Abstract
N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase (GalNAc4S-6ST) is a sulfotransferase responsible for biosynthesis of chondroitin sulfate E (CS-E). CS-E plays important roles in numerous biological events, such as neurite outgrowth. However, the role of GalNAc4S-6ST in tumor progression remains unknown. In the present study, we analyzed expression of GalNAc4S-6ST mRNA in colorectal cancer by combining real-time RT-PCR with in situ hybridization (ISH) using archived formalin-fixed and paraffin-embedded tissue sections. In 57.5% of 40 patients, expression of GalNAc4S-6ST mRNA was increased in cancer tissues compared with paired normal mucosa. ISH using an RNA probe specific for GalNAc4S-6ST revealed that it was expressed in colorectal cancer cells. Analysis of the relationship between expression of GalNAc4S-6ST as determined by real-time RT-PCR assay and various clinicopathological variables revealed that GalNAc4S-6ST was associated with vessel invasion, although a statistically significant difference was not seen (P=0.125 for lymphatic vessel invasion and P=0.242 for venous invasion). Taken together, we show that real-time RT-PCR combined with ISH is useful to investigate quantitatively GalNAc4S-6ST mRNA expression in formalin-fixed and paraffin-embedded tissue sections, and that GalNAc4S-6ST expressed by colorectal cancer cells plays a minor role in tumor progression.
Keywords: chondroitin sulfate E, colorectal cancer, in situ hybridization, real-time RT-PCR, sulfotransferase
I. Introduction
Complex carbohydrates such as glycoproteins, glycolipids, and proteoglycans present on the cell surface or extracellular matrix play important roles in various biological events not only under physiological conditions but in pathological states such as inflammation and tumor progression [6, 7, 21, 25]. Since attached glycans are synthesized by concerted reactions of glycosyltransferases and sulfotransferases, the analysis of glycogenes, which encode these glycosyltransferases and sulfotransferases, will be helpful to understand the biological functions of glycan chains, as shown in an example of the analysis of O-GlcNAc transferase in diabetic rats [1]. Approximately 180 human glycogenes have been cloned so far, and thus molecular and biochemical information regarding their activity is available [19]. However, the study of glycogenes at the mRNA level using archived formalin-fixed and paraffin-embedded tissue blocks for routine pathological examination has been limited due to overall low expression of these transcripts [3].
Glycan chains comprising proteoglycans, called glycosaminoglycans (GAG), are variably sulfated by specific sulfotransferases [8, 14]. Chondroitin sulfate (CS) is a typical GAG subtype, and the negatively charged nature of GAG is implicated in several biological processes. In particular, chondroitin sulfate E (CS-E), which is composed of repeating GlcAβ1-3GalNAc(4,6-bis-SO4) disaccharide units with a β1,4-linkage and were originally identified in squid cartilage [10], has been reported to play diverse roles in vitro. Among these are release of histamine from mast cells [5], inhibition of procoagulant activity [16], promotion of neural outgrowth [2], and binding to heparin binding growth factors such as midkine and pleiotrophin [4, 26], basic fibroblast growth factors [4], E/P-selectin, and chemokines [11]. In addition, CS-E is known to be increased under pathological conditions, such as liver cirrhosis developed in the Long-Evans Cinnamon rat animal model of this disease [13]. In tumors, Monzavi-Karbassi et al. demonstrated that CS-E units inhibited P-selectin binding to a human breast cancer cell line in vitro [17]. Since P-selectin (also called GMP-140 and PADGEM) is found within the Weibel-Palade bodies of endothelial cells and α-granules of platelets [15], CS-E on the tumor cells may facilitate the metastasis by binding to P-selectin present in endothelial cells and/or platelets. However, the clinicopathological significance of CS-E expression in tumors in vivo remains unknown.
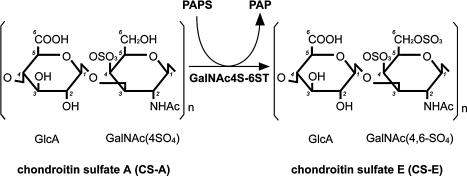
Recently, we purified N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase (GalNAc4S-6ST) protein from squid cartilage [9] and subsequently cloned its human orthologue [20]. GalNAc4S-6ST is responsible for the biosynthesis of CS-E from chondroitin sulfate A (CS-A) by transferring a sulfate group to the position C6 of GalNAc(4SO4) in CS-A (Fig. 1). Since the specific antibody directed to CS-E has not been available, analyses of GalNAc4S-6ST gene are helpful to understand the biological roles of CS-E.
Fig. 1.
Biosynthesis of CS-E. CS-A is converted to CS-E by GalNAc4S-6ST, which catalyzes sulfation at the C6 position of GalNAc(4SO4) residues of CS-A from a sulfate donor, 3'-phosphoadenosine 5'-phosphosulfate (PAPS). The position of carbon atoms is numbered in italics.
In the present study, we quantitatively analyzed expression of GalNAc4S-6ST mRNA detected in colorectal cancer tissue sections prepared from archived formalin-fixed and paraffin-embedded tissue blocks used for routine pathological examination by real-time RT-PCR assay. Since real-time RT-PCR assays cannot identify cell type(s) expressing a specific mRNA, we also carried out in situ hybridization (ISH) with a specific GalNAc4S-6ST RNA probe in the same colorectal cancer tissues for comparison. Finally, to determine a possible role of GalNAc4S-6ST in tumor progression, we compared expression levels of GalNAc4S-6ST mRNA detected in colorectal cancer with clinicopathological variables.
II. Materials and Methods
Patient samples
Formalin-fixed and paraffin-embedded tissue blocks of surgically resected primary colorectal cancers were retrieved from the pathology files of the Department of Laboratory Medicine, Shinshu University Hospital, Matsumoto, Japan. They included 40 patients (22 male and 18 female with ages ranging from 49 to 85 years (average 66.5 years)) operated on at that hospital. In each patient, a representative portion of colorectal cancer and its normal counterpart at the cut end were examined. All tissue samples were fixed in 20% formalin buffered with 0.1 M phosphate buffer (pH 7.4) at room temperature for 48 hr and then embedded in paraffin. Clinicopathological data analyzed in the present study were based on the original pathology reports, in which venous invasion and lymphatic invasion were assessed by Victoria blue-H&E staining and H&E staining, respectively. The experimental protocol for this study was approved by the Ethical Committee of Shinshu University School of Medicine.
Isolation of RNA from formalin-fixed, paraffin-embedded tissue blocks and cDNA synthesis
Total RNA was isolated from tumor portion of colorectal cancer tissues embedded in paraffin blocks. To avoid possible contamination of surrounding connective tissues or transitional mucosa from the tumor, the border between tumor and non-tumor portion was marked on H&E-stained tissue slides with a marker pen. By referring to the marked tissue slides, the tumor border was again marked on the relevant tissue blocks, and shallow incision was then carefully made into the tissue blocks using a razor blade along the marked border. After insection, 6 tissue slices of 5 µm thickness were prepared and transferred to a sterile 1.5 ml tube. Similarly, total RNA was also prepared from the normal colorectal mucosa, making another shallow incision between the mucosal layer and submucosa of paraffin blocks containing normal colorectal mucosa, and 6 slices of 5 µm thickness were transferred in the same manner. For deparaffinization, 1 ml of Hemo De (FALMA, Tokyo, Japan) was added to each tube and agitated. Tubes were tilted on a wave shaker at room temperature for 20 min and then centrifuged at 20,000×g for 20 min. After centrifugation, the supernatant was removed immediately and the step repeated 3 times. Next, 1 ml of 100% ethanol was added to the same tube and agitated. All tubes were shaken again at room temperature for 15 min, centrifuged at 20,000×g for 20 min, and immediately the supernatant was discarded, a step repeated three times. The pellet was dried completely and dissolved in 50 µl of proteinase K solution containing 1 µg/µl proteinase K (Nacalai Tesque, Kyoto, Japan), 20 mM Tris-HCl (pH 8.0), 0.15 M NaCl, 5 mM EDTA, and 1% sodium dodecyl sulfate. Tubes were capped and incubated at 37°C overnight. Total RNA was isolated using an RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. To remove genomic DNA, 15 µl of purified total RNA was digested with 2.5 µl of 10 U/µl RNase-free DNase I (Roche, Penzberg, Germany) at 37°C for 2 hr and heated at 70°C to inactivate the enzyme. Samples were then denatured at 70°C for 10 min and hybridized with 1 µl of 0.5 µg/µl random primers (Promega, Madison, WI, USA). For single-strand cDNA synthesis, total RNA was reverse transcribed using 1 µl of 200 U/µl SuperScript III (Invitrogen, Carlsbad, CA, USA), 2.5 µl of a 2.5 mM dNTP mixture, 0.5 µl of 0.1 M dithiothreitol, and 1 µl of 40 U/µl RNasin Plus RNase inhibitor (Promega) at 50°C for 1 hr and then heated to 70°C for 10 min. First-strand cDNA was used as a template for real-time RT-PCR.
Real-time RT-PCR analysis of GalNAc4S-6ST mRNA
Real-time RT-PCR analysis of GalNAc4S-6ST mRNA was achieved by using the 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Premixed reagents containing primers and TaqMan probes for GalNAc4S-6ST (Hs00248144_m1) and β2-microglobulin (B2M) (Hs99999907_m1) as a standard housekeeping gene were purchased from Applied Biosystems. To construct a standard curve, regions amplified using premixed primers for GalNAc4S-6ST or B2M were cloned into the pGEM-T vector (Promega), and a 10-fold dilution series of plasmid DNA ranging from 100 ag/µl to 1 pg/µl together with 50 ag/µl concentration of the same plasmid DNA were measured.
Using 96-well optical plates, a 50 µl solution containing 25 µl of 2×TaqMan universal master mix (Applied Biosystems), 0.5 µl of cDNA, and 2.5 µl of premixed reagents containing primers and TaqMan probe were added to each well. Plates were heated to 50°C for 2 min and 95°C for 10 min and then subjected to 55 thermal cycles (95°C for 15 sec, 60°C for 1 min). Absence of genomic DNA contamination was confirmed by amplifying samples without reverse transcriptase. GalNAc4S-6ST mRNA expression was normalized to that of B2M mRNA of the corresponding sample. Relative expression of GalNAc4S-6ST was defined by GalNAc4S-6ST mRNA/B2M mRNA ratios multiplied by 1×103. Assays were performed in duplicate, and median value and 25–75 percentile were indicated.
Preparation of a GalNAc4S-6ST-specific RNA probe for ISH
A digoxigenin (DIG)-labeled RNA probe was used to detect GalNAc4S-6ST mRNA in colorectal cancer by ISH. Using pFLAGGalNAc4S-6ST as a template [20], a GalNAc4S-6ST-specific nucleotide sequence (nucleotides 801–950; the first nucleotide of the initiation codon is defined as 1) was amplified by PCR. According to the published sequence (DDBJ/EMBL/GenBank accession number AB062423), 5'- and 3'-primers were 5'-CCCAAGