Abstract
Aims
Some children with malaria and convulsions also have concurrent bacterial meningitis. Chloramphenicol is used to treat the latter whereas phenytoin is used for convulsions. Since chloramphenicol inhibits the metabolism of phenytoin in vivo, we studied the effects of chloramphenicol on phenytoin pharmacokinetics in children with malaria.
Methods
Multiple intravenous (i.v.) doses of chloramphenicol succinate (CAP) (25 mg kg−1 6 hourly for 72 h) and a single intramuscular (i.m.) seizure prophylactic dose of fosphenytoin (18 mg kg−1 phenytoin sodium equivalents) were concomitantly administered to 15 African children with malaria. Control children (n= 13) with malaria received a similar dose of fosphenytoin and multiple i.v. doses (25 mg kg−1 8 hourly for 72 h) of cefotaxime (CEF). Blood pressure, heart rate, respiratory rate, oxygen saturation, level of consciousness and convulsion episodes were monitored. Cerebrospinal fluid (CSF) and plasma phenytoin concentrations were determined.
Results
The area under the plasma unbound phenytoin concentration-time curve (AUC(0,∞); means (CAP, CEF): 58.5, 47.6 µg ml−1 h; 95% CI for difference between means: −35.0, 11.4), the peak unbound phenytoin concentrations (Cmax; medians: 1.12, 1.29 µg ml−1; 95% CI: −0.5, 0.04), the times to Cmax(tmax; medians: 4.0, 4.0 h; 95% CI: −2.0, 3.7), the CSF:plasma phenytoin ratios (means: 0.21, 0.22; 95% CI: −0.8, 0.10), the fraction of phenytoin unbound (means: 0.06, 0.09; 95% CI: −0.01, 0.07) and the cardiovascular parameters were not significantly different between CAP and CEF groups. However, mean terminal elimination half-life (t1/2,z) was significantly longer (23.7, 15.5 h; 95% CI: 1.71, 14.98) in the CAP group compared with the CEF group. Seventy per cent of the children had no convulsions during the study period.
Conclusions
Concomitant administration of chloramphenicol and a single i.m. dose of fosphenytoin alters the t1/2,z but not the other pharmacokinetic parameters or clinical effects of phenytoin in African children with severe malaria. Moreover, a single i.m. dose of fosphenytoin provides anticonvulsant prophylaxis in the majority of the children over 72 h. However, a larger study would be needed to investigate the effect of concomitant administration of multiple doses of the two drugs in this population of patients.
Keywords: children, chloramphenicol, fosphenytoin, interaction, malaria, pharmacokinetics, phenytoin
Introduction
Severe falciparum malaria is a major cause of childhood deaths in sub-Saharan Africa [1, 2]. Seizures are a common complication of severe disease, and occur in 30% of all the children admitted with malaria [3, 4]. Approximately 80% of children with cerebral malaria (CM) have a history of seizures prior to hospitalization, whereas about 60% will develop seizures after admission [2, 5]. A small proportion (25%) of children with CM also has subtle seizure activity [5] that would be difficult to detect in most health facilities in resource poor countries. Seizures that are unresponsive to treatment with first line anticonvulsants are usually treated with phenytoin [6]. Some of the children (4–8%) admitted to our hospital with severe malaria also have concurrent bacteraemia, including bacterial meningitis [7, 8]. However, in such severely ill children, it is not clinically easy to distinguish CM from meningitis [9, 10]. Even in situations where lumbar puncture (LP) is necessary, the procedure may be delayed since there is a risk of brain herniation in comatose children [11]. However, the high risk of bacterial infection and potential serious consequences if not treated, justify immediate administration of antibiotics [7]. In resource poor countries, antibiotic cover with chloramphenicol and crystalline penicillin is standard practice, since chloramphenicol is cheap and effective. Thus, some children who receive chloramphenicol also receive phenytoin as an anticonvulsant.
Prolonged or repetitive seizures are associated with increased risk of neurological sequelae in children with severe malaria [12, 13]. The potential adverse consequences of seizures and the occurrence of subtle seizures has led to the consideration of anticonvulsant prophylaxis as a rational therapeutic option in children with CM. Phenobarbitone is an effective anticonvulsant prophylaxis in children with CM at a dose of 20 mg kg−1, but is associated with increased mortality in African children with CM [14]. Phenytoin would be a suitable alternative to phenobarbitone due to its long elimination half-life at the usual therapeutic doses. We have previously shown that the pharmacokinetics and clinical effects of multiple intravenous (i.v.) doses of phenytoin were comparable with those of in vivo generated phenytoin following multiple i.v. and intramuscular (i.m.) doses of its water-soluble pro-drug, fosphenytoin, in children with severe malaria and convulsions [15]. For seizure prophylaxis, a single dose of fosphenytoin would have practical advantages, if it could be shown to be effective. Therefore, the first aim of the present study was to investigate the pharmacokinetics and safety of a single i.m. dose of fosphenytoin in children with severe malaria but who were seizure-free at the start of the study.
Several studies have reported that chloramphenicol inhibits the metabolism of phenytoin, in some cases leading to toxic concentrations in vivo[16–20]. In resource poor countries, most of the children who would receive fosphenytoin as an anticonvulsant prophylaxis would also receive chloramphenicol for bacterial meningitis or bacteraemia. The alternatives to chloramphenicol are the third generation cephalosporins, but they are more expensive. The second aim of this study was to investigate the effect of concomitant administration of chloramphenicol on the pharmacokinetics and safety of phenytoin in children with CM. We used cefotaxime, a third generation cephalosporin that is effective in the treatment of bacterial meningitis [21] in the control arm of the study. The only reported interaction between this class of antibiotics and phenytoin is the displacement of the latter from plasma protein binding sites by ceftriaxone [22]. We assumed that cefotaxime does not inhibit the metabolism of phenytoin.
Methods
Study site
The study was approved by the Kenya Medical Research Institute (KEMRI) Ethics Committee, and carried out at the KEMRI/Wellcome Trust high dependency Unit at the Kilifi District Hospital on the Kenyan coast. Children were recruited into the study after their parents/guardians had signed a written informed consent form.
Subjects
Children admitted to the Unit were recruited into the study if they: (i) were aged 9 months to 13 years [children < 9 months are unable to localize a painful stimulus which is used to indicate resolution of coma, while those> 13 years are admitted to the adult ward], (ii) had a blood slide positive for Plasmodium falciparum, and (iii) if their parents/guardian gave a written informed consent. They were excluded if: (i) consent was refused, (ii) they had received phenytoin or chloramphenicol prior to recruitment into the study, (iii) they had detectable phenytoin or chloramphenicol in the predose sample (excluded at the data analysis stage). They were withdrawn from the study if: (i) consent was withdrawn, and (ii) lumbar puncture indicated that they had meningitis.
Protocol
This was an open-label parallel design study in which one group of children received fosphenytoin and chloramphenicol while the control group received fosphenytoin and cefotaxime. Children were given fosphenytoin for prevention of seizures then sequentially randomized to receive either chloramphenicol or cefotaxime to cover the possibility that the coma was caused by bacterial meningitis. A clinical history was obtained and physical examination performed on all the children on admission. Venous access was obtained by fixing Teflon cannulae (Jelco ®, Ethicon S.p.A, Italy) into the arm veins, one for i.v. administration of fluids, and the other (in the opposite arm) for blood sampling. On admission a blood sample (6 ml) was drawn for malaria parasite count, blood culture, and the determination of glucose, electrolytes, plasma proteins, plasma albumin and a differential blood cell count. A portion of the blood was centrifuged (1500 g; 3 min) and the plasma separated and stored at −20 °C until assayed for baseline phenytoin and chloramphenicol. Details of other clinical management have been described elsewhere [6]. Chloramphenicol sodium succinate (Lincoln Pharmaceuticals Ltd, Nirav Complex, Ahmedabad, India; 25 mg kg−1 every 6 h for 72 h) or cefotaxime (Cardila Pharmaceuticals Ltd, Ghodasar, Ahmedabad, India; 25 mg kg−1 every 8 h for 72 h) was administered as an i.v. bolus dose, while fosphenytoin sodium (Pro-Epanutin ®, Parke-Davis, Eastleigh, UK; 18 mg kg−1 phenytoin sodium equivalents), was administered undiluted as a single i.m. injection into the anterior aspect of the thigh, followed by rubbing of the area for 30 s.
Blood and CSF sampling and drug assays
A blood sample (0.4 ml) for measuring plasma fosphenytoin, phenytoin, chloramphenicol succinate and chloramphenicol concentrations was withdrawn through the sampling cannula at 5, 10, 15, 20, 30, 40, 60 min, and at 2, 4, 6, 12, 24, 36, 48, 60 and 72 h after drug administration. The cannula was flushed with sterile heparinized normal saline (1 ml; 20 i.u. ml−1) after each sample collection. Residual saline in the cannulae was removed before each sample collection. Blood was collected in lithium heparin-coated tubes and centrifuged (1500 g; 3 min) to separate plasma. Plasma was stored in polyvinyl vials at −20 °C until assayed for drug concentrations. An additional blood sample (1.5 ml) obtained at 4 h post drug administration was used to obtain plasma for the determination of unbound phenytoin concentrations. Plasma (1 ml) was placed in a Centrifree ®micropartition filter unit (Amicon Inc., Beverly, MA, USA) and centrifuged (1500 g; 15 min at 4 °C) to obtain plasma water which was stored at −20 °C until assayed for phenytoin. In those patients who had a lumber puncture performed for clinical purposes, an aliquot (100 µl) of CSF was obtained and stored at −20 °C until assayed for phenytoin.
Plasma fosphenytoin [23], phenytoin [24], chloramphenicol and chloramphenicol succinate [25] and CSF phenytoin [24] concentrations were determined according to previously published h.p.l.c. methods. The intra-and interassay coefficients of variation for the determina-tion of chloramphenicol, chloramphenicol succinate, phenytoin and fosphenytoin were all < 10%, and there were no interferences with the assays from other concomitantly administered drugs (antibiotics, antipyretics and antimalarials).
Pharmacodynamic measurements
Changes in heart rate (HR), blood pressure (BP), respiratory rate (RR) and transcutaneous oxygen saturation were recorded at every sampling time for 72 h, using a specifically designed proforma. Hypotension was defined as mean BP < 50 mmHg, bradycardia as HR < 80 beats min−1, and respiratory depression as RR < 20 breaths min−1 and transcutaneous oxygen saturation of < 96%.
Pharmacokinetic analysis
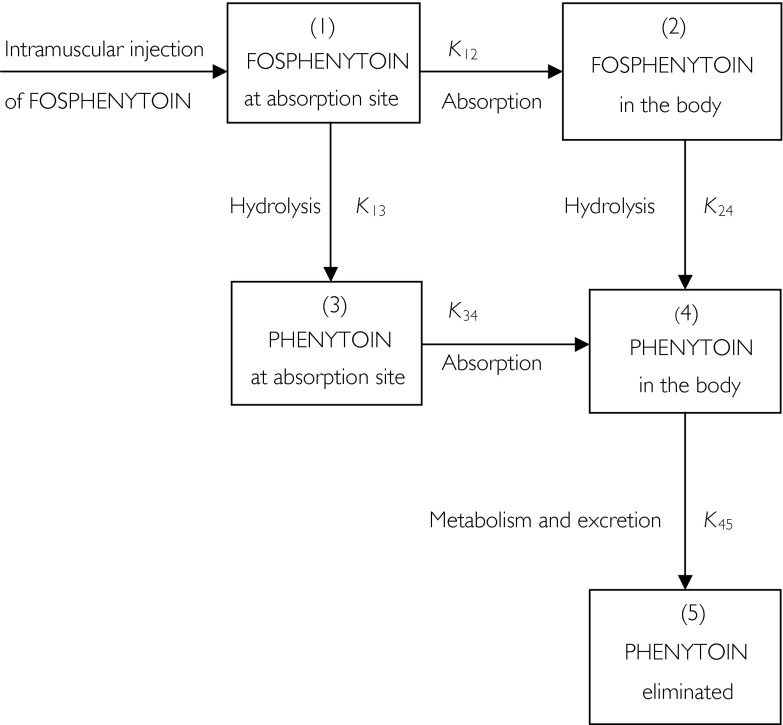
Peak plasma concentrations (Cmax) and concentration peak times (tmax) of phenytoin were derived directly from measured values. The area under the plasma phenytoin concentration-time curve (AUC) was calculated by use of the trapezoidal method with extrapolation to infinity. The pharmacodynamic measurements were expressed in terms of area under the effect-time curve (AUE), using the change from baseline (time zero) values [26]. In the present case, this was achieved by first determining the change from baseline value (x) at various times post drug administration, normalizing the baseline value to an arbitrary value of 100 and finally expressing the ‘normalised’ effects as (100 + x) or (100 − x), depending on whether the change was a decrease (–) or increase (+) over the baseline value. The advantage of using the transformed data is that all the values are positive and the numerical value of AUE is a direct measure of the magnitude of change. Thus, the smaller the value of AUE, the greater the reduction in a particular parameter. AUE was calculated using the trapezoidal method. Following administration of a single dose of phenytoin, differentiating between linear and nonlinear compartment models may be difficult [27]. In the present study, we assumed that linear pharmacokinetics applied, and used a five-compartment user-defined model (Figure 1) to simultaneously fit the plasma fosphenytoin and phenytoin concentration-time data. The model assumes that hydrolysis of fosphenytoin to phenytoin takes place both at the site of injection and in the rest of the body. The model was chosen on the basis of the best-fit [28] when compared to alternative models. We used the estimated terminal elimination rate constant (K45 in Figure 1) to determine the half-life (t1/2,z) for phenytoin. The pharmacokinetic program TopFit [29] was used to fit models to the data. The estimated CSF:plasma phenytoin ratios were assumed to reflect steady state conditions.
Figure 1.
Schematic representation of the disposition of fosphenytoin following intramuscular administration.
Statistical analysis
Results were expressed as mean values ± s.d., except for CSF:plasma phenytoin concentration ratio, Cmax and tmax which were expressed as median (range). Parasite counts were expressed as the geometric means. The Mann–Whitney U-test was used to compare demographic, pharmacodynamic and pharmacokinetic parameters for phenytoin between the chloramphenicol-treated group and the cefotaxime-treated group. A value of P< 0.05 was considered statistically significant. The results were also compared by determining the 95% confidence interval (CI) using a commercially available computer program [30].
Results
Fifteen children [11 males, four females, median (range) age: 43 (10–108) months] were recruited into the chloramphenicol group and 13 children [seven males, six females, median (range) age: 39 (18–96) months] were recruited into the cefotaxime group. The biochemical parameters at admission were comparable between the groups except for the white blood cell count which was significantly higher in the cefotaxime compared with the chloramphenicol treated patients. However, this is unlikely to have affected the outcome of the study.
All the children received the antimalarials quinine and sulfadoxine/pyrimethamine, with the latter being administered on discharge. All the children in the chloramphenicol group also received benzylpenicillin, whereas seven children (five in the cefotaxime group and two in the chloramphenicol group) received phenobarbitone. Diazepam was administered to one child in the chloramphenicol group.
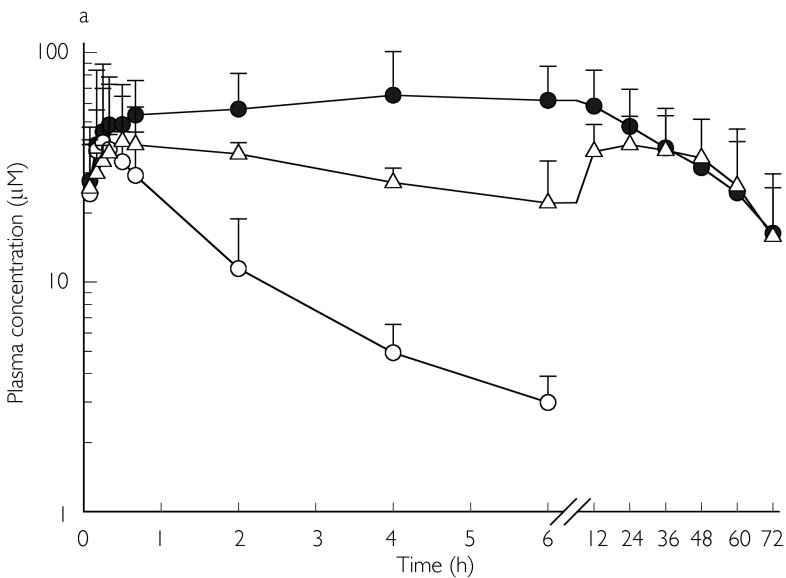
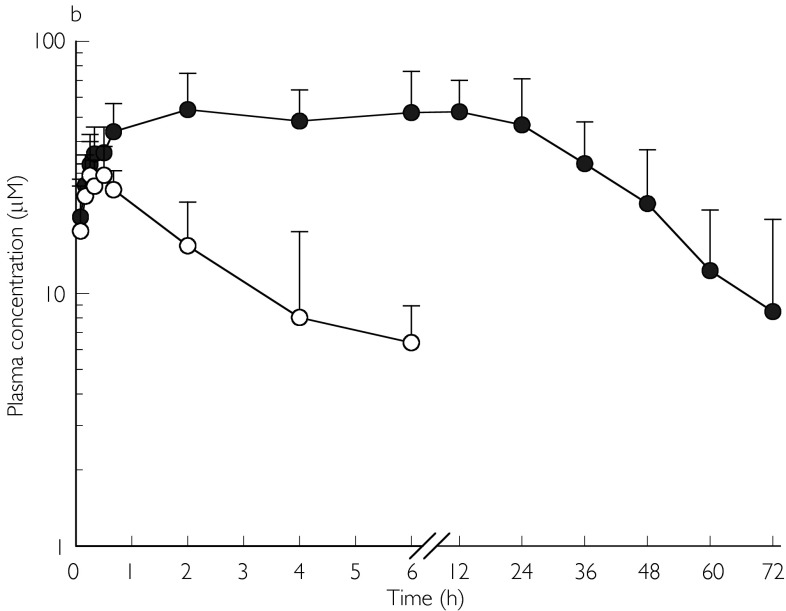
Fosphenytoin appeared to be rapidly hydrolysed to phenytoin in both groups, with appreciable amounts of phenytoin detectable in plasma within 5 min after fosphenytoin administration (Figure 2a, b). Plasma fosphenytoin concentrations declined rapidly in both groups of patients, and were below the detection limit after 6 h. Plasma concentration-time profiles for both fosphenytoin and phenytoin were adequately fitted to the model described in Figure 1. Using this model, the estimated mean rate constant for hydrolysis of fosphenytoin in the systemic circulation (K24) was smaller than that at the site of injection (K13), and the rate constant for absorption (K12). This suggests that K24 partly reflected absorption kinetics (‘flip-flop’ phenomenon) in some of the children. Using the values for K13 to represent the true in vivo hydrolysis rate constant, the mean half-life for hydrolysis of fosphenytoin to phenytoin was approximately 4 min in both groups of patients. Peak plasma phenytoin concentrations were rapidly achieved in some patients, but delayed in others. Mean con-centrations were sustained at approximately 50 µm(13.7 µg ml−1) for a considerable period of time, whereas mean chloramphenicol concentrations were approximately 40 µm(12.9 µg ml−1) (Figure 2a, b). Mean unbound phenytoin concentrations were maintained at approximately 1 µg ml−1(3.96 µm) for the first 24 h in both groups, whereas CSF phenytoin concentrations greater than 1 µg ml−1 were achieved in all patients. Apart from t1/2,z(Table 1) which was significantly longer in the chloramphenicol-treated group, there was no difference between the two groups with regard to the mean pharmacokinetic parameters or vital signs (respiratory rate, heart rate, transcutaneous oxygen saturation and blood pressure). Five patients in the chloramphenicol group and four in the cefotaxime group had more than one episode of a decrease in systolic blood pressure by more 20 mmHg. However, this was not associated with an appreciable decrease in mean arterial blood pressure.
figure 2.
a) Mean (s.d.) plasma concentrations of fosphenytoin (○; n= 7), phenytoin (•; n= 11), and chloramphenicol (▵ n= 12) following a single intramuscular dose (18 mg kg−1 phenytoin sodium equivalents) of fosphenytoin plus multiple intravenous doses (25 mg kg−16 hourly for 72 h) of chloramphenicol sodium succinate in children. b) Mean (s.d.) plasma concentrations of fosphenytoin (○; n= 5) and phenytoin (•; n= 13) following a single intramuscular dose (18 mg kg−1 phenytoin sodium equivalents) of fosphenytoin plus multiple intravenous doses (25 mg kg−1 8 hourly for 72 h) of cefotaxime in children.
Table 1.
Pharmacokinetic parameters of phenytoin following administration of a single intramuscular dose (18 mg kg−1 phenytoin sodium equivalents) of fosphenytoin plus multiple intravenous doses (25 mg kg−16 hourly for 72 h) of chloramphenicol sodium succinate or multiple intravenous doses (25 mg kg−1 8 hourly for 72 h) of cefotaxime in children.
| Mean (s.d.) or median (range)‡ | |||
|---|---|---|---|
| parameter | Chloramphenicol group | Cefotaxime group | 95% CI of difference between means |
| AUC(0,∞; total) (µg ml−1 h) | 841.1 (425.7); n= 11 | 562.8 (224.6); n= 8 | −633.7, 65.3 |
| AUC(0, ∞; unbound) (µg ml−1 h) | 58.5 (24.2); n= 7 | 47.6 (9.4); n= 6 | −35.0, 11.4 |
| Fraction unbound (fu) | 0.06 (0.03); n= 7 | 0.09 (0.04); n= 6 | −0.01, 0.07 |
| K45(h−1) | 0.03 (0.01); n= 10 | 0.05 (0.01); n= 9 | −0.02, −0.003 |
| t1/2,z(h) | 23.7 (9.1); n= 10 | 15.4 (2.8); n= 9 | 1.71, 14.99 |
| CSF:plasma phenytion‡ | 0.21 (0.14–0.40); n= 12 | 0.22 (0.01–0.41); n= 10 | −0.08, 0.10 |
| Cmax(total; µg ml−1)‡ | 14.98 (7.5–34.6); n= 15 | 14.45 (8.4–34.9); n= 9 | −5.0, 6.6 |
| Cmax(unbound; µg ml−1)‡ | 1.12 (0.6–1.8); n= 12 | 1.29 (0.96–1.95); n= 10 | −0.5, 0.04 |
| tmax(h)‡ | 4.0 (0.33–12.0); n= 15 | 4.0 (0.33–12.0); n= 13 | −2.0, 3.7 |
Only 30% of the children had convulsions requiring treatment with another anticonvulsant, all of which occurred within the first 24 h following fosphenytoin administration. Four children (two from each group) died before completion of the study and all had features of intracranial hypertension. Data from these children are not included in the analysis. None of the surviving children developed neurological sequelae at the time of discharge from hospital.
Discussion
A single i.m. dose of fosphenytoin rapidly achieved and sustained plasma total phenytoin concentrations of more than 10 µg ml−1( 39.6 µm) over 72 h, and unbound concentrations of more than 1 µg ml−1( 3.96 µm) for 24 h. Seventy per cent of the children in the study did not have convulsions that required further treatment during the 72 h period, suggesting that fosphenytoin may be useful for anticonvulsant prophylaxis in children with severe malaria. Furthermore it can be administered i.m., a practical advantage for use at peripheral health facilities. The recurrence of convulsions was not related to the lack of achievement of accepted effective plasma phenytoin concentrations since in all the children who had recurrence of convulsions, plasma unbound phenytoin concentrations within the reported therapeutics range (1–2 µg ml−1) were rapidly achieved. However, the number of children in this study was too small, and the effectiveness of fosphenytoin for prophylaxis needs further investigation in a larger study.
Concurrent administration of chloramphenicol in multiple therapeutic doses similar to those used for the treatment of bacterial meningitis resulted in an increase in the elimination half-life of phenytoin, but this had no significant effect on plasma phenytoin concentrations or the cardiovascular effects of phenytoin. The loading dose of fosphenytoin used in this study [18 mg kg−1 phenytoin sodium equivalents (PE)] is the same as the intravenous dose we use for treatment of acute and prolonged convulsions [15].
The absorption of fosphenytoin appeared to be slow in some of the children (as indicated by the apparent ‘flip flop’ between absorption and systemic hydrolysis rate constants). A decreased rate of drug absorption from the i.m. site of injection would be expected in some children with severe malaria, since this condition is known to be associated with compromised peripheral circulation [31]. In general, provided the drug is in solution, absorption from i.m. site is perfusion rate-limited [32]. Median CSF:plasma phenytoin concentration ratios were approximately 0.2 in both groups (Table 1), close to previously reported values in children [33].
According to Figure 1, phenytoin could have been formed from hydrolysis of fosphenytoin at the site of absorption or in systemic circulation. In the former case, phenytoin could have precipitated at the site of absorption, leading to subsequent slow and variable absorption. There is no simple expression for AUC based on a model describing first order absorption followed by capacity-limited elimination, since AUC depends on the rate of absorption [34]. In either case, estimation of AUC by the trapezoidal method, as in the present study, may underestimate the bioavailability of phenytoin when elimination is capacity-limited [35]. Moreover, because we could not keep most patients in the hospital for more than 72 h, the t1/2,z for phenytoin was estimated over a shorter than ideal period. Estimation of t1/2,z may also have been influenced by protracted absorption of phenytoin in some patients. However, since the concentrations of chloramphenicol fluctuated little during most of the study period, and since fosphenytoin was administered via the same route and at the same dose to both the chloramphenicol and cefotaxime-treated groups, any effect of chloramphenicol on phenytoin elimination would still have been detected irrespective of the model used to describe phenytoin disposition. Hence, the simple linear disposition model was used in the present study.
Owing to the nonlinear pharmacokinetics of phenytoin, a lack of interaction with the other drug following a single dose does not exclude the occurrence of an interaction when multiple doses are administered [36]. Phenytoin is eliminated mainly by hepatic metabolism [37, 38], and has a low hepatic extraction ratio [39]. Therefore, its clearance is susceptible to inhibition irrespective of route of administration [40]. The significant increase in elimination half-life, and the higher (though not statistically significant) AUC of unbound phenytoin noted in the chloramphenicol-treated group suggest that some inhibition of metabolism had occurred. The small number of patients in this study may have precluded detection of a significant difference in other pharmacokinetic parameters.
Concomitantly administered antimalarial drugs (quinine and sulfadoxine/pyrimethamine) are unlikely to have affected the pharmacokinetics of phenytoin in this study. Sulphonamides are known to inhibit the metabolism of phenytoin [41], a substrate of CYP 2C9 [42, 43], but sulfadoxine/pyrimethamine was only administered on discharge. Quinine is principally metabolized by cytochrome CYP3A isoforms [44]. Some children received phenobarbitone which can inhibit or induce the metabolism of phenytoin [45]. These effects may neutralize each other [45], or the combination may lead to a decrease in phenytoin concentrations [46, 47]. Two children in the chloramphenicol group and five children in the cefotaxime group received phenobarbitone. However, even after exclusion of the two children from the chloramphenicol group, the mean t1/2,z of phenytoin in this group was still significantly longer compared with that in the cefotaxime group. Diazepam and benzyl penicillin, the other two drugs coadministered with phenytoin, are not substrates or inhibitors of CYP2C9.
CYP2C9 is known to exhibit genetic polymorphism [48, 49] and phenytoin toxicity has been reported in CYP 2C9 poor metabolizers (PMs) [50, 51]. A study of the frequency of common CYP2C9 alleles (CYP2C9*1, CYP2C9*2 and CYP2C9*3) in Italians and Ethiopians concluded that the relative distribution of CYP2C9 alleles in the Ethiopian population was different from that described in other ethnic groups [52]. The proportion of PMs and the influence of CYP2C9 allele distribution among other black African populations, including children, are still unknown. For PMs, even a single dose of phenytoin may result in toxicity, which may be enhanced by concurrent administration of chloramphenicol. The exact mechanism by which chloramphenicol affects CYP2C9 has also not been fully elucidated, although it may involve inactivation of the enzyme [53].
In conclusion, we have shown that following concurrent administration of a single dose of fosphenytoin and multiple doses of chloramphenicol, there are apparently no significant changes in the pharmacokinetics or clinical effects of phenytoin. Children with severe malaria who are receiving chloramphenicol for concurrent bacterial infections can safely receive single doses of phenytoin (or fosphenytoin) for seizure control or prophylaxis. However, these conclusions may not be applicable in situations where a higher loading dose of phenytoin is used. Moreover, whether it is safe to use chloramphenicol in those children receiving multiple doses of phenytoin needs further investigation. The single dose of fosphenytoin used in the present study rapidly achieves and maintains adequate plasma phenytoin concentrations for at least 24 h, suggesting that i.m. fosphenytoin may offer a practical way of administering phenytoin for seizure prophylaxis.
Acknowledgments
This work is published with the permission of the Director of Kenya Medical Research Institute. This study was supported by a Collaborative Research Initiative Grant from The Wellcome Trust (grant no. 057978/Z/99/Z), a Research Capability Strengthening Grant from W.H.O (TDR/MIM grant no. 980074), [both to Professor Kokwaro] and a Wellcome Trust Senior Clinical Research Fellowship (grant no. 050533) to Dr Newton. Dr Ogutu is a trainee in Clinical Pharmacology supported by W.H.O (TDR/MIM) and KEMRI. We are grateful to Professor Kevin Marsh (Director, KEMRI-Wellcome Trust Collaborative Research Programme) and Dr Norbert Peshu (Director, KEMRI-Centre for Geographic Medicine Research-Coast) for their support. We also wish to thank all our clinical, nursing and laboratory colleagues in Kilifi and Nairobi who contributed immensely to this work. Finally, we are grateful to Dr Neil Hounslow (Parke-Davies, UK), for providing the fosphenytoin for this study.
References
- 1.Warrell D, Molyneux M, Beales P. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84(Suppl 2):1–65. [PubMed] [Google Scholar]
- 2.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 3.Waruiru CM, Newton CRJC, Forster D, et al. Epileptic seizures and malaria in Kenyan children. Trans R Soc Trop Med Hyg. 1996;90:152–155. doi: 10.1016/s0035-9203(96)90120-0. [DOI] [PubMed] [Google Scholar]
- 4.Wattanagoon Y, Srivilairit S, Looareesuwan S, White NJ. Convulsions in childhood malaria. Trans R Soc Trop Med Hyg. 1994;88:426–428. doi: 10.1016/0035-9203(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 5.Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epilepticus in childhood cerebral malaria. Q J Med. 1996;89:591–597. doi: 10.1093/qjmed/89.8.591. [DOI] [PubMed] [Google Scholar]
- 6.Ogutu BR, Newton CRJC, Crawley J, et al. Pharmacokinetics and anticonvulsant effects of diazepam in children with severe falciparum malaria and convulsions. Br J Clin Pharmacol. 2002;53:49–57. doi: 10.1046/j.0306-5251.2001.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkley JA, Mwangi I, Ngetsa CJ, et al. Diagnosis of acute bacterial meningitis in children at a district hospital in sub-Saharan Africa. Lancet. 2001;357:1753–1757. doi: 10.1016/S0140-6736(00)04897-2. [DOI] [PubMed] [Google Scholar]
- 8.Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93:283–286. doi: 10.1016/s0035-9203(99)90024-x. [DOI] [PubMed] [Google Scholar]
- 9.Berkley JA, Mwangi I, Mellington F, Mwarumba S, Marsh K. Cerebral malaria versus bacterial meningitis in children with impaired consciousness. Q J Med. 1999;92:151–157. doi: 10.1093/qjmed/92.3.151. [DOI] [PubMed] [Google Scholar]
- 10.Wright PW, Avery WG, Ardill WD, McLarty JW. Initial clinical assessment of the comatose patient: cerebral malaria versus meningitis. Pediatr Inf Dis J. 1993;12:37–41. doi: 10.1097/00006454-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Newton CRJC, Kirkham FJ, Winstanley PA, et al. Intracranial pressure in African children with cerebral malaria. Lancet. 1991;337:573–576. doi: 10.1016/0140-6736(91)91638-b. [DOI] [PubMed] [Google Scholar]
- 12.Newton CJRC, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79:1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 13.Holding PA, Stevenson J, Peshu N, Marsh K. Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg. 1999;93:529–534. doi: 10.1016/s0035-9203(99)90368-1. [DOI] [PubMed] [Google Scholar]
- 14.Crawley J, Waruiru C, Mithwani S, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised controlled intervention study. Lancet. 2000;355:701–706. doi: 10.1016/S0140-6736(99)07148-2. [DOI] [PubMed] [Google Scholar]
- 15.Ogutu BR, Newton CRJC, Muchohi SN, et al. Pharmacokinetics and clinical effects of phenytoin in children with malaria and convulsions. Br J Clin Pharmacol. doi: 10.1046/j.1365-2125.2003.01829.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen LK, Skovsted L. Inhibition of drug metabolism by chloramphenicol. Lancet. 1969;ii:1397–1399. doi: 10.1016/s0140-6736(69)90937-4. [DOI] [PubMed] [Google Scholar]
- 17.Ballek RE, Reidenberg MM, Orr L. Inhibition of diphenylhydantoin metabolism by chloramphenicol. Lancet. 1973;i:150. doi: 10.1016/s0140-6736(73)90219-5. [DOI] [PubMed] [Google Scholar]
- 18.Koup JR, Gibaldi M, McNamara P, Hilligoss DM, Colburn WA, Bruck E. Interaction of chloramphenicol with phenytoin and phenobarbital: Case report. Clin Pharmacol Ther. 1978;24:571–575. doi: 10.1002/cpt1978245571. [DOI] [PubMed] [Google Scholar]
- 19.Greenlaw CW. Chloramphenicol–phenytoin interaction. Drug Intell Clin Pharm. 1979;13:609–610. [Google Scholar]
- 20.Salter M, Stephens NM. Phenytoin–chloramphenicol interaction. Drug Intell Clin Pharm. 1980;14:221. [Google Scholar]
- 21.Landesman SH, Corrado MS, Shah PM, et al. Past and current roles of cephalosporin antibiotics in the treatment of meningitis. Am J Med. 1981;71:693–703. doi: 10.1016/0002-9343(81)90240-0. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta A, Dennen DA, Dean R, McLawhon RW. Displacement of phenytoin from serum protein carriers by antibiotics: studies with ceftriaxone, nafcillin, and sulfamethoxazole. Clin Chem. 1991;37:98–100. [PubMed] [Google Scholar]
- 23.Cwik MJ, Liang M, Deyo K, Andrews C, Fischer J. Simultaneous rapid high-performance liquid chromatographic determination of phenytoin and its pro-drug fosphenytoin in human plasma and ultrafiltrate. J Chromatogr. 1997;693:407–414. doi: 10.1016/s0378-4347(97)00057-1. [DOI] [PubMed] [Google Scholar]
- 24.Aynacioglu AS, Brockmoller J, Bauer S, et al. Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol. 1999;48:409–415. doi: 10.1046/j.1365-2125.1999.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velagapudi R, Smith RV, Ludden T, Sagraves R. Simultaneous determination of chloramphenicol and chloramphenicol succinate in plasma using high performance liquid chromatography. J Chromatogr. 1982;228:423–428. doi: 10.1016/s0378-4347(00)80467-3. [DOI] [PubMed] [Google Scholar]
- 26.Drewe J, Ball HA, Beglinger C, et al. Effect of P-glycoprotein modulation on the clinical pharmacokinetics and adverse effects of morphine. Br J Clin Pharmacol. 2000;50:237–246. doi: 10.1046/j.1365-2125.2000.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzel G, Degner B. Pharmacokinetic Fitting of Data Exhibiting Nonlinear (Michaelis-Menten) Kinetics. In: Heinzel G, Woloszczak R, Thomann P, editors. Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. Stuttgart: Gustav Fischer; 1993. pp. 5.101–5.102. [Google Scholar]
- 28.Akaike A. Posterior probabilities for choosing a regression model. Annal Instit Math Stat. 1978;30:A9. [Google Scholar]
- 29.Heinzel G, Woloszczak R, Thomann P. TopFit Version 20Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. Stuttgart: Gustav Fischer; 1993. pp. 3.72–3.86. [Google Scholar]
- 30.Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with Confidence. 2. Bristol: BMJ Books; 2000. pp. 15–27. [Google Scholar]
- 31.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 32.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications. 2. Philadelphia: Lea & Febiger; 1989. p. 115. [Google Scholar]
- 33.Koran G, Barzilay Z, Schachar E, et al. Kinetics of CSF phenytoin in children. Can J Neurol Sci. 1983;10:195–197. doi: 10.1017/s0317167100044917. [DOI] [PubMed] [Google Scholar]
- 34.Wagner JG. Pharmacokinetics for the Pharmaceutical Scientist. 1. Lancaster, Pennsylvania: Technomic Publishing Co.; 1993. pp. 128–129. [Google Scholar]
- 35.Jusko WG, Koup JR, Alvan G. Nonlinear assessment of phenytoin bioavailability. J Pharmacokin Biopharm. 1976;4:327–336. doi: 10.1007/BF01063122. [DOI] [PubMed] [Google Scholar]
- 36.Nation RL, Evans AM, Milne RW. Pharmacokinetic drug interactions with phenytoin. Clin Pharmacokinet. 1990;18:37–60. doi: 10.2165/00003088-199018010-00003. [DOI] [PubMed] [Google Scholar]
- 37.Glazko AJ. Antiepileptic drugs: Biotransformation, metabolism, and serum half-life. Epilepsia. 1975;16:367–391. doi: 10.1111/j.1528-1157.1975.tb06064.x. [DOI] [PubMed] [Google Scholar]
- 38.Witkin KM, Bius DL, Teague BL, et al. Determination of 5-(hydroxyphenyl)-5-phenylhydantoin and studies relating to the disposition of phenytoin in man. Ther Drug Monit. 1979;1:11–34. doi: 10.1097/00007691-197901000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications. 2. Philadelphia: Lea & Febiger; 1989. p. 154. [Google Scholar]
- 40.Powell JR, Cate EW. Induction and inhibition of drug metabolism. In: Spokane WA, editor; Evans WE, Schentag JJ, Jusko WJ, editors. Applied Pharmacokinetics: Principles of Therapeutic Drug Monitoring. Applied Therapeutics Inc; 1986. pp. 139–186. [Google Scholar]
- 41.Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand. 1979;624(Suppl):106–110. doi: 10.1111/j.0954-6820.1979.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 42.Veronese ME, Doecke CJ, Mackenzie PI, et al. Site-directed mutation studies of human liver cytochrome P450 isoenzymes in the CYP2C subfamily. Biochem J. 1993;289:533–538. doi: 10.1042/bj2890533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doecke CJ, Veronese ME, Pond SM, et al. Relationship between phenytoin and tolbutamide hydroxylations in human liver microsomes. Br J Clin Pharmacol. 1991;31:125–130. doi: 10.1111/j.1365-2125.1991.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Coville PF, Walker RJ, et al. Evidence for involvement of human CYP3A in the 3-hydroxylation of quinine. Br J Clin Pharmacol. 1997;43:245–252. doi: 10.1046/j.1365-2125.1997.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dodson WE. Nonlinear kinetics of phenytoin in children. Neurology. 1982;32:42–48. doi: 10.1212/wnl.32.1.42. [DOI] [PubMed] [Google Scholar]
- 46.Buchanan RA, Heffelfinger JC, Weiss CF. The effect of phenobarbital on diphenylhydantoin metabolism in children. Paediatrics. 1969;43:114–116. [PubMed] [Google Scholar]
- 47.Garretson LK, Dayton PG. Disappearance of phenobarbital and diphenylhydantoin from serum of children. Clin Pharmacol Ther. 1970;11:674–679. doi: 10.1002/cpt1970115674. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan-Klose TH, Ghanayem BI, Bell DA, et al. The role of the CYP2C9-Leu 359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics. 1996;6:341–349. doi: 10.1097/00008571-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Rettie AE, Wienkers LC, Gonzalez FJ, et al. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics. 1994;4:39–42. doi: 10.1097/00008571-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Nimomiya H, Mamiya K, Matsuo S, et al. Genetic polymorphism of CYP2C subfamily and excessive serum phenytoin concentration with nervous system intoxication. Ther Drug Monit. 2000;22:230–232. doi: 10.1097/00007691-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Goldsten JA. Clinical relevance of genetic polymorphism in the human CYP2C subfamily. Br J Clin Pharmacol. 2001;52:349–355. doi: 10.1046/j.0306-5251.2001.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scordo MG, Aklillu E, Yasar U, Dahl ML, Spina E, Ingelman-Sundberg M. Genetic polymorphism of cytochrome P4502C9 in a Caucasian and a black African population. Br J Clin Pharmacol. 2001;52:447–450. doi: 10.1046/j.0306-5251.2001.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halpert JR, Miller NE, Gorsky LD. On the mechanism of the inactivation of the major phenobarbital-induced isoenzyme of rat liver cytochrome P-450 by chloramphenicol. J Biol Chem. 1985;260:8397–8403. [PubMed] [Google Scholar]