Abstract
Poliovirus neuropathogenicity depends on sequences within the 5′ nontranslated region of the virus. Exchange of the poliovirus internal ribosomal entry site with its counterpart from human rhinovirus type 2 resulted in attenuation of neurovirulence in primates. Despite deficient virus propagation in cells of neuronal origin, nonpathogenic polio recombinants retain excellent growth characteristics in cell lines derived from glial neoplasms. Susceptibility of malignant glioma cells to poliovirus may be mediated by expression of a poliovirus receptor, CD155, in glial neoplasms. Intergeneric polio recombinants with heterologous internal ribosomal entry site elements unfolded strong oncolytic potential against experimentally induced gliomas in athymic mice. Our observations suggest that highly attenuated poliovirus recombinants may have applicability as biotherapeutic antineoplastic agents.
Malignant gliomas are the most common primary tumors of the central nervous system (1). Available treatment is of limited utility for these tumors, and prognosis is therefore poor (1). The resistance of malignant gliomas to conventional therapies has inspired the search for novel strategies, and recently, these strategies have involved animal viruses, either as attenuated variants of pathogenic species with direct oncolytic properties (2, 3) or as delivery vehicles for foreign genetic material (4–6).
Poliovirus is a nonenveloped plus-stranded RNA virus belonging to Picornaviridae and is the causative agent of paralytic poliomyelitis. The vast majority of poliovirus infections remain asymptomatic, but 1–2% of cases result in neurologic complications (7). Restriction of poliovirus cell tropism to lower motor neurons resident within the spinal cord and brainstem gives rise to a highly characteristic clinical syndrome dominated by flaccid paralysis. Selective targeting of motor neurons by poliovirus is most likely determined by the distribution of its cellular receptor, the Ig superfamily molecule CD155. This assumption is supported by the observation that mice transgenic for CD155 develop a polio-like syndrome after poliovirus infection (8–10). In addition, cell-internal conditions favoring viral replication may contribute to poliovirus cell-type specificity.
The neuropathogenicity of poliovirus depends on the cell-type-specific function of its internal ribosomal entry site (IRES) element in cells of neuronal origin (10). The IRES is part of the 5′ nontranslated region not only of picornaviruses (11) but also of hepatitis C virus (12, 13). IRES elements assure initiation of translation in a 5′ end-independent, cap-independent manner (14–16). We have demonstrated that IRES elements encode strong cell-type-specific restrictions toward virus propagation.
This restriction is illustrated by the highly attenuated phenotype of intergeneric recombinant polioviruses carrying IRES elements derived from human rhinovirus type 2 (10, 17). We have demonstrated that a prototype intergeneric poliovirus chimera [called PV1(RIPO)] is characterized by exceedingly poor growth in tissue culture cell lines of neuronal origin and avirulence in mice transgenic for CD155 (10). We confirmed the nonpathogenic phenotype of PV1(RIPO) to extend to primates where intraspinal inoculation into Cynomolgus monkeys showed a high degree of attenuation (17). Comprehensive neurovirulence testing of PV1(RIPO) in nonhuman primates was performed at the Center for Biologics Evaluation and Research of the Food and Drug Administration. Tests that were carried out according to the World Health Organization's guidelines for neurovirulence assessment of the life-attenuated vaccine strains of poliovirus confirmed the nonneuropathogenic phenotype (K. Chumakov, M.G, and E. W., unpublished observations).
In this study, we show that PV1(RIPO) can infect and propagate in cell lines derived from malignant gliomas. Treatment of athymic mice bearing s.c. or intracerebral glioma xenografts with PV1(RIPO) halted tumor progression and resulted in tumor elimination. We propose that oncolytic poliovirus recombinants with ablated neuropathogenicity may be suitable agents in oncologic therapy.
Materials and Methods
Primary Tissue Cell Culture.
Tumor material was obtained during craniotomy for glioma resection. Immediately after removal, tissue fragments were immersed in PBS containing 75 μg of kanamycin, 100 μg of streptomycin, 10 units of penicillin, and 175 μg of amphotericin B per ml. Tissue fragments were freed of debris and transferred into PBS containing 0.25% crude Trypsin (type II-S; Sigma) at 4°C for 6 h. Subsequently, fragments were placed at 37°C for 20 min and gently dispersed in MEM [containing the antibiotics mentioned above at given concentrations and 10% (vol/vol) FBS]. The cell suspension was spread on tissue culture dishes and passaged by following standard procedures. One-step growth curves were performed as described (17). Briefly, tissue culture monolayers were infected with PV1(RIPO) at a multiplicity of infection of 10 by incubation for 30 min at room temperature. Culture dishes were thoroughly rinsed with five changes of growth medium to remove unbound particles and placed at 37°C thereafter. At the indicated intervals, culture dishes were removed and treated with three consecutive freeze/thaw cycles. Virus titers in infected monolayer cultures were quantified by plaque assay as described (17).
Tumor Xenotransplantation.
HTB-15 cells were grown in MEM containing 10% (vol/vol) FBS. Cells were harvested, washed twice with MEM, and resuspended in sterile PBS. The concentration of cells in the suspension was determined with a hemacytometer. s.c. inoculation of 5 × 106 HTB-15 cells was performed with insuject (Becton Dickinson) injection syringes (28 gauge). Tumor cells were xenografted into anesthetized male homozygous NCr nude mice (Taconic Farms) bilaterally in a region overlaying the flank. PV1(RIPO) was propagated and purified according to established protocols (10). Virus concentrations were determined with the plaque assay (10). Virus was suspended at the desired concentration in sterile PBS and injected into a tail vein, the gastrocnemius muscle, or intraneoplastically with insuject syringes. For intracranial xenotransplantation, HTB-14 cells were grown in tissue culture by following standard procedures. Cells were prepared for intracranial injection as outlined above. Anesthetized male NCr nude mice were inoculated with 5 × 105 cells into the target area. The cerebral region targeted was the right anterolateral portion of the fornix. Stereotactic deposition of HTB-14 cells was performed by following standard procedures (18). Mice were carefully examined for 3 days to exclude the occurrence of neurological sequelae stemming from the inoculation procedure. PV1(RIPO) was injected intramuscularly or i.v. as described above. For intraneoplastic virus inoculations, coordinates for stereotactic deposition of tumor cells were used to release 2 × 107 plaque-forming units (pfu) of PV1(RIPO) into the area of the tumor xenograft. With the appearance of signs of neurological dysfunction, mice were killed, and the brain and upper spinal cord were processed for histopathological analysis. Resected neural tissues were processed histologically as described (10). All procedures involving experimental animals were conducted according to protocols approved by the institutional committees on animal welfare.
Reverse Transcription–PCR Amplification of Viral Sequences.
PV1(RIPO), recovered from intracerebral glioma xenografts, was grown on HTB-14 cells (the cell line constituting the xenograft) for the isolation of viral RNA by established procedures (17). Reverse transcription with oligo(dT) as a primer and subsequent PCR amplification were performed with a First-Strand cDNA Synthesis Kit (Roche Molecular Biochemicals). Primers used for PCR amplification (5′-cttagaagtttttcacaaag-3′ and 5′-cccatggtgccaatatatatattg-3′) encompass the entire IRES segment (nucleotides 110–620) of PV1(RIPO). Sequencing of PCR product was accomplished by standard procedures (17).
Immunohistochemistry.
Fresh-frozen sections from human gliomas were prepared as follows. Glioma tissue was obtained during craniotomy. Tissue samples were rinsed in sterile PBS immediately after removal, freed of surrounding debris, and submerged into OCT compound (Sakura, Torrance). The embedded tissue samples were then frozen in powdered dry ice and cryosectioned at a thickness of 14 μm. Samples from normal human brain were obtained during autopsy and prepared for cryostat sectioning as described above. Immunohistochemical staining for CD155 was performed with a monoclonal anti-CD155 antibody (D171; ref. 19), horseradish peroxidase-conjugated anti-mouse IgG secondary antibody (Roche Molecular Biochemicals), and the horseradish peroxidase substrate amino-ethyl-carbonate (Sigma) according to published protocols (20).
Results
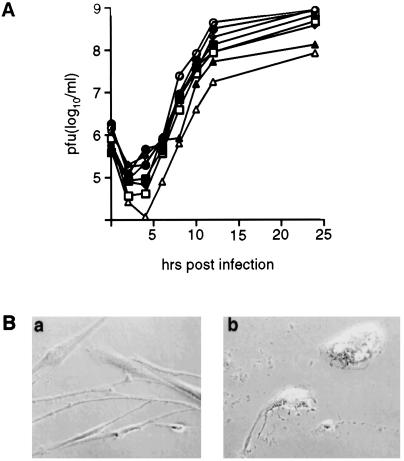
Despite the inability of PV1(RIPO) to propagate efficiently in neuronal cell lines, growth in cell lines derived from glial neoplasms and in primary cultures of glial tumors was unaffected by the presence of the human rhinovirus type 2 IRES (Fig. 1A). Virus propagation in infected primary glioma cells caused a prominent cytopathic effect and their rapid lytic destruction (Fig. 1B).
Figure 1.
Propagation and toxicity of PV1(RIPO) in malignant glioma tissue culture cell lines. (A) One-step growth curves of PV1(RIPO) were obtained after synchronized infection of monolayer cultures at a multiplicity of infection of 10 according to previous reports (10). Glioma cell lines tested included SF188 (open square; glioblastoma multiforme), SF295 (closed square; glioblastoma multiforme), SF767 (open triangle; anaplastic astrocytoma), SF763 (closed triangle; anaplastic astrocytoma), HTB-14 (open circle; anaplastic astrocytoma), HTB-15 (closed circle; anaplastic astrocytoma), DU54 (open diamond; glioblastoma multiforme), and DU65 (closed triangle; glioblastoma multiforme). (B) Synchronized infection of primary cultured glioblastoma multiforme cells. Cells were photographed before (a) and 12 h after (b) infection with PV1(RIPO) at a multiplicity of infection of 10.
To test the oncolytic properties of PV1(RIPO) in vivo, s.c. xenografts of HTB-15 cells, derived from a human anaplastic astrocytoma were established in the hind flank of athymic mice. After s.c. deposition of tumor cells, 90% of treated animals developed growing tumors. When tumors reached an average size of 8-mm cross diameter (6–8 weeks after tumor cell implantation), the mice were treated with a single i.v. inoculation of 2 × 107 pfu of PV1(RIPO) in PBS. Control animals were injected with PBS alone.
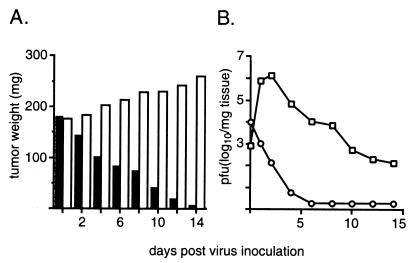
The single injection of PV1(RIPO) resulted in marked suppression of tumor growth and tumor resolution in all treated mice by 2 weeks after treatment (Figs. 2 and 3). In contrast, xenotransplants in untreated mice increased in size by 45% in the same period (Fig. 3A). Similar results were seen after intramuscular administration of virus (data not shown). In mice implanted with bilateral flank tumors, inoculation into one xenograft resulted in synchronous regression of the contralateral tumor. These observations identify the specific targeting properties of PV1(RIPO) and suggest that intratumoral replication of PV1(RIPO) results in release of sufficient numbers of infectious particles to destroy distant tumor foci. This assumption is supported by analyses of the kinetics of intratumoral virus propagation that revealed viral replication after i.v. administration at levels indicating efficient tumor cell lysis (Fig. 3B).
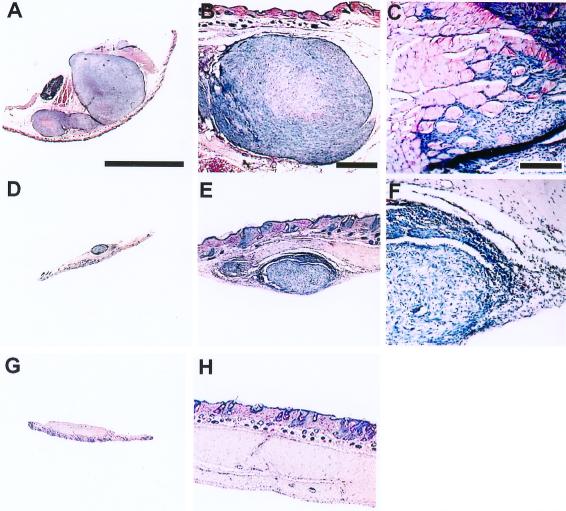
Figure 2.
Effect of PV1(RIPO) on s.c. HTB-15 tumor xenografts in athymic mice. Mice received bilateral implants. When tumors reached an average cross diameter of 8 mm (6–8 weeks after implantation), the mice were given a single i.v. injection of PV1(RIPO) (n = 10) or PBS (n = 10). An s.c. tumor of a PBS-control-treated animal 2 weeks after PBS treatment (A) infiltrated into surrounding tissues (B) and skeletal muscle (C). The tumors regressed 2 weeks after i.v. administration of 2 × 107 pfu PV1(RIPO) (D), revealing remaining tumor mass encircled by necrotic debris and inflammatory infiltrates invading the tumor from the periphery (E and F). At 3 weeks after PV1(RIPO) treatment, the tumors had been replaced by a fibrotic patch (G) with no evidence of residual neoplastic cells (H). Sections are 12 μm thick; hematoxylin/eosin stain. [A, Bar = 8 mm (applies to A, D, and G); B, Bar = 1 mm (applies to B, E, and H); C, Bar = 0.2 mm (applies to C and F)].
Figure 3.
Intratumoral propagation of PV1(RIPO) in s.c. HTB-15 xenografts grown in athymic mice. (A) Tumor growth in mock-treated (open bars) animals and xenograft regress in mice treated with PV1(RIPO) (closed bars). Mice (n = 4) with similarly sized tumors (8-mm cross diameter) were treated either with a single i.v. inoculation of 2 × 107 pfu of PV1(RIPO) or with PBS alone and were killed at the indicated intervals. Tumors were dissected, weighed, and processed for determination of the viral load by plaque assay (10). The data shown represent mean values for all four animals comprising an experimental group. At 2 weeks after virus treatment, tumor tissue could no longer be macroscopically discerned (compare Fig. 2). (B) Intratumoral (squares) and i.v. (circles) virus load after i.v. administration of PV1(RIPO) to xenografted athymic mice.
In another series of experiments, HTB-14 cells, derived from a human anaplastic astrocytoma, were used to generate intracerebral tumors in athymic mice. We chose HTB-14 cells, because HTB-15 cells, used to produce s.c. xenotransplants, do not form tumors after intracerebral xenografting. The tumors, if left untreated, produced neurologic symptoms and death within 21–26 days (Fig. 4). Experimental groups of 25 mice each were either left untreated or treated with PV1(RIPO) by intramuscular, i.v., or intratumoral inoculation 12 days after tumor implantation (Fig. 4).
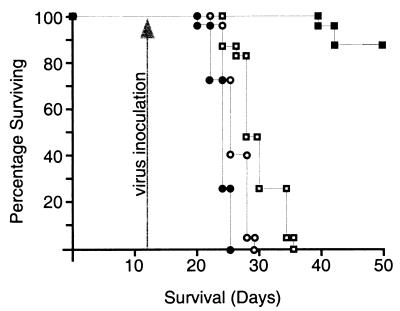
Figure 4.
Survival of athymic mice with intracerebral HTB-14 xenografts. Tumor implantation was carried out on day 0, and animals were treated on day 12 (arrow) with a single intramuscular (open circles), i.v. (open squares), or intratumoral (closed squares) inoculation of 2 × 107 pfu PV1(RIPO). Closed circles represent untreated control mice. The number of surviving animals was recorded daily and plotted against time.
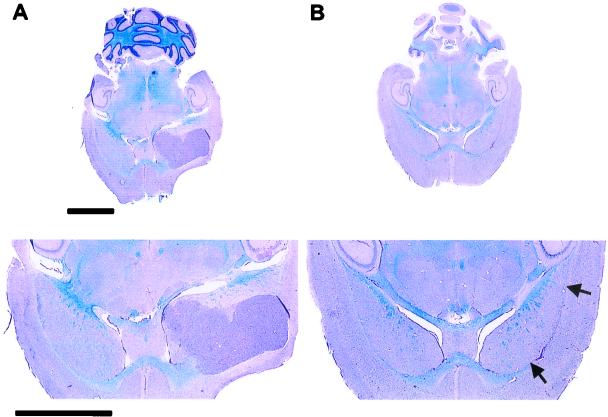
Intramuscular injection of 2 × 107 pfu of PV1(RIPO) had little effect on progression of neurologic symptoms or survival, whereas i.v. administration of the same amount of virus only delayed progression of neurologic symptoms and death up to 11 days (Fig. 4). However, intratumoral inoculation of 2 × 107 pfu of PV1(RIPO) resulted in improvement of neurologic symptoms and a significant increase in survival (Fig. 4). Only 3 of 25 animals given an intratumoral inoculation of virus succumbed to the growing tumor. Histological examination of all animals participating in the study revealed successful xenograft implantation, indicating a tumor take rate of 100%. At the time the experiment was terminated (50 days after tumor implantation), all surviving animals were killed. In animals that had received an intratumoral inoculation of PV1(RIPO), glioma xenografts had receded dramatically (Fig. 5). Only 4 of 22 surviving animals had evidence of residual tumor.
Figure 5.
Histopathological evaluation of intracerebral tumor implantation sites. (Lower) Details of the respective cross sections of the brain. (A) A transversal midcephalic section reveals a large xenograft originating in the area of the anterolateral fornix of an untreated control mouse 22 days after tumor implantation. (B) A midcephalic section through the brain of an athymic mouse treated with a single injection of PV1(RIPO) 12 days after xenograft implantation. The image shows the brain 50 days after tumor induction and 38 days after virus treatment. A large tissue defect delineating the fornix indicates the region of the implantation site (arrows). Apart from this lesion and the presence of reactive infiltrates within the area, no tissue abnormalities or residual tumor can be distinguished. Sections are 12 μm thick (luxol fast blue/periodic-acid-Schiff/hematoxylin stain). (Bars = 3 mm.)
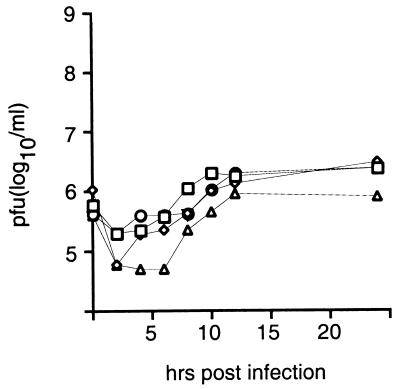
We investigated whether replication within glioma cells led to the emergence of variants of PV1(RIPO) with altered neuropathogenic phenotype. Athymic mice that had received intracerebral xenografts and were treated with intratumoral inoculation of PV1(RIPO) according to the regimen described in Fig. 4 were killed 7 days after virus administration. Brain tissue from the hemisphere harboring the tumor xenograft in three athymic mice was homogenized. Virus recovered from the tissue suspension was analyzed by one-step growth curves in SK-N-MC neuroblastoma cells (10) and subjected to sequencing analysis of the 5′ nontranslated region. Virus isolated from nude mouse xenografts exhibited exceedingly poor growth in SK-N-MC neuroblastoma cells characteristic of the nonneuropathogenic phenotype of PV1(RIPO) (Fig. 6; ref. 10). To confirm these observations in vivo, virus isolated from treated tumor xenografts was propagated in the glioma cell line constituting the xenograft (HTB-14) and used to inoculate CD155 transgenic mice by the intracerebral route. All three virus isolates, like the original PV1(RIPO) (10), failed to induce paralytic symptoms, even after intracerebral inoculation of doses exceeding 109 pfu. Sequencing of the IRES region of PV1(RIPO) recovered from treated athymic mice revealed nucleotide sequences to be identical to the original inoculum.
Figure 6.
Growth curve analyses of the original PV1(RIPO) inoculum (squares) and three isolates recovered from treated intracranial glioma xenografts (circles, diamonds, and triangles). One-step growth curves of all three isolates derived from treated xenografts revealed the deficient propagation in SK-N-MC neuroblastoma cells that characterizes the nonneuropathogenic phenotype of PV1(RIPO) (10).
Efficient treatment of malignant gliomas by poliovirus recombinants may depend on expression of the human poliovirus receptor CD155, previously known as hPVR (19, 21). Immunohistochemical analysis of fresh-frozen biopsy material demonstrated CD155 expression in 19 of 25 malignant glial tumors examined (Table 1). Susceptibility to poliovirus indicated expression of CD155 in all eight glioma cell lines tested (Fig. 1). Furthermore, the presence of CD155 was demonstrated immunohistologically in HTB-14 and HTB-15 xenografts (data not shown). Our observations have been confirmed in a recent study of differential gene expression in cancer, in which an association of CD155 with neuroectodermal tumors was identified through microarray analyses (22).
Table 1.
Immunohistochemical analyses of expression of glial acidic fibrillary protein (GFAP) and the human poliovirus receptor (CD155) in human malignant gliomas*
| No. of tumors tested | Histological grade | No. of tumors positive for
|
|
|---|---|---|---|
| GFAP | CD155 | ||
| 6 | I/II | 6 | 6 |
| Low-grade astrocytoma | |||
| 6 | III | 6 | 5 |
| Anaplastic astrocytoma | |||
| Anaplastic oligodendroglioma | |||
| 13 | IV | 13 | 8 |
| Glioblastoma multiforme | |||
A physiological role for CD155 and its rodent relatives has been proposed to involve intercellular adhesion (23, 24). Cell adhesion molecules of the Ig superfamily are recognized increasingly for their association with cancer and malignant gliomas in particular (25, 26). A close relative of CD155 in rodents, the tumor-associated glycoprotein E4, has been identified because of its association with gastrointestinal malignancies (27). Our observations suggest that deregulated expression of CD155 in malignancy may contribute to an increased susceptibility toward the attenuated yet highly oncolytic poliovirus chimera PV1(RIPO).
Discussion
Oncolytic viruses offer advantages over viral gene delivery vehicles for the treatment of malignant disease. Limitations in target specificity and transduction efficiencies combined with low expression levels restrict the usefulness of virus-mediated gene transfer against aggressively growing invasive malignancies (28). Oncolytic viruses such as the poliovirus derivative described herein rapidly destroy tumor cells through their own cell-killing devices without the need for expression of foreign genes. The multiple mechanisms of poliovirus-induced cytolysis are not completely understood, but the combination of shutoff of cellular protein synthesis, inhibition of transport of cellular glycoproteins, and the proteolytic digestion of transcription factors (29) led to the complete destruction of primary cell lines derived from malignant gliomas within 12 h.
Efficient oncolysis of s.c. tumor xenografts in athymic mice after i.v. administration of PV1(RIPO) demonstrated the remarkable ability of this recombinant poliovirus to target neoplastic cells. Even established intracerebral xenografts were eliminated after a single intratumoral inoculation of PV1(RIPO). However, whereas peripheral virus administration was sufficient to suppress s.c. xenograft growth, it proved ineffective against intracerebral neoplasms, possibly reflecting a relative inaccessibility of intracerebral targets for circulating infectious agents. Thus, intratumoral PV1(RIPO) administration may be required for maximum efficacy against malignant gliomas. Genotypic and phenotypic analyses demonstrated PV1(RIPO) replicating in tumor xenografts in athymic mice to retain the nonneuropathogenic phenotype.
Susceptibility of glioma cells to poliovirus depends on expression of the human poliovirus receptor, the Ig superfamily molecule CD155. We have shown susceptibility to PV1(RIPO) of all eight glioma cell lines used in this study. Furthermore, we have demonstrated CD155 to be expressed in a majority of malignant gliomas of varying histopathologic classification. Recently, an association of CD155 with neuroectodermal malignancies has been confirmed independently (J. M. Olson, A. Strand, M.G., L. P. Zhao, J. R. Geyer, R. Ellenbogen, J. Biegel, and S. J. Tapscott, unpublished work).
Although entry into malignant glioma cells is likely mediated by the cellular receptor for poliovirus CD155, other glioma-specific conditions that support PV1(RIPO) replication are likely to be equally important and may explain efficient lytic destruction of glioma cells.
This study provides evidence that highly attenuated poliovirus/human rhinovirus type 2 chimeras possess strong oncolytic activity against malignant gliomas. The neuropathogenic properties inherent to poliovirus are greatly reduced in the recombinant poliovirus described (17). This phenotype, combined with a natural tropism for malignant glioma cells that express CD155, suggests that polio/human rhinovirus type 2 chimeras may be suitable for the treatment of malignant disease of the central nervous system.
Acknowledgments
We are grateful to Dr. D. Bigner of Duke University for providing the glioma cell lines used in this study as well as to the Brain Tumor Research Tissue Bank at the University of California, San Francisco. Human neural tissue specimens were donations from the Neurological Research Specimen Bank (Los Angeles), which is sponsored by National Institute of Neurological Disorders and Stroke–National Institute of Mental Health, the National Multiple Sclerosis Society, and the Veterans Health Services and Research Administration, Department of Veterans Affairs. This work was supported by grants from The Brain Tumor Society, the New York Center for Biotechnology, and the National Institutes of Health. M.G. is a recipient of a Burroughs Wellcome Career Award in the Biomedical Sciences.
Abbreviations
- IRES
internal ribosomal entry site
- pfu
plaque-forming unit
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 6242.
References
- 1.Levin V A, Gutin P H, Leibel S. In: Cancer: Principles & Practice of Oncology. DeVita V, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1993. pp. 1679–1737. [Google Scholar]
- 2.Martuza R L, Malick A, Markert J M, Ruffner K L, Coen D M. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 3.Coffey M C, Strong J E, Forsyth P A, Lee P W K. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 4.Culver K W, Ram Z, Wallbridge S, Ishii H, Oldfield E H, Blaese R M. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 5.Eck S L, Alavi J B, Alavi A, Davis A, Hackney D, Judy M, Mollman J, Phillips P C, Wheeldon E B, Wilson J M. Hum Gene Ther. 1996;7:1465–1482. doi: 10.1089/hum.1996.7.12-1465. [DOI] [PubMed] [Google Scholar]
- 6.Parr M J, Manome Y, Tanaka T, Wen P, Kufe D W, Kaelin W G, Jr, Fine H A. Nat Med. 1997;3:1145–1149. doi: 10.1038/nm1097-1145. [DOI] [PubMed] [Google Scholar]
- 7.Bodian D. In: Pathology of the Nervous System. Minckler J, editor. New York: McGraw–Hill; 1972. pp. 2323–2344. [Google Scholar]
- 8.Ren R, Costantini F, Gorgacz E J, Lee J J, Racaniello V R. Cell. 1990;63:353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- 9.Koike S, Taya C, Kurata T, Abe W, Ise I, Yonekawa H, Nomoto A. Proc Natl Acad Sci USA. 1991;88:951–955. doi: 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gromeier M, Alexander L, Wimmer E. Proc Natl Acad Sci USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wimmer E, Hellen C U T, Cao X M. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 12.Tsushima-Kohara K, Iizuka N, Kohara M, Nomoto A. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H H, Wimmer E. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang S K, Kraeusslich H G, Nicklin M J, Duke G M, Palmenberg A C, Wimmer E. J Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelletier J, Sonenberg N. Nature (London) 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 16.Molla A, Jang S K, Paul A V, Reuer Q, Wimmer E. Nature (London) 1992;356:255–257. doi: 10.1038/356255a0. [DOI] [PubMed] [Google Scholar]
- 17.Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E. J Virol. 1999;72:958–964. doi: 10.1128/jvi.73.2.958-964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreansky S, Soroceanu L, Flotte E R, Chou J, Markert J M, Gillespie G Y, Roizman T, Whitley R J. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- 19.Mendelsohn C, Wimmer E, Racaniello V R. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 20.Shepley M P, Sherry B, Weiner H L. Proc Natl Acad Sci USA. 1988;85:7743–7747. doi: 10.1073/pnas.85.20.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wimmer E, Harber J J, Bibb J A, Gromeier M, Lu H H, Bernhardt G. In: Cellular Receptor for Animal Viruses. Wimmer E, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 101–127. [Google Scholar]
- 22.Bernhardt G, Bibb J A, Bradley J, Wimmer E. Virology. 1994;199:105–113. doi: 10.1006/viro.1994.1102. [DOI] [PubMed] [Google Scholar]
- 23.Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Exp Cell Res. 1997;15:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- 24.Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P. Blood. 1998;92:4602–4611. [PubMed] [Google Scholar]
- 25.Izumoto S, Ohnishi T, Arita N, Hiraga S, Taki T, Hayakawa T. Cancer Res. 1996;56:1440–1444. [PubMed] [Google Scholar]
- 26.Sasaki H, Yoshida K, Ikeda E, Asou H, Inaba M, Otani M, Kawase T. Cancer. 1998;82:1921–1931. doi: 10.1002/(sici)1097-0142(19980515)82:10<1921::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Denis M G. Int J Cancer. 1998;12:997–1005. doi: 10.3892/ijo.12.5.997. [DOI] [PubMed] [Google Scholar]
- 28.Kornblith P K, Welch W C, Bradley M K. Surg Neurol. 1993;39:538–543. doi: 10.1016/0090-3019(93)90041-x. [DOI] [PubMed] [Google Scholar]
- 29.Rueckert R R. In: Fields Virology. Fields B N, editor. New York: Raven; 1990. pp. 1204–1234. [Google Scholar]