the technique and has the theoretical potential to lead to systematic overestimation of intravascular volume via extravasate of unbound 99mTc. Furthermore, if binding varies between different subjects results would not be comparable. Therefore it is essential that red cell binding is as close to 100% as possible with minimal variation between individuals.
Principally there are three methods of labelling [53]:
In vivo technique: Stannous (such as Amerscan Stannous Agent from Amersham International) is injected intravenously followed by 99mTc pertechnetate injection after approximately 20 min. This is the simplest technique. However this technique has not only the lowest binding efficacy but also a high interindividual variation. In our validation studies [54] binding efficacy using this technique was 79.8 [16.7% (s.e. mean, n= 20)].
In vitro technique: A sample of blood is taken and red cells are separated and incubated first with stannous and than with 99mTc pertechnetate. The cells are washed with saline before and after each step to eliminate unbound material. The cells are re-injected into the patient with little or no free pertechnetate, approaching a labelling efficacy of 100%. This technique however, is technically difficult and requires a higher level of logistic organization.
Modified in vivo technique: This technique (described above) is easier to perform and results in high labelling efficacy and little interindividual variability. In our own validation studies we achieved a mean binding efficacy of 96.8 (0.6% (varying from 96 to 98%, n= 20) [54]. To establish the reliability of binding throughout lengthy studies we also assessed unbinding from erythrocytes over a 3 h period (Figure 10). We found no significant unbinding over this period (% labelling efficiency was determined by the ratio: Ac× 100/Ac+ As, where Acis the activity in the labelled cell suspension (middle panel in Figure 10) and As is the activity remaining in the plasma supernatant – lower panel in Figure 10).
Figure 10.

Blood samples taken over a 3 h period (left to right; Baseline, 1 h, 2 h, 3 h). The upper panel shows whole blood-activity, the middle panel concentrated erythrocyte-activity, and the lower panel the activity in plasma. The single ROI between upper and middle panel serves to correct for background radiation.
Whilst correction for physical decay is easily performed by a computer program on the basis of the tracer half-life (6 h for technetium) and the study length, biological decay (biological decay refers to changes in the count rate due to loss of the isotope from the erythrocyte or change in haematocrit) may have to be taken in account depending on the study setting and nature of the intervention. For example when performing exercise studies it is good practice to correct for changes in radioactive counts ml−1 as this may change significantly due to release of erythrocytes from the spleen [6] that may escape effective labelling. We previously observed a 10–12% fall in radioactive counts/ml blood in healthy individuals during maximal exercise.
Effects of arterial inflow on venous volume
A crucial question is whether changes in regional blood volume are influenced by changes in arterial inflow. The vast majority of vasoactive drugs effect both arterial and venous tone and the possibility exists that changes in venous volume are at least in part consequence of the changes in arterial inflow. To address this problem, we investigated the effect of brachial artery infusion of hydralazine on both forearm blood flow (FBF) and the venous P/V relation. Hydralazine is a selective arterial vasodilator, the peak effect of which is delayed for 30–45 min after administration [55, 56]. Five healthy subjects had FBF measured by strain gauge plethysmography for 30 min following an intra-arterial infusion of 800 µg hydralazine in 8 ml at 1 ml min−1. Hydralazine caused progressive arterial vasodilatation, with a peak increase in FBF of 262 ± 102% at 30 min, but there was no change in the forearm venous P/V relation [42]. Our findings are in keeping with previous studies. Wathan et al.[57] compared the effects of hydralazine (10 mg i.v.) and GTN (0.6 mg s.l.) using radiolabelled albumin and a collimated scintillation probe to assess calf venous volume. Both drugs reduced vascular resistance. Whereas GTN increased calf venous volume, hydralazine did not change it. Wang et al.[9] compared the effects of systemic hydralazine, enalaprilat and GTN on the intestinal venous P/V relation in a canine acute heart failure model. Enalaprilat and GTN both induced an upward shift of the P/V relation (= venodilation) and markedly reduced LVEDP, whereas hydralazine had no effect on the P/V relation and minimal effect on LVEDP. Manyari et al.[52, 58] compared the effects of systemic nifedipine and GTN administration. Both agents reduced blood pressure. GTN caused an upward shift in the P/V relation (= venodilation), but nifedipine had no effect. Further strong, albeit indirect evidence is derived from the observation that leg blood volume remains virtually unchanged during a work load increase between 50% to 100% of maximal VO2[6] whilst there is a remarkable increase in blood flow [59]. Given the fact that radionuclide plethysmography is able to detect blood volume changes of as little as 5 ml [49], these data support the view that changes in the venous P/V relation are largely independent of changes in arterial inflow. Measurement of venous tone is therefore valid even when arterial inflow is markedly altered.
Limitations of radionuclide plethysmography
Beside the obvious disadvantages of the radiation exposure (roughly 6.4 mSV), the financial aspects (expensive equipment), the considerable logistic efforts, regulatory, ethical, and health and safety aspects involved when performing studies using radioactive substances, the major disadvantage is the inability to express the changes in volume in absolute (volume) units. Thus changes are expressed as a percentage of control measurements. Simultaneous measurement of volume changes using radionuclide and strain gauge plethysmography has the theoretical potential to overcome the latter limitation. Whilst invasive assessment of venous pressure is not necessary as there is a close relationship between cuff pressure and invasively assessed conduit vein pressure [60], the radionuclide technique shares the inherent limitation of all venous occlusion plethysmographic techniques, that the pressure considered to plot venous P/V relations is an ‘upstream’ pressure and not that of the small veins and venules. However the pressure drop between the small veins and venules and the ‘upstream’ veins is in most situations minimal. Finally, radionuclide plethysmography does not provide a measure of capillary filtration. Therefore increased tissue attenuation as a consequence of increased capillary filtration can lead, at least theoretically, to underestimation of the vasodilating potency of the studied agent.
Comparison with available methods to assess venous function and capacitance
As outlined above measurement of changes in venous capacitance is essential to describe venous actions of cardiovascular drugs and key to assessing the importance of the venous system in cardiovascular haemostasis. Because the vast majority of blood is contained in microscopic vessels, hidden in tissue, blood volume and distending pressure are notoriously difficult to assess.
Established techniques
Dorsal hand vein technique
The principal method used for in vivo evaluation of human venous tone is the dorsal hand vein technique described by Aellig [35–37]. The internal diameter of a single superficial hand vein is recorded by measuring the linear displacement of a lightweight probe resting on the skin over the summit of the vein, when the pressure in a congesting cuff placed around the upper arm is lowered from 45 mmHg to 0 mmHg. Changes in vein diameter following various interventions can be measured in order to determine the effects of these interventions on venous tone. However, this technique does not quantify intravascular volume, nor does it permit construction of a complete P/V relation. Thus it is not possible to distinguish whether an intervention is affecting tone alone, or whether it is causing alterations in compliance, passively altering volume, or having a combination of these effects. More importantly this technique measures diameter in a superficial conduit vein, and results from such studies should not be extrapolated to the small veins and venules (and vice versa) in which a much larger proportion of the blood volume lies.
Plethysmographic techniques for volume measurement
As outlined and illustrated in an earlier review of this series [61] conventional venous occlusion plethysmography (water displacement or strain gauge) can be used to assess changes in ‘limb venous volume’ [33, 34, 62, 63] and when modifying the protocol accordingly to assess capillary filtration [64] or permeability [65] and microvascular monitoring [66]. Measuring changes of the total volume of tissue, by placing the tissue (conventionally the forearm) in a chamber (plethysmograph) or measuring circumferential changes of a cross-sectional area of a limb (strain gauge) is the classical approach. Whilst this technique can provide a highly reproducible and accurate measure of tissue volume [62], several assumptions have to be made before concluding that the change in total tissue volume is representative of a change in vascular volume.
Strain gauge plethysmography
This measures changes in circumference of a cross-sectional area in a limb. It is based on the (usually reliable) assumption that circumferential changes reflect limb volume changes. It does not measure intravascular volume alone and assumes that the rate of venous pressure rise in the assessed cross-section equals the rate of capacity pressure rise, not taking account of varying viscoelastic properties of different veins leading to different filling pressures. Measurement of the entire P/V relationship is not possible, nor is it possible to be sure that the preparation has returned to the same control volume after experimental stimuli [4]. Whilst the technique is well validated and reproducible it is highly sensitive to limb movements which can lead to error over a prolonged period. When assessing vascular compliance using strain gauges, venous outflow is either obstructed abruptly or stepwise and the progressive change in volume (producing a lengthening of the tube and thus a thinning of the mercury column consequently leading to an increase in electrical resistance) is derived from the different sections (slopes) of the curve reflecting circumferential extension. After an early (seconds) rapid phase of circumferential increase, reflecting the rate of arterial inflow, the slope flattens out. This slope reflects a change in forearm volume, believed to be mainly caused by venous expansion. This is followed by a final slow steady state increase believed to represent interstitial fluid volume accumulation (leakage, lymph, secretion, viscoelastic creep). During this latter phase intravascular volume may actually start to decrease due to a change in transmural pressure. The difference between the asymptotic volume and steady state rate of change and the moment by moment changes in volume tend to follow a single exponential pattern between about 30 s and less than 5 min after a sudden change in venous pressure [4]. In other words the difference in volume of the studied limb before and after inflation of the occlusion cuff consequently reflects the blood pooled in the limb at the occlusion pressure applied. It does, however, not represent the total blood volume of this segment, but only the volume increase from basal volume after the occlusion [36]. Furthermore the small continued increase in forearm volume following prolonged venous occlusion is sometimes used to assess capillary filtration [64, 67]. Finally, there are some caveats to be considered when applying this assumptions; although vascular capacitance refers to a static, time independent relation, veins and capacitance vessels in particular also elicit some stress relaxation [68, 69] and delayed compliance [70], the time course of which extends to minutes [69, 70]. The technique of VOP itself however, is at its best when limited to relatively short time intervals because repeated and/or prolonged obstruction of venous outflow (via inflation of upper arm cuff) itself can cause a paradoxical decrease in forearm (vascular) volume for reasons outlined below.
Comparing radionuclide plethysmography and strain gauge plethysmography
Whilst P/V relations and acute changes in venous volume are highly correlated and reproducible [39, 52] using both techniques detailed analysis shows that they are not identical. Clements et al.[39] performed a series of simple but elegant experiments comparing both techniques. They recorded simultaneous changes in forearm volume and forearm count during sequential 1 min acquisitions, in which they inflated an upper arm cuff (‘collecting cuff’) in a stepwise manner, from 0 to 80 mmHg, followed by deflation back to 0 mmHg. Equilibration for 1 min was allowed after each pressure change at each 20 mmHg step before measurements were taken. A close correlation (r= 0.94 to r= 0.99) between percentage changes in count rate and forearm volume changes was present in each subject (n= 6). However, whereas forearm count rates had returned to baseline following final deflation, forearm circumferential volume remained slightly elevated, probably due to tissue fluid accumulation. To investigate this finding further they obstructed venous outflow for 18 min in a single subject. After 2 min of continuous cuff pressure, forearm circumferential volume continued to increase but forearm count rate decreased. Following cuff deflation the count rate returned to baseline within 1 min whilst arm volume remained elevated 5 min after cuff deflation. In a second experiment they occluded arterial inflow using a second cuff inflated to 300 mmHg proximal to the collecting cuff. Whilst in this setting inflation of the collecting cuff had no effect on count rate it produced a sustained upward deflection in the strain gauge measurement, most likely due to forearm distortion. Other groups reported a similar inflation artifact [71]. These findings are in keeping with earlier work by Zelis and coworkers [72] showing that ‘in normal human subjects short-term venous congestion and subsequent oedema fluid accumulation can result in qualitatively similar changes in limb vascular dynamics as are shown in heart failure patients’.
Whilst strain gauge plethysmography is the gold standard for assessing FBF, in our view the technique is suboptimal to assess venous capacitance when performing prolonged studies, dose–response studies with cumulative drug infusion, or assessing capacitance effects of drugs with marked effects on vascular conductivity, such as natriuretic and other vasoactive peptides, which have well documented effects on capillary filtration [64, 67, 73]. Indeed, the results of previous studies of venous vascular volume and tone in response to ANP in health [73] and CHF [74] using VOP contrast with the marked changes observed with radionuclide plethysmography in health [75] and CHF [76, 77]. These observations requires further confirmation. The issue of increased vascular permeability must be considered especially in disease states with established increased capillary permeability such as type-1 diabetes [78], CHF or sepsis [65].
More importantly conventional VOP does not provide a measure of unstressed volume or change in unstressed volume. In contrast radionuclide plethysmography provides a direct measure of intravascular unstressed volume and relative changes in unstressed volume.
Conclusion and perspective
Despite some theoretical limitations strain gauge plethysmography is regarded as the gold standard for assessment of changes in limb volume and has been used extensively to study human limb veins. This use is justified to assess the effect of drugs on venous physiology and pharmacology. Importantly, when modifying protocols accordingly, the technique is able to assess the effect of vasoactive drugs on capillary permeability. Whilst VOP, without the need of complex technology, serves a similar purpose as radionuclide plethysmography when assessing limb haemodynamics and the effects of local drug infusions, the latter technique has the potential to assess simultaneously further vascular beds, such as the splanchnic and pulmonary circulation, inaccessible to VOP. Furthermore, radionuclide plethysmography allows precise assessment of right and left ventricular function at baseline and during/following physiological (e.g. exercise or lower body negative pressure) and pharmacological stimuli. Combining the advantages of equilibrium blood pool scintigraphy and conventional occlusive techniques has therefore the potential to be used for complex (central and peripheral) haemodynamic assessment of cardiovascular drugs, a prerequisite for optimizing and tailoring medical treatment in conditions such as chronic heart failure and essential hypertension. Finally, as a research tool, radionuclide plethysmography has the advantage of providing a direct measure of changes in unstressed intravascular volume and may be the more suitable technique for prolonged studies, and when assessing changes in vascular volume in disease states with known increased capillary filtration such as diabetes and heart failure, albeit at the cost of not providing a measure of the degree of capillary filtration. The latter weakness could be overcome by combining both techniques.
MS is supported by the British Cardiac Society – De Bono Research Fellowship. JRC and MPF are supported by the British Heart Foundation. The authors wish to thank Mrs Jan Sharpe for her help with the illustrations.
References
- 1.Wilkins RW, Bradley SE. Changes in arterial and venous blood pressure and flow distal to a cuff inflated on the human arm. Am J Physiol. 1946;147:260–269. doi: 10.1152/ajplegacy.1946.147.2.260. [DOI] [PubMed] [Google Scholar]
- 2.Webb DJ, Hand MF. Assessment of the effects of drugs on the peripheral vasculature. In: Nimmo WS, Tucker GT, editors. Clinical Measurement in Drug Evaluation. London: John Wiley & Sons Ltd; 1995. pp. 136–150. [Google Scholar]
- 3.Hokanson DE, Sumner DS, Strandness DE., Jr An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng. 1975;22:25–29. doi: 10.1109/tbme.1975.324535. [DOI] [PubMed] [Google Scholar]
- 4.Rothe C. Venous system: Physiology of the capacitance vessels. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology: The Cardiovascular System Peripheral Circulation. Washington DC: American Physiological Society; 1983:. pp. 397–452. [Google Scholar]
- 5.Abboud FM, Mark AL, Heidstadd DD, Schmid PG, Barnes RW. The venous system. In: Levine H, editor. Cardiovascular Pathophysiology. New York: Grune and Stratton; 1976. pp. 207–257. [Google Scholar]
- 6.Flamm SD, Taki J, Moore R, et al. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation. 1990;81:1550–1559. doi: 10.1161/01.cir.81.5.1550. [DOI] [PubMed] [Google Scholar]
- 7.Thomson HL, Atherton JJ, Khafagi FA, Frenneaux MP. Failure of reflex venoconstriction during exercise in patients with vasovagal syncope. Circulation. 1996;93:953–959. doi: 10.1161/01.cir.93.5.953. [DOI] [PubMed] [Google Scholar]
- 8.Thomson HL, Morris-Thurgood J, Atherton J, McKenna W, Frenneaux MP. Reflex responses of venous capacitance vessels in patients with hypertophic cardiomyopathy. Clin Sci. 1998;94:339–346. doi: 10.1042/cs0940339. [DOI] [PubMed] [Google Scholar]
- 9.Wang SY, Manyari DE, Scott-Douglas N, Smiseth OA, Smith ER, Tyberg JV. Splanchnic venous pressure–volume relation during experimental acute ischemic heart failure. Differential effects of hydralazine, enalaprilat, and nitroglycerin. Circulation. 1995;91:1205–1212. doi: 10.1161/01.cir.91.4.1205. [DOI] [PubMed] [Google Scholar]
- 10.Wang SY, Manyari DE, Tyberg JV. Cardiac vagal reflex modulates intestinal vascular capacitance and ventricular preload in anesthetized dogs with acute myocardial infarction. Circulation. 1996;94:529–533. doi: 10.1161/01.cir.94.3.529. [DOI] [PubMed] [Google Scholar]
- 11.Atherton JJ, Moore TD, Lele SS, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349:1720–1724. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- 12.Atherton JJ, Thomson HL, Moore TD, et al. Diastolic ventricular interaction: a possible mechanism for abnormal vascular responses during volume unloading in heart failure. Circulation. 1997;96:4273–4279. doi: 10.1161/01.cir.96.12.4273. [DOI] [PubMed] [Google Scholar]
- 13.Atherton JJ, Blackman DJ, Moore TD, et al. Diastolic ventricular interaction in chronic heart failure: relation to heart rate variability and neurohumoral status. Heart Vessels. 1998;13:269–277. doi: 10.1007/BF03257231. [DOI] [PubMed] [Google Scholar]
- 14.Tyberg JV, Belenkie I, Manyari DE, Smith ER. Ventricular interaction and venous capacitance modulate left ventricular preload. Can J Cardiol. 1996;12:1058–1064. [PubMed] [Google Scholar]
- 15.Webb-Peploe MM. The isovolumetric spleen: index of reflex changes in splanchnic vascular capacity. Am J Physiol. 1969;216:407–413. doi: 10.1152/ajplegacy.1969.216.2.407. [DOI] [PubMed] [Google Scholar]
- 16.Kjekshus H, Risoe C, Scholz T, Smiseth OA. Methods for assessing hepatic distending pressure and changes in hepatic capacitance in pigs. Am J Physiol. 2000;279:H1796–H1803. doi: 10.1152/ajpheart.2000.279.4.H1796. [DOI] [PubMed] [Google Scholar]
- 17.Kjekshus H, Risoe C, Scholz T, Smiseth OA. Regulation of hepatic vascular volume: contributions from active and passive mechanisms during catecholamine and sodium nitroprusside infusion. Circulation. 1997;96:4415–4423. doi: 10.1161/01.cir.96.12.4415. [DOI] [PubMed] [Google Scholar]
- 18.Hadjiminas J, Oberg B. Effects of carotid baroreceptor reflexes on venous tone in skeletal muscle and intestine of the cat. Acta Physiol Scand. 1968;72:518–532. doi: 10.1111/j.1748-1716.1968.tb03876.x. [DOI] [PubMed] [Google Scholar]
- 19.DiSalvo J, Parker PE, Scott JB, Haddy FJ. Carotid baroreceptor influence on total and segmental resistances in skin and muscle vasculatures. Am J Physiol. 1971;220:1970–1978. doi: 10.1152/ajplegacy.1971.220.6.1970. [DOI] [PubMed] [Google Scholar]
- 20.Dow RW, Fry WJ. Regulation of venous capacity: the carotid sinus mechanism. Surgery. 1968;64:346–350. [PubMed] [Google Scholar]
- 21.Gero J, Gerova M. Sympathetic regulation of collecting vein. Experientia. 1968;24:811–812. doi: 10.1007/BF02144886. [DOI] [PubMed] [Google Scholar]
- 22.Brender D, Webb-Peploe MM. Influence of carotid baroreceptors on different components of the vascular system. J Physiol. 1969;205:257–274. doi: 10.1113/jphysiol.1969.sp008963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karim F, Ali H. Effect of electrical stimulation of the carotid sinus on venomotor tone of the superior vena cava in dogs. Life Sci. 1969;8:791–798. doi: 10.1016/0024-3205(69)90139-8. [DOI] [PubMed] [Google Scholar]
- 24.Brooksby GA, Donald DE. Dynamic changes in splanchnic blood flow and blood volume in dogs during activation of sympathetic nerves. Circ Res. 1971;29:227–238. doi: 10.1161/01.res.29.3.227. [DOI] [PubMed] [Google Scholar]
- 25.Vanhoutte PM. Adjustments in the peripheral circulation in chronic heart failure. Eur Heart J. 1983;4(Suppl A):67–83. doi: 10.1093/eurheartj/4.suppl_a.67. [DOI] [PubMed] [Google Scholar]
- 26.Sheperd JT, Vanhoutte PM. Participation of the veins in cardiovascular reflexes. In: Sheperd JT, Vanhoutte PM, editors. Veins and Their Control. Philadelphia: W.B. Saunders; 1975. pp. 153–170. [Google Scholar]
- 27.Johnson JM, Rowell LB. Forearm skin and muscle vascular responses to prolonged leg exercise in man. J Appl Physiol. 1975;39:920–924. doi: 10.1152/jappl.1975.39.6.920. [DOI] [PubMed] [Google Scholar]
- 28.Nadel ER, Cafarelli E, Roberts MF, Wenger CB. Circulatory regulation during exercise in different ambient temperatures. J Appl Physiol Respirat Environ Exerc Physiol. 1979;46:430–437. doi: 10.1152/jappl.1979.46.3.430. [DOI] [PubMed] [Google Scholar]
- 29.Nadel ER, Fortney SM, Wenger CB. Effect of hydration state on circulatory and thermal regulations. J Appl Physiol Respirat Environ Exerc Physiol. 1980;49:715–721. doi: 10.1152/jappl.1980.49.4.715. [DOI] [PubMed] [Google Scholar]
- 30.Roberts MF, Wenger CB. Control of skin blood flow during exercise by thermal reflexes and baroreflexes. J Appl Physiol Respirat Environ Exerc Physiol. 1980;48:717–723. doi: 10.1152/jappl.1980.48.4.717. [DOI] [PubMed] [Google Scholar]
- 31.Fortney SM, Wenger CB, Bove JR, Nadel ER. Effect of blood volume on forearm venous and cardiac stroke volume during exercise. J Appl Physiol Respirat Environ Exerc Physiol. 1983;55:884–890. doi: 10.1152/jappl.1983.55.3.884. [DOI] [PubMed] [Google Scholar]
- 32.Tripathi A, Mack GW, Nadel ER. Cutaneous vascular reflexes during exercise in the heat. Med Sci Sports Exerc. 1990;22:796–803. doi: 10.1249/00005768-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Carpentier PH. Methods for vascular exploration in man: microcirculation and veins. Therapie. 1999;54:369–374. [PubMed] [Google Scholar]
- 34.Whitney RJ. The measurement of volume changes in human limbs. J Physiol. 1953;121:1–27. doi: 10.1113/jphysiol.1953.sp004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aellig WH. A new technique for recording compliance of human hand veins. Br J Clin Pharmacol. 1981;11:237–243. doi: 10.1111/j.1365-2125.1981.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins – 1. Br J Clin Pharmacol. 1994;38:181–196. doi: 10.1111/j.1365-2125.1994.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins – 2. Br J Clin Pharmacol. 1994;38:289–305. doi: 10.1111/j.1365-2125.1994.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutlen DL, Wackers FJ, Zaret BL. Radionuclide assessment of peripheral intravascular capacity: a technique to measure intravascular volume changes in the capacitance circulation in man. Circulation. 1981;64:146–152. doi: 10.1161/01.cir.64.1.146. [DOI] [PubMed] [Google Scholar]
- 39.Clements IP, Strelow DA, Becker GP, Vlietstra RE, Brown ML. Radionuclide evaluation of peripheral circulatory dynamics: new clinical application of blood pool scintigraphy for measuring limb venous volume, capacity, and blood flow. Am Heart J. 1981;102:980–983. doi: 10.1016/0002-8703(81)90480-4. [DOI] [PubMed] [Google Scholar]
- 40.Robinson VJ, Manyari DE, Tyberg JV, Fick GH, Smith ER. Volume–pressure analysis of reflex changes in forearm venous function. A method by mental arithmetic stress and radionuclide plethysmography. Circulation. 1989;80:99–105. doi: 10.1161/01.cir.80.1.99. [DOI] [PubMed] [Google Scholar]
- 41.Manyari DE, Wang Z, Cohen J, Tyberg JV. Assessment of the human splanchnic venous volume–pressure relation using radionuclide plethysmography. Effect of nitroglycerin. Circulation. 1993;87:1142–1151. doi: 10.1161/01.cir.87.4.1142. [DOI] [PubMed] [Google Scholar]
- 42.Blackman DJ, Morris-Thurgood JA, Atherton JJ, et al. Endothelium-derived nitric oxide contributes to the regulation of venous tone in humans. Circulation. 2000;101:165–170. doi: 10.1161/01.cir.101.2.165. [DOI] [PubMed] [Google Scholar]
- 43.Atherton JJ, Dryburgh LG, Thomson HL, et al. Forearm vasoconstriction during dynamic leg exercise in patients with chronic heart failure. Heart Vessels. 1998;13:278–289. doi: 10.1007/BF03257232. [DOI] [PubMed] [Google Scholar]
- 44.Thomson H, Morris-Thurgood J, Atherton J, Frenneaux MP. Forearm vascular responses during semierect dynamic leg exercise in patients following myocardial infarction. Heart Vessels. 1998;13:87–94. doi: 10.1007/BF01744591. [DOI] [PubMed] [Google Scholar]
- 45.Thomson HL, Lele SS, Atherton JJ, Wright KN, Stafford W, Frenneaux MP. Abnormal forearm vascular responses during dynamic leg exercise in patients with vasovagal syncope. Circulation. 1995;92:2204–2209. doi: 10.1161/01.cir.92.8.2204. [DOI] [PubMed] [Google Scholar]
- 46.Ablad B, Mellander S. Comparative effects of hydralazine, sodium nitrate, and acetylcholine on resistance and capacitance blood vessels and capillary filtration on skeletal muscle in the cat. Acta Physiol Scand. 1963;58:319–324. doi: 10.1111/j.1748-1716.1963.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 47.Baker CH, O'Brien LJ. Vascular volume changes in the dog forelimb. Am J Physiol. 1964;206:1291–1296. [Google Scholar]
- 48.Callahan JR, Froelich JW, McKusick KA, Leppo J, Strauss HW. A modified method for the in vivo labelling of red cells with Tc-99m. J Nucl Med. 1982;23:315–318. [PubMed] [Google Scholar]
- 49.Scott-Douglas NW, Manyari DE, Smiseth OA, et al. Measurement of intestinal vascular capacitance in dogs: an application of blood pool scintigraphy. J Appl Physiol. 1995;78:232–238. doi: 10.1152/jappl.1995.78.1.232. [DOI] [PubMed] [Google Scholar]
- 50.Manyari DE, Rose S, Tyberg JV, Sheldon RS. Abnormal reflex venous function in patients with neuromediated syncope. J Am Coll Cardiol. 1996;27:1730–1735. doi: 10.1016/0735-1097(96)00051-4. [DOI] [PubMed] [Google Scholar]
- 51.Nightingale AK, Blackman DJ, Ellis GR, et al. Preservation of venous endothelial function in the forearm venous capacitance bed of patients with chronic heart failure despite arterial endothelial dysfunction. J Am Coll Cardiol. 2001;37:1062–1068. doi: 10.1016/s0735-1097(01)01142-1. [DOI] [PubMed] [Google Scholar]
- 52.Manyari DE, Malkinson TJ, Robinson V, Smith ER, Cooper KE. Acute changes in forearm venous volume and tone using radionuclide plethysmography. Am J Physiol. 1988;255:H947–H952. doi: 10.1152/ajpheart.1988.255.4.H947. [DOI] [PubMed] [Google Scholar]
- 53.Pennel DJ, Prvulovich E. Nuclear Cardiology. 1. Hatfield/UK: Impact Healthcare – Marketing Communication Group Ltd; 1995. Radionuclide Ventriculography; pp. 163–188. [Google Scholar]
- 54.Blackman DJ. Birmingham, England: University of Birmingham: Nitric oxide and venous tone in health and heart failure. Thesis. [Google Scholar]
- 55.Robinson BF, Collier JG, Dobbs RJ. Acquired tolerance to dilator action of hydralazine during oral administration. Br J Clin Pharmacol. 1980;9:407–412. doi: 10.1111/j.1365-2125.1980.tb01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collier JG, Lorge RE, Robinson BF. Comparison of effects of tolmesoxide (RX71107), diazoxide, hydrallazine, prazosin, glyceryl trinitrate and sodium nitroprusside on forearm arteries and dorsal hand veins of man. Br J Clin Pharmacol. 1978;5:35–44. doi: 10.1111/j.1365-2125.1978.tb01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wathen CG, Hannan WJ, Adie CJ, Muir AL. A radionuclide method for the simultaneous study of the effects of drugs on central and peripheral haemodynamics. Br J Clin Pharmacol. 1983;16:45–50. doi: 10.1111/j.1365-2125.1983.tb02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manyari DE, Smith ER, Spragg J. Isosorbide dinitrate and glyceryl trinitrate: demonstration of cross tolerance in the capacitance vessels. Am J Cardiol. 1985;55:927–931. doi: 10.1016/0002-9149(85)90719-2. [DOI] [PubMed] [Google Scholar]
- 59.Saltin B. Hemodynamic adaptations to exercise. Am J Cardiol. 1985;55:42–47. doi: 10.1016/0002-9149(85)91054-9. [DOI] [PubMed] [Google Scholar]
- 60.Christ F, Gamble J, Baschnegger H, Gartside IB. Relationship between venous pressure and tissue volume during venous congestion plethysmography in man. J Physiol. 1997;503:463–467. doi: 10.1111/j.1469-7793.1997.463bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631–646. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ensink FB, Baller D, Wolpers HG, Zipfel J, Hellige G. The reliability of venous capacity and blood flow determination by plethysmograph. In: Jagenau AHM, editor. Non-Invasive Methods on Cardiovascular Haemodynamics. Amsterdam: Elsevier/North Holland; 1981. pp. 215–226. [Google Scholar]
- 63.Ensink FBM, Hellige G. History and principle of strain gauge plethysmography. In: Jagenau AHM, editor. Non-Invasive Methods on Cardiovascular Haemodynamics. Amsterdam: Elsevier/North Holland; 1981. pp. 169–183. [Google Scholar]
- 64.Ando S, Imaizumi T, Harada S, Hirooka Y, Takeshita A. Atrial natriuretic peptide increases human capillary filtration and venous distensibility. J Hypertens. 1992;10:451–457. doi: 10.1097/00004872-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Christ F, Gamble J, Gartside IB, Kox WJ. Increased microvascular water permeability in patients with septic shock, assessed with venous congestion plethysmography (VCP) Intensive Care Med. 1998;24:18–27. doi: 10.1007/s001340050509. [DOI] [PubMed] [Google Scholar]
- 66.Christ F, Gartside IB, Kox WJ, Gamble J. Microvascular monitoring using mercury in silastic strain gauge plethysmography (MSG) Infusionsther Transfusionsmed. 1993;20:253–259. [PubMed] [Google Scholar]
- 67.Groban L, Cowley AW, Ebert TJ. Atrial natriuretic peptide augments forearm capillary filtration in humans. Am J Physiol. 1990;259:H258–H263. doi: 10.1152/ajpheart.1990.259.1.H258. [DOI] [PubMed] [Google Scholar]
- 68.Gauer OH, Thron HL. Properties of veins in vivo: integrated effects of their smooth muscle. Physiol Rev. 1962;42(Suppl 5):283–303. [PubMed] [Google Scholar]
- 69.Alexander RS, Edwards WS, Ankeney JL. The distensibility characteristics of the portal vascular bed. Circ Res. 1953;1:271–277. doi: 10.1161/01.res.1.3.271. [DOI] [PubMed] [Google Scholar]
- 70.Porciuncula CI, Armstrong GG, Guyton AC, Stone HL. Delayed compliance in external jugular vein of the dog. Am J Physiol. 1964;207:728–732. doi: 10.1152/ajplegacy.1964.207.3.728. [DOI] [PubMed] [Google Scholar]
- 71.Bevegard BS, Sheperd JT. Reaction in man of resistance and capacitance vessels in forearm and hand to leg exercise. J Appl Physiol. 1966;21:123–132. doi: 10.1152/jappl.1966.21.1.123. [DOI] [PubMed] [Google Scholar]
- 72.Zelis R, Capone R, Mansour E, Field JM. The effects of short term venous congestion on forearm venous volume and reactive hyperaemia blood flow in human subjects. Circulation. 1978;57:1001–1003. doi: 10.1161/01.cir.57.5.1001. [DOI] [PubMed] [Google Scholar]
- 73.Doorenbos CJ, Blauw GJ, van Brummelen P. Arterial and venous effects of atrial natriuretic peptide in the human forearm. Am J Hypertens. 1991;4:333–340. doi: 10.1093/ajh/4.4.333. [DOI] [PubMed] [Google Scholar]
- 74.Ikenouchi H, Sato H, Hirata Y, Iizuka M, Serizawa T, Sugimoto T. Lack of venodilative activity of alpha human atrial natriuretic polypeptide in patients with congestive heart failure. Jpn Heart J. 1989;30:443–457. doi: 10.1536/ihj.30.443. [DOI] [PubMed] [Google Scholar]
- 75.Schmitt M, Nightingale AK, Broadley A, et al. Atrial natriuretic peptide regulates venous capacitance in man. Circulation. 2001;104(Suppl 2) [Google Scholar]
- 76.Schmitt M, Broadley AJ, Middleton GW, et al. reserved venous ANP responsiveness in chronic heart failure is partially mediated via endothelial nitric oxide. J Am Coll Cardiol. 2002;39(Suppl B):4327. [Google Scholar]
- 77.Schmitt M, Taylor J, Broadley A, et al. ANP but not CNP modulates forearm capacitance in CHF. Eur J Heart Fail. 2002;1:364. [Google Scholar]
- 78.Jaap AJ, Shore AC, Gartside IB, Gamble J, Tooke JE. Increased microvascular fluid permeability in young type 1 (insulin-dependent) diabetic patients. Diabetologia. 1993;36:648–652. doi: 10.1007/BF00404075. [DOI] [PubMed] [Google Scholar]