Abstract
Aims
Angiotensin II is a putative mediator in bronchial asthma. There have been very few studies investigating the involvement of angiotensin II receptors in bronchial hyper-responsiveness in asthmatic patients. We examined the effect of candesartan cilexetil, a specific angiotensin II type 1 (AT1) receptor antagonist, on bronchial responsiveness to inhaled methacholine in patients with asthma.
Methods
Bronchial responsiveness to methacholine, assessed as the concentration of methacholine producing a 20% fall in FEV1 (PC20–FEV1), was measured on three occasions 2 weeks apart in 11 stable asthmatic patients. Candesartan cilexetil (8 mg once a day) or a placebo was orally administered for 1 week before the methacholine provocation test in a double-blind, randomized, crossover manner.
Results
Although there were no significant differences between treatment periods in FEV1 values at baseline, the geometric mean (95% CI) PC20–FEV1 values increased significantly (P= 0.041) from 0.691 (0.379, 1.259) mg ml−1 with placebo to 0.837 (0.506, 1.384) mg ml−1 with candesartan. Candesartan decreased the mean (95% CI) arterial blood pressure (placebo: 95.6 (89.0, 102.2) mmHg, candesartan: 86.4 (79.8, 93.1) mmHg, P= 0.015). There was no correlation between the change in blood pressure and the change in PC20–FEV1.
Conclusions
We conclude that AT1 receptors are involved in bronchial hyper-responsiveness in asthmatic patients.
Keywords: angiotensin II type 1 receptor, angiotensin II, asthma, bronchial hyper-responsiveness, candesartan cilexetil, methacholine
Introduction
Angiotensin II mediates most of the biological effects of the renin-angiotensin system (RAS), and is formed from angiotensin I by the angiotensin-converting enzyme (ACE), which is highly expressed in the lungs [1]. There are two major receptor binding sites for angiotensin II that can be defined pharmacologically by losartan and PD123177, and act as angiotensin II type 1 (AT1) and type 2 (AT2) receptors [2, 3]. Most of the known physiological functions of angiotensin II, including vasoconstriction, aldosterone release, enhanced noradrenaline release, feedback control of renin release, and drinking behaviour, are mediated by the AT1 receptor subtype [4]. Both Northern [5] and Western blot [6] analyses have demonstrated that the AT1 receptor expression in human lung tissue.
Some animal studies have demonstrated that AT1 receptors are involved in angiotensin II-induced bronchoconstriction in guinea pigs [7, 8], peptide leukotriene (LT) production in guinea pig airways [7], potentiating effect of angiotensin II on endothelin-1-induced contraction of bovine bronchial smooth muscle [9], and Cl secretion by canine tracheal epithelium [10]. We previously demonstrated that candesartan, an AT1 receptor antagonist, prevented antigen-induced airway hyper-responsiveness and eosinophil accumulation in the guinea pig airway [11]. These observations suggest that angiotensin II is a putative mediator in bronchial asthma. Indeed, in asthmatic patients, activation of the RAS with an elevation in plasma renin and angiotensin II levels is observed during severe acute attacks [12, 13]. Angiotensin II causes bronchoconstriction in mildly asthmatic patients [14] and angiotensin II in subthreshold concentrations increases bronchial responsiveness to methacholine both in human bronchi in vitro and in mildly asthmatic patients in vivo[14]. We demonstrated that losartan, an AT1 receptor antagonist, slightly reduced methacholine airway hyper-responsiveness in patients with asthma [15]. Recently, Tanaka et al.[16] reported that bronchial hyper-responsiveness has a tendency to improve in asthmatic patients treated with candesartan, but with no statistically significant difference. In their study, however, the dose of candesartan may have been insufficient and 95% of asthmatic patients were treated with inhaled corticosteroids of 200 µg to 1600 µg, which may mask the reducing effect of candesartan on bronchial hyper-responsiveness. In this study, to determine the effect of candesartan on bronchial hyper-responsiveness in patients with asthma, we used the recommended maximum dose of candesartan, and enrolled asthmatic patients who were not taking high dose inhaled corticosteroids.
Methods
Patients
Eleven asthmatic patients (eight males, three females, aged 29–68 years) with baseline FEV1 values of 54.9–100.5% of predicted values were studied (Table 1). None of the patients had ever smoked or experienced any occupational exposure, and each patient satisfied the American Thoracic Society definition of asthma, with symptoms of episodic wheezing, cough, and shortness of breath responding to bronchodilators and reversible airflow obstruction (more than 15% reversibility in terms of FEV1) documented in at least one pulmonary function study [17]. The patients had no history of excessive mucus expectoration, and no low-attenuation areas on thin-slice chest. None of the patients had taken theophylline, antihistamines, sodium cromoglycate, or oral corticosteroids for at least 2 months prior to the study, and none had experienced an upper respiratory tract infection in the preceding month or during the study. The study was performed while symptoms were mild and stable. Written informed consent was obtained from all patients. The study was approved by the ethics committee of the university hospital.
Table 1.
Subject characteristics.
| Subject | Age(years) | Sex | FEV1(% predicted) | FEV1/FVC(%) | Treatment |
|---|---|---|---|---|---|
| 1 | 61 | M | 80.5 | 54.2 | S |
| 2 | 29 | M | 100.5 | 79.7 | S |
| 3 | 63 | M | 83.1 | 62.5 | S, B (400) |
| 4 | 35 | M | 80.8 | 71.7 | S |
| 5 | 61 | M | 100.4 | 79.8 | S |
| 6 | 47 | F | 97.2 | 73.2 | S |
| 7 | 64 | F | 92.3 | 70.9 | S, B (800) |
| 8 | 57 | M | 89.0 | 70.2 | S |
| 9 | 66 | M | 76.9 | 53.6 | S, B (400) |
| 10 | 41 | F | 71.8 | 72.0 | S, B (400) |
| 11 | 68 | M | 54.9 | 54.2 | S, B (800) |
| Mean | 54 | 84.3 | 67.5 | ||
| 95% CI | 45, 63 | 75.1, 93.5 | 60.9, 74.0 |
Be (mg) = beclomethasone dipropionate via metered dose inhaler (daily inhaled dose); S = salbutamol via metered dose inhaler on demand.
Study protocol
The study was performed in a randomized, double-blind, placebo-controlled, two-period crossover manner. The methacholine concentration producing a 20% fall in FEV1 (PC20–FEV1) was measured on three occasions 2 weeks apart. The first methacholine test was at a run-in period, and the second and third tests were in the crossover phase. Candesartan cilexetil was administered orally at a dose of 8 mg once a day, 30 min after breakfast, for 6 days, and at 08.00 h on the 7th day (test day). At the time of crossover from the first to the second treatment regimen, administration of the test drug was suspended for 1 week. Permitted medication, which remained unchanged during the study, included inhaled β2-adrenoceptor agonists and inhaled corticosteroids up to 800 µg day−1. Inhaled β2–adrenoceptor agonists and inhaled corticosteroids were stopped at 13.00 h on the previous day to allow a washout time of at least 24 h. The bronchial responsiveness to inhaled methacholine was then determined at 13.00 h after blood pressure was measured. Blood pressure was measured on the same arm at each visit with a mercury sphygmomanometer with a cuff of appropriate size. After 5 min of rest, sitting systolic blood pressure and diastolic blood pressure were measured to the nearest 2 mmHg.
Bronchial responsiveness to inhaled methacholine
Methacholine was dissolved in physiological saline solution to produce concentrations of 0.04, 0.08, 0.16, 0.31, 0.63, 1.25, 2.5, 5, 10, 20, 40, and 80 mg ml−1. Saline and each solution were inhaled from a DeVilbiss 646 nebulizer (DeVilbiss Co., Somerset, PA) operated by compressed air at 5 l min−1. The nebulizer output was 0.14 ml min−1. Saline was inhaled first for 2 min and FEV1(Chestac 55, Chest Ltd, Nagoya, Japan) was measured. If the change in FEV1 from the baseline value was 10% or less, inhalation of methacholine was started, and if the saline solution induced a change in FEV1> 10%, the test was stopped or postponed. Methacholine was inhaled for 2 min by tidal mouth breathing with the patients wearing a nose clip, followed immediately by three measurements of FEV1 at 1 min intervals; the curve with the largest FEV1 was retained for analysis. Increasing concentrations of methacholine were inhaled until a fall of 20% or more in FEV1 occurred.
Data analysis
All data were expressed as mean and 95% confidence intervals (95% CI). PC20-FEV1 was determined by linear interpolation from the log dose–response curve, and was logarithmically transformed for analysis. Statistical analyses of PC20-FEV1 values were performed on logarithmically transformed data. Factorial repeated measure anova was used for testing for direct effect, period effect and treatment by period interaction (carry-over effect). Change in PC20-FEV1 and blood pressure between candesartan day and placebo day was calculated as (baseline values after candesartan minus baseline values after placebo)/baseline values after placebo. Statistical differences between two-period crossover examinations were determined by Student's paired t-test. Correlations were obtained using Pearson's correlation coefficient. A value of P< 0.05 was considered to be statistically significant.
Results
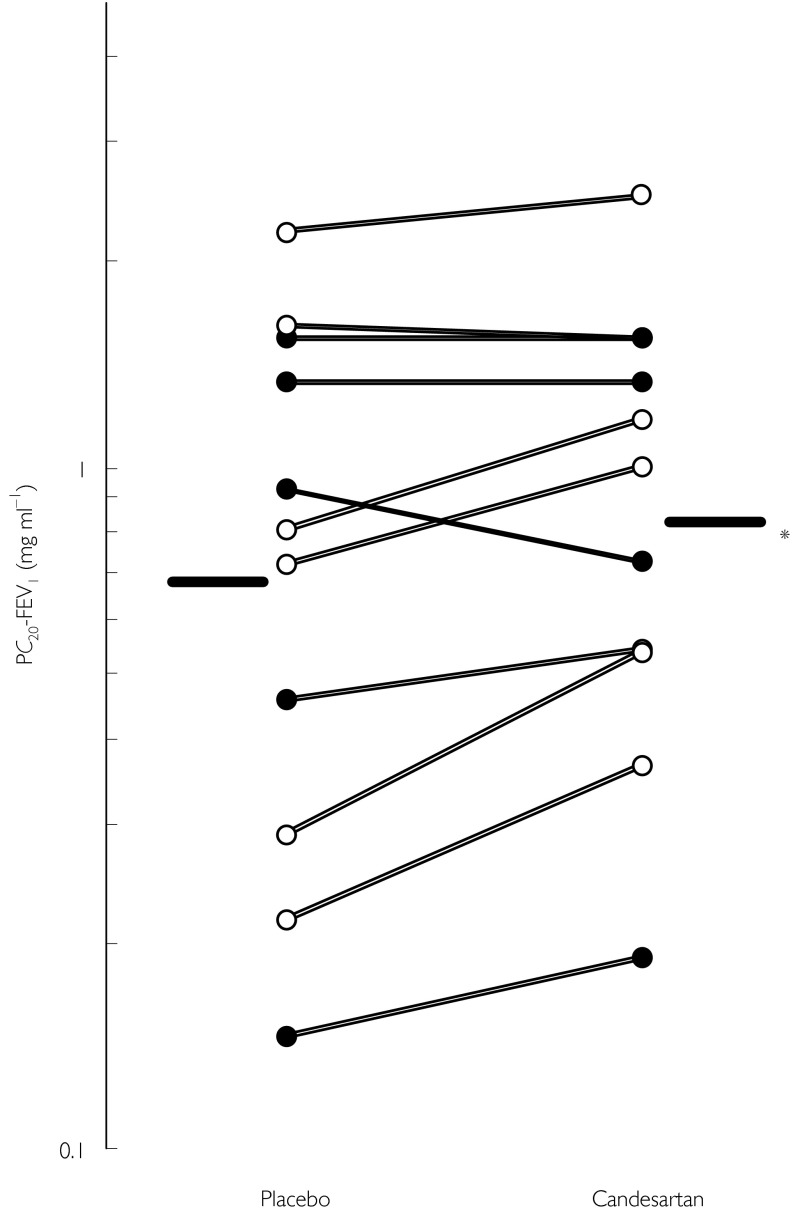
Six and five patients received candesartan and placebo for the first treatment period, respectively. Baseline (run-in period) values of PC20-FEV1 did not differ between the groups (P= 0.242). Results of analyses are shown in Table 2. Treatment by period interaction (carry-over effect) was not significant (P= 0.09). PC20-FEV1 values in the first period did not significantly differ from the values in the second period (P= 0.395). The geometric mean PC20-FEV1 values increased significantly (direct treatment effect, P= 0.041) from 0.691 (0.379, 1.259) mg ml−1 with placebo to 0.837 (0.506, 1.384) mg ml−1 with candesartan (Figure 1). There were no significant differences in baseline FEV1 values between treatments (placebo: 2.31 (1.82, 2.79) l, candesartan: 2.38 (1.91, 2.86) l, P= 0.184).
Table 2.
Summary of data on bronchial hyper-responsiveness.
| First treatment | Number of patients | Baseline (run-in period) | Candesartan | Placebo |
|---|---|---|---|---|
| Candesartan | 6 | 0.534 (0.187, 1.574) | 0.689 (0.256, 1.854) | 0.561 (0.171, 1.845) |
| Placebo | 5 | 0.889 (0.445, 1.778) | 1.059 (0.637, 1.758) | 0.887 (0.482, 1.637) |
| Total | 11 | 0.679 (0.386, 1.194) | 0.837 (0.506, 1.384) | 0.691 (0.379, 1.259) |
Data are shown as geometric mean (95% CI). Treatment by period interaction: P= 0.09. Period effect: P= 0.395. Direct effect: P= 0.041 (candesartan > placebo).
Figure 1.
The effect of candesartan cilexetil on bronchial hyper-responsiveness to methacholine in asthmatic patients. ○: patients not taking inhaled steroids. •: patients taking inhaled steroids. PC20-FEV1: The methacholine concentration producing a 20% fall in FEV1. Bars: geometric mean PC20-FEV1. *P= 0.034.
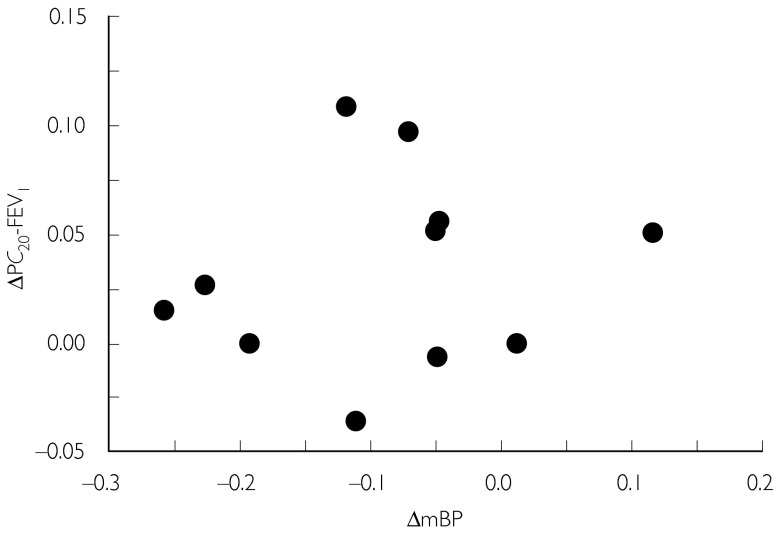
Candesartan decreased the mean blood pressure (placebo: 95.6 (89.0, 102.2) mmHg, candesartan: 86.4 (79.8, 93.1), mmHg, P= 0.015) without affecting heart rate (placebo: 80.5 (72.4, 88.5) min−1, candesartan: 80.0 ± (71.9, 88.1) beats min−1, P= 0.922). There was no correlation between the change in blood pressure and the change in PC20-FEV1 (r= 0.171, P= 0.850; Figure 2). No patient complained of adverse effects.
