Abstract
Aims In rodent and primate studies, urotensin II is an extremely potent vasoconstrictor peptide with effects in the central aortic and arterial vasculature as well as on cardiac function. The aim of the present study was to assess systemic haemodynamic responses to intravenous urotensin II infusion in humans.
Methods In 10 healthy male volunteers, intravenous urotensin II (3, 30 and 300 pmol min−1) and saline placebo were given on separate occasions in a single-blind randomized manner. Systemic haemodynamics and arterial stiffness were assessed by sphygmomanometry, transthoracic bioimpedance, and pulse wave analysis. Plasma urotensin II immuno-reactivity was measured by radio-immunoassay.
Results Intravenous urotensin II infusions were well tolerated with no adverse clinical effects and no electrocardiographic changes. Circulating plasma urotensin II immuno-reactivity increased from baseline of 16 ± 1 to 1460 ± 82 pmol l−1 (mean ± s.e. mean) during infusion of urotensin II at 300 pmol min−1 (P < 0.001). However, there were no significant placebo adjusted changes in heart rate (95% confidence intervals: −3.6, +4.4 min−1), mean arterial pressure (−5.8, +1.7 mmHg) or cardiac index (−0.1, +0.4 l min−1 m−2). There were also no changes in augmentation index (−4.1, +5.2%) or pulse wave velocity (−1.3, +0.3 m s−1).
Conclusions Intravenous urotensin II infusion did not affect systemic haemodynamics or arterial stiffness, despite achieving an ∼100-fold increase in plasma immuno-reactivity. We conclude that urotensin II is unlikely to have a physiological role in the short term regulation of vascular tone or blood pressure in man. Further confirmatory studies with urotensin II receptor antagonists are required.
Keywords: arterial pressure, cardiac output, peptides, vascular resistance, vasoconstriction
Introduction
Urotensin II (UII) is a vasoactive peptide found in the circulation of humans and many animal species [1–4]. In man it has 11 amino acids differentiating it from other species with 12 and 13 amino acids such as the fish and frog [1]. Urotensin II is the most potent arterial vasoconstrictor yet discovered, having sustained effects in in vitro studies in animals [5]. In addition, it has profound and potentially lethal pressor and vasoconstrictor effects in nonhuman primates in vivo[2].
Human UII was first isolated in man from subgroups of motor neurones in the spinal cord [2]. Outwith the central nervous system, the kidney has the highest expression of human preproUII mRNA and this therefore appears to be the most likely source of circulating UII in man [4]. The distribution of UII receptors has been mapped using immuno-histochemistry, confirming target binding sites in cardiovascular tissues; including coronary arteries, internal mammary arteries and ventricular cardiomyocytes [3, 6]. Thus, it can be considered likely that UII functions as an endocrine hormone with cardiovascular actions [1].
Both the anatomical location and species appear to dictate the observed vascular response to UII administration [5]. In the rat, there is a marked vasoconstrictor response in the proximal aorta with continuous reduction in activity progressively down the arterial tree [7]. Previous human in vivo studies, carried out in our laboratory, show that high concentrations of UII delivered by the intrabrachial route have no effect on local vascular tone in the forearm [8]. This is in contrast to a similar, but not placebo-controlled, study performed recently by Böhm & Pernow [9]. However, these studies primarily aimed to assess responses of the resistance arterioles and did not specifically examine the integrated response of the arterial system. This omission may be important because the extreme pressor and myocardial ischaemic responses seen in nonhuman primates may have resulted from large artery stiffening or vasoconstriction [3].
The arterial pressure waveform alters with progression down the arterial tree. This is due to local variations in vascular stiffness as well as superimposition of the reflected pressure waveform that returns to the central arteries and aorta in diastole [10]. Augmentation index is dependent on three components: pulse wave velocity, site of wave reflection in the vascular tree and amplitude of the reflected wave. Increased stiffness of small arteries causes an increase in the amplitude of the reflected wave and effectively moves the site of wave reflection proximally. However, increasing large artery stiffness is manifested as a rise in pulse wave velocity. Aortic pulse pressure depends on aortic stiffness and the degree of wave reflection. These stiffness-related effects produce an increase in central aortic pressure and cardiac afterload, and a reduction in coronary perfusion pressure due to the movement of the reflected wave into systole. By measuring augmentation index a composite measure of central arterial stiffness can be obtained, whereas aortic pulse wave velocity examines the contribution of large arterial stiffness [10–12].
Given the data from studies in nonhuman primates, we hypothesized that systemic administration of UII would act physiologically as a circulating hormone to increase large arterial stiffness and blood pressure. Our aim was therefore to investigate the effects of intravenous UII infusion on a range of systemic haemodynamic parameters including blood pressure and central arterial stiffness, in vivo in healthy humans.
Methods
Subjects
Ten healthy men, mean age 42 ± 4 years (range 22–55), were recruited into the study, which was conducted with the approval of the local research ethics committee (Lothian Research Ethics Committee) and the written informed consent of each subject. Subjects abstained from caffeine containing drinks, alcohol and tobacco over the preceding 24 h and were fasted from midnight prior to the study.
Drugs
Human UII (Peptide Institute, Osaka, Japan) was dissolved in saline (0.9% Baxter Healthcare Ltd, Norfolk, UK) and administered intravenously at 1 ml min−1 via a constant rate infusion pump (IVAC). Purity and fidelity of human UII from the Peptide Institute was established by high performance liquid chromatography and microsequencing. Biological activity and potency of the human UII peptide was confirmed in the rat proximal aorta (data not shown). Doses used in study protocols were based on our initial studies giving UII via the intrabrachial route [8].
Augmentation index and pulse wave velocity
Augmentation index (AIx) was determined from the radial artery using the technique of pulse wave analysis (SphygmoCor 2000 version 6.2; PWV Medical PTY Ltd, Sydney, Australia) as previously described [10]. Pulse wave velocity (PWV) was determined using pulse wave analysis (SphygmoCor 2000 version 6.2) combined with electrocardiographic monitoring at the carotid artery (adjacent to the thyroid cartilage) and femoral artery (immediately below the inguinal ligament). The separation of the pulse waveforms was defined as the difference between the distances from the sternal notch to the inguinal ligament and to the thyroid cartilage. All measurements were made in duplicate and mean values used in subsequent data analysis. Recordings with systolic and diastolic variability in excess of 5% were excluded and the measurement repeated.
Haemodynamic variables
Blood pressure (BP) was recorded in the noninfused arm using a validated oscillometric sphygmomanometer (HEM 705CP, Omron, Japan) [13]. Cardiac index (CI) was assessed using a validated transthoracic electrical bioimpedance technique [14] (NCCOM3, BoMed Irvine CA, USA). Mean arterial pressure (MAP) was defined as the diastolic pressure plus a third of the pulse pressure. Systemic vascular resistance index (SVRI) was defined as the MAP divided by the CI and then converted from Wood units to dyn s m2/cm5 on the basis that 1 Wood unit approximates to 80 dyn s m2/cm5. Throughout the study continuous electrocardiographic monitoring was employed and a 12 lead electrocardiogram (ECG) recorded at baseline, during the last 2 min of the 300 pmol min−1 infusion of UII and at the end of the final saline infusion.
Urotensin II immunoreactivity assay
Venous blood (10 ml) was drawn during the last 2 min of each infusion period from a cannula sited in the noninfused arm. Samples were collected into ethylene diamine tetra-acetic acid, immediately centrifuged at 3300 gfor 10 min at 4 °C and the plasma stored at −80 °C until subsequent analysis. Plasma concentrations of UII immuno-reactivity were determined using an acetic acid extraction technique and radio-immunoassay, with rabbit antiflounder UII, as described previously [8, 15] and are expressed in the results as pmol l−1. The antibody had equal specificity for human and flounder UII, and there was no cross-reactivity in the assay with ET-1, ANGII or somatostatin-14 [8] (Sigma Chemical Co, UK). Recovery of hUII in plasma extracts was 63% and intra-and inter-assay coefficients of variation in our laboratory were 7.6% and 13.3%, respectively; the sensitivity of the assay was 1 fmol hUII ml−1 plasma [8].
Study protocol
Each subject attended on two occasions at least 1 week apart and received an initial 30 min saline infusion during which baseline recordings were performed at 15, 22 and 25 min. Baseline bloods and ECG were obtained at 28 min, just prior to UII or placebo infusion. This was followed by a single-blind randomized administration of either UII (3, 30 and 300 pmol min−1 for 20 min at each dose) or saline for 1 h, before a final 30 min saline infusion. BP, heart rate (HR), CI and AIx were recorded at 5 and 12 min, PWV at 15 min, and blood samples obtained and a 12 lead electrocardiogram taken at 18 min of each infusion period.
Statistical analysis
All results are expressed as mean ± s.e. mean. The AIx and PWV values represent change from baseline. Data were analysed using anova with repeated measures. Statistical significance was taken at the 5% level. Previous studies carried out in our department using noradrenaline infusions had 98% power to detect a change of 7% in eight volunteers at a significance level of 0.05 [11].
Results
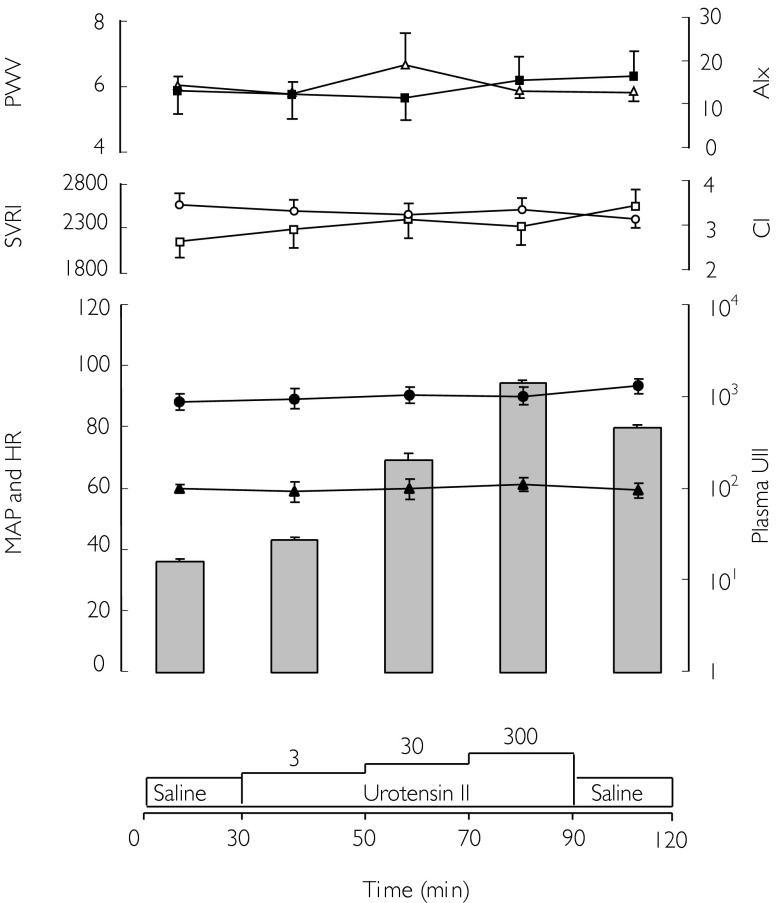
Baseline HR, CI, BP, AIx, PWV and plasma UII concentrations were similar on the two study days. The baseline AIx and PWV raw data recordings were 13 ± 4% and 5.9 ± 0.3 m s−1, respectively. Both values were consistent with other healthy subjects from our local population with similar demographics. All subjects were symptom-free throughout the studies: specifically, there were no reports of chest pain, headache or abdominal pain. There were also no changes in continuous single lead cardiac monitoring and 12 lead electrocardiograms throughout the studies. There were no significant changes in systemic haemodynamic parameters or central aortic stiffness during either saline (placebo) or UII infusion (Figure 1). At the highest infusion rate of 300 pmol min−1, plasma UII immuno-reactivity increased 91-fold (16 ± 1 to 1460 ± 82 pmol l−1) in the systemic venous plasma (n = 10; P < 0.001). Despite this, there were no significant placebo adjusted changes in HR (0.4, −3.6, +4.4 min−1: mean difference, 95% confidence interval), MAP (−2.0, −5.8, +1.7 mmHg), CI (0.2, −0.1, +0.4l min−1 m−2) and systemic vascular resistance index (−160, −396, +76 dyn s m2/cm5). Moreover, arterial stiffness was unaffected, with no demonstrable alterations in AIx (+ 0.5, −4.1, +5.2%) or PWV (−0.5, −1.3, +0.3 m s−1).
Figure 1.
Mean pulse wave data, haemodynamic parameters (displayed as lines) and plasma urotensin II concentration (UII; displayed as histogram; pmol l−1) during intravenous UII infusion. Mean augmentation index (AIx, ▵%), pulse wave velocity (PWV, ▪ m s−1), cardiac index (CI, ○ l min−1 m−2), systemic vascular resistance index (SVRI, □ dyn m−2 cm−5), mean arterial pressure (MAP, • mmHg), and heart rate (HR, ▴ beats min−1).
Discussion
This is the first in vivo study of which we are aware in which systemic intravenous administration of UII has been used to increase circulating peptide concentrations in man. There were no demonstrable effects of UII on systemic haemodynamics or arterial stiffness, although plasma UII immuno-reactivity increased by nearly 100-fold. This contrasts with the modest two-fold elevation of plasma UII in renal disease [16].
Our findings contrast with in vivo studies in nonhuman primates, where UII caused potent pressor and vasoconstrictor effects [2]. Moreover, the in vivo human studies done by Böhm & Pernow and ourselves, an intra-arterial UII infusion of 300 pmol min−1 did not alter systemic blood pressure [8, 10], an intra-arterial infusion rate that we demonstrated to raise systemic plasma UII immuno-reactivity by 30-fold [8]. Such an increase is not always sufficient to cause peripheral haemodynamic effects, as can be seen with vasopressin, which requires a 10–100-fold rise in plasma concentrations [17, 18]. In vitro studies have shown that the rat aorta is highly responsive to UII, particularly in its proximal region [5, 6]. However, against the reproducible in vitro pharmacological response to UII in cardiovascular tissues from animals, findings reported in human in vitro studies are inconsistent [1, 19]. Our results may reflect a fundamental difference in species response, although the in vitro human aortic response is currently unknown. As it appears likely that UII is an endocrine hormone with receptors located in human cardiovascular tissues, the question remains as to its function in human vascular physiology.
We have previously demonstrated dose-dependent increases in AIx with intravenous infusion of pressor hormones, including angiotensin II and noradrenaline [11]. Moreover, intravenously infused peptides, such as angiotensin II and endothelin-1, cause a significant rise in mean arterial blood pressure for only a 2 and 3-fold rise in plasma concentrations, respectively [20, 21]. In the present study, we administered 300 pmol min−1 (total dose of 85 pmol kg−1) of UII for 20 min and achieved a 91-fold increase in plasma UII immuno-reactivity. This was associated with no symptoms, no electrocardiographic changes, and no alterations in systemic haemodynamic parameters. When given intravenously, UII caused profound ischaemic electrocardiographic changes in nonhuman primates in association with cardiac dysfunction and even death [3]. Ames et al. used doses of UII up to 3000 pmol kg−1and reported that doses <30 pmol kg−1 increased cardiac output, while doses>30 pmol kg−1 increased vascular resistance and decreased myocardial function [3]. However, due to safety concerns, we did not use either bolus injections or the higher doses of UII that were used in the nonhuman primate in vivo studies. It may be the case that UII has a role in cardiovascular regulation in man that is not addressed directly in our studies. For instance, it has recently been suggested that UII may influence atherogenesis by augmenting the mitogenic activity of subfractions of oxidized low density lipoprotein [22] and even that UII might have a role in the regulation of insulin release [23].
In conclusion, we have observed no change in arterial stiffness or systemic haemodynamic parameters, including blood pressure in response to intravenous UII infusion in vivo in man despite a nearly 100-fold increase in plasma UII immuno-reactivity. These findings indicate that UII is unlikely to have a major physiological role in the regulation of vascular tone and blood pressure in man. Further confirmatory studies using UII receptor antagonists will be required before firm conclusions can be drawn about the possible role of UII in human vascular physiology and disease.
Dr Jonathan Affolter is a British Heart Foundation Junior Research Fellow (Grant FS2000/021). Professor D.J. Webb was the recipient of a Research Leave Fellowship from the Wellcome Trust (WT 0526330) at the time of this work. Our thanks goes to Neil R Johnston, Fiona Howie and Louise Coppard for their technical support in the analysis of the blood samples, and to Donna Miller for her help during the studies. We are grateful to Drs Steve Douglas and Eliot Ohlstein at SmithKline Beecham, Pennsylvania, USA for their help in the authentication of the UII. We are grateful to Dr Ian Megson for confirming the biological activity of UII in the rat proximal aorta.
References
- 1.Affolter J, Webb DJ. Urotensin II: a new mediator in cardiopulmonary regulation? Lancet. 2001;358:774–775. doi: 10.1016/S0140-6736(01)06005-6. [DOI] [PubMed] [Google Scholar]
- 2.Coulouarn Y, Lihrmann I, Jegou S, et al. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motorneurones of the spinal cord. Proc Natl Acad Sci USA. 1988;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames RS, Sarau HM, Chambers JK, et al. Human urotensin II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 4.Nothacker HP, Wang Z, McNeil AM, et al. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nature Cell Biol. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- 5.Douglas SA, Sulpizio AC, Piercy V, et al. Differential vasoconstrictor activity of human urotensin II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maguire JJ, Kuc RE, Davenport AP. Orphan-receptor ligand human urotensin II. receptor localisation in human tissues and comparison of vascular responses with endothelin-1. Br J Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh H, Itoh Y, Rivier J, Lederis K. Contraction of major artery segments of rat by fish neuropeptide urotensin II. Am J Physiol. 1987;252:R361–R366. doi: 10.1152/ajpregu.1987.252.2.R361. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson IB, Affolter JT, De Haas SL, et al. High plasma concentrations of human urotensin II do not alter local or systemic haemodynamics in man. Cardiovasc Res. 2002;53:341–347. doi: 10.1016/s0008-6363(01)00485-0. [DOI] [PubMed] [Google Scholar]
- 9.Böhm F, Pernow J. Urotensin II evokes potent vasoconstriction in humans in vivo. Br J Pharmacol. 2002;135:25–27. doi: 10.1038/sj.bjp.0704448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. London: Arnold; 1998. [Google Scholar]
- 11.Wilkinson IB, MacCallum H, Hupperetz PC, van Thoor CJ, Cockcroft JR, Webb DJ. Changes in the derived central pressure waveform and pulse pressure in response to angiotensin II and noradrenaline in man. J Physiol. 2001;530:541–550. doi: 10.1111/j.1469-7793.2001.0541k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol. 2001;51:507–522. doi: 10.1046/j.0306-5251.2001.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien E, Mee F, Atkins N, Thomas M. Evaluation of three devices of blood pressure according to the revised British Hypertension Society Protocol: The Omron HEM-705CP, Philips HP5332 and Nissei DS-175. Blood Pressure Monitoring. 1996;1:55–61. [PubMed] [Google Scholar]
- 14.Northridge DB, Findlay IN, Wilson J, Henderson E, Dargie HJ. Non-invasive determination of cardiac output by Doppler echocardiography and electrical bioimpedance. Br Heart J. 1990;63:93–97. doi: 10.1136/hrt.63.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter MJ, Hubbard PC, McCrohan CR, Balment RJ. A homologous radioimmunoassay for the measurement of urotensin II in the euryhaline flounder, Platichthys flesus. Gen Comp Endocrinol. 1999;114:249–256. doi: 10.1006/gcen.1998.7245. [DOI] [PubMed] [Google Scholar]
- 16.Totsune K, Takahashi K, Arihara Z, et al. Role of urotensin II in patients on dialysis. Lancet. 2001;358:810–811. doi: 10.1016/S0140-6736(01)06002-0. [DOI] [PubMed] [Google Scholar]
- 17.Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345:588–595. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- 18.Aylward PE, Floras JS, Leimbach WN, Jr, Abboud FM. Effects of vasopressin on the circulation and its baroreflex control in healthy men. Circulation. 1986;73:1145–1154. doi: 10.1161/01.cir.73.6.1145. [DOI] [PubMed] [Google Scholar]
- 19.Hillier C, Berry C, Petrie MC, et al. Effects of urotensin II in human arteries and veins of varying caliber. Circulation. 2001;103:1378–1381. doi: 10.1161/01.cir.103.10.1378. [DOI] [PubMed] [Google Scholar]
- 20.Motwani JG, Struthers AD. Dose–response study of the redistribution of intra-vascular volume by angiotensin II in man. Clin Sci. 1992;82:397–405. doi: 10.1042/cs0820397. [DOI] [PubMed] [Google Scholar]
- 21.Kaasjager KAH, Koomans HA, Rabelink TJ. Effectiveness of enalapril versus nifedipine to antagonize blood pressure and the renal response to endothelin in humans. Hypertension. 1995;25:620–625. doi: 10.1161/01.hyp.25.4.620. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Rajbabu P, Takashi K, Benedict CR. Synergistic effect of urotensin II with mildly oxidised LDL on DNA synthesis in vascular smooth muscle cells. Circulation. 2001;104:16–18. doi: 10.1161/hc2601.092848. [DOI] [PubMed] [Google Scholar]
- 23.Silvestre RA, Rodriguez-Gallardo J, Egido EM, Marco J. Inhibition of insulin release by urotensin II: a study on the perfused rat pancreas. Horm Metab Res. 2001;33:379–381. doi: 10.1055/s-2001-15414. [DOI] [PubMed] [Google Scholar]