Figure 2.
Relationship between change in mean blood pressure (ΔmBP) and PC20-FEV1(ΔPC20-FEV1). There was no significant correlation between them. r= 0.171, P= 0.850.
Discussion
The present study investigated the effect of candesartan cilexetil on bronchial hyper-responsiveness to methacholine in 11 patients with asthma. The results indicated that the PC20-FEV1 was attenuated by candesartan. The effects of candesartan on PC20-FEV1 appear to be specific, because the drug alone did not affect baseline FEV1. Therefore, the effect of candesartan on PC20-FEV1 cannot be attributed to bronchodilatory effects of the compound. As the sample size of this study was small, the power was calculated retrospectively. The calculation showed that the sample size would give a 70% probability of detecting a true effect of candesartan in the ratio of PC20-FEV1 with candesartan to PC20-FEV1 with placebo (>1.25) when using a test at the 5% significance level and the standard deviation of the ratio investigated.
Two distinct subtypes of the angiotensin II receptor have been defined based on their pharmacologic and biochemical properties and are designated as AT1 and AT2 receptors [2, 3]. Candesartan cilexetil is the prodrug form of CV-11974, a new AT1 receptor antagonist with high affinity in a receptor binding assay and high potency in a rabbit aorta contraction assay [18]. We [15] previously reported that losartan inhibits bronchial hyper-responsiveness in asthmatic patients. Losartan interacts with TXA2/prostaglandin H2 (PGH2) receptors [19] and inhibits induction of platelet aggregation and vasoconstriction in rats by the TXA2 analogue U46619 [20] which augments bronchial responsiveness in asthmatic patients [21]. This inhibition is specific for losartan, and CV-11974 does not interact with TXA2 receptors [20]. Furthermore, the prodrug form of candesartan has no effect on vascular contraction induced by noradrenaline, potassium chloride, serotonin, PGF2α, or endothelin [22], indicating that candesartan is a highly selective angiotensin II inhibitor. These observations suggest that AT1 receptors are involved in bronchial hyper-responsiveness in asthmatic patients.
Although angiotensin II increases bronchial responsiveness to methacholine both in human bronchi in vitro and in mildly asthmatic patients in vivo[14], Dicpinigaitis et al.[23] reported that losartan had no effect on PC20-FEV1 in stable mildly asthmatic patients. In their study [23], however, cardiovascular effects, which are useful parameters for confirming the dose of losartan administered, were not assessed. Indeed, we [15] recently demonstrated that losartan at doses sufficient to decrease blood pressure attenuated methacholine hyper-responsiveness in asthmatic patients. Recently, Tanaka et al.[16] demonstrated that 4 mg candesartan had a tendency to reduce bronchial hyper-responsiveness in asthmatic patients. Recommended doses of candesartan are 4–8 mg day−1 in Japan, and we used 8 mg candesartan in this study. Furthermore, in their study, 57 of 60 patients received inhalation of 200 µg to 1600 µg beclomethasone. In this study, we excluded patients receiving more than 800 µg beclomethasone. These differences might be the reason for our positive results with candesartan. However, the usefulness of candesartan for the treatment of asthma is thought to be unclear because the reducing effect of candesartan on bronchial hyperesponsiveness is not very strong. On the other hand, more marked effects were observed in patients without inhaled steroids compared with patients with inhaled steroids. Therefore, inhalation of steroids could explain why there were differences in the patients with more marked/less marked effects. However, 11 patients are too small a sample from which to draw a conclusion.
In the present study, candesartan decreased blood pressure, which has a potential effect on airway responsiveness through a number of reflex mechanisms, such as a rise in circulating adrenaline. Indeed, Fish et al.[24] reported that calcium channel blocker, verapamil, inhibited the asthmatic airway response to methacholine, but not histaminergic or allergic stimuli. In this study, there was no correlation between the change in blood pressure and the change in PC20-FEV1, suggesting that the attenuating effect of candesartan on PC20-FEV1 is not due to a decrease in blood pressure. It cannot be concluded, however, that the effect observed is specific for the AT1 receptor antagonist. Additional studies using another antihypertensive compound such as calcium antagonists are required to determine the specificity of the AT1 receptor antagonist.
In conclusion, the present results suggest that AT1 receptors are involved in bronchial hyper-responsiveness to methacholine in patients with asthma.
We thank Dr Suling Li (Loyola University at Chicago, IL) for statistical analysis.
References
- 1.Johnson AR, Ashton J, Schulz WW, Erdos EG. Neutral metalloendopeptidase in human lung tissue and cultured cells. Am Rev Respir Dis. 1985;132:564–568. doi: 10.1164/arrd.1985.132.3.564. [DOI] [PubMed] [Google Scholar]
- 2.Whitebread S, Mele M, Kamber B, de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;163:284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- 3.de Gasparo M, Husain A, Alexander W, et al. Proposed update of angiotensin receptor nomenclature. Hypertension. 1995;25:924–927. doi: 10.1161/01.hyp.25.5.924. [DOI] [PubMed] [Google Scholar]
- 4.Chung O, Stoll M, Unger T. Physiologic and pharmacologic implications of AT1 versus AT2 receptors. Blood Press Suppl. 1996;2:47–52. [PubMed] [Google Scholar]
- 5.Mauzy CA, Hwang O, Egloff AM, Wu LH, Chung FZ. Cloning expression, and characterization of a gene encoding the human angiotensin II type 1A receptor. Biochem Biophys Res Commun. 1992;186:277–284. doi: 10.1016/s0006-291x(05)80804-6. [DOI] [PubMed] [Google Scholar]
- 6.Rakugi H, Okamura A, Kamide K, et al. Recognition of tissue-and subtype-specific modulation of angiotensin II receptors using antibodies against AT1 and AT2 receptors. Hypertens Res. 1997;20:51–55. doi: 10.1291/hypres.20.51. [DOI] [PubMed] [Google Scholar]
- 7.Kanazawa H, Kurihara N, Hirata K, et al. Angiotensin II stimulates peptide leukotriene production by guinea pig airway via the AT1 receptor pathway. Prostaglandins Leukot Essent Fatty Acids. 1995;52:241–244. doi: 10.1016/0952-3278(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 8.Kanazawa H, Kurihara N, Hirata K, Fujiwara H, Takeda T. Angiotensin II stimulates production of nitric oxide in guinea pig airways via AT1 receptor activation. Life Sci. 1995;56:1427–1431. doi: 10.1016/0024-3205(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 9.Nally JE, Clayton RA, Wakelam MJ, Thomson NC, McGrath JC. Angiotensin II enhances responses to endothelin-1 in bovine bronchial smooth muscle. Pulm Pharmacol. 1994;7:409–413. doi: 10.1006/pulp.1994.1048. [DOI] [PubMed] [Google Scholar]
- 10.Tamaoki J, Yamauchi F, Konno K. Effects of angiotensin peptides on cholinergic neurotransmission in rabbit tracheal smooth muscle. Res Commun Chem Pathol Pharmacol. 1992;77:259–272. [PubMed] [Google Scholar]
- 11.Myou S, Fujimura M, Kurashima K, Tachibana H, Watanabe K, Hirose T. Type 1 angiotensin II receptor antagonism reduces antigen-induced airway reactions. Am J Respir Crit Care Med. 2000;162:45–49. doi: 10.1164/ajrccm.162.1.9907128. [DOI] [PubMed] [Google Scholar]
- 12.Millar EA, Angus RM, Hulks G, Morton JJ, Connell JM, Thomson NC. Activity of the renin–angiotensin system in acute severe asthma and the effect of angiotensin II on lung function. Thorax. 1994;49:492–495. doi: 10.1136/thx.49.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsay SG, Dagg KD, McKay IC, Lipworth BJ, McSharry C, Thomson NC. Investigations on the renin–angiotensin system in acute severe asthma. Eur Respir J. 1997;10:2766–2771. doi: 10.1183/09031936.97.10122766. [DOI] [PubMed] [Google Scholar]
- 14.Millar EA, Nally JE, Thomson NC, Angiotensin II. potentiates methacholine-induced bronchoconstriction in human airway both in vitro and in vivo. Eur Respir J. 1995;8:1838–1841. doi: 10.1183/09031936.95.08111838. [DOI] [PubMed] [Google Scholar]
- 15.Myou S, Fujimura M, Kamio Y, et al. Effect of losartan, a type 1 angiotensin II receptor antagonist, on bronchial hyperresponsiveness to methacholine in patients with bronchial asthma. Am J Respir Crit Care Med. 2000;162:40–44. doi: 10.1164/ajrccm.162.1.9907127. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka H, Teramoto S, Oashi K, et al. Effects of candesartan on cough and bronchial hyperresponsiveness in mildly to moderately hypertensive patients with symptomatic asthma. Circulation. 2001;104:281–285. doi: 10.1161/01.cir.104.3.281. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Standard for diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 18.Noda M, Shibouta Y, Inada Y, et al. Inhibition of rabbit aortic angiotensin II (AII) receptor by CV-11974, a new nonpeptide AII antagonist. Biochem Pharmacol. 1993;46:311–318. doi: 10.1016/0006-2952(93)90420-2. [DOI] [PubMed] [Google Scholar]
- 19.Bertolino F, Valentin JP, Maffre M, Jover B, Bessac AM, John GW. Prevention of thromboxane A2 receptor-mediated pulmonary hypertension by a nonpeptide angiotensin II type 1 receptor antagonist. J Pharmacol Exp Ther. 1994;268:747–752. [PubMed] [Google Scholar]
- 20.Li P, Ferrario CM, Brosnihan KB. Losartan inhibits thromboxane A2-induced platelet aggregation and vascular constriction in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1998;32:198–205. doi: 10.1097/00005344-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Jones GL, Saroea HG, Watson RM, O'Byrne PM. Effect of an inhaled thromboxane mimetic (U46619) on airway function in human subjects. Am Rev Respir Dis. 1992;145:1270–1274. doi: 10.1164/ajrccm/145.6.1270. [DOI] [PubMed] [Google Scholar]
- 22.Shibouta Y, Inada Y, Ojima M, et al. Pharmacological profiles of TCV-116, a high potent and long acting angiotensin II receptor antagonist. J Hypertens. 1992;10(Suppl):143. [Google Scholar]
- 23.Dicpinigaitis PV, Dobkin JB. Effect of angiotensin II receptor blockade on bronchial responsiveness in asthmatic subjects. J Allergy Clin Immunol. 1998;102:521–522. doi: 10.1016/s0091-6749(98)70143-5. [DOI] [PubMed] [Google Scholar]
- 24.Fish JE, Norman PS. Effects of the calcium channel blocker, verapamil, on asthmatic airway responses to muscarinic, histaminergic, and allergenic stimuli. Am Rev Respir Dis. 1986;133:730–734. doi: 10.1164/arrd.1986.133.5.730. [DOI] [PubMed] [Google Scholar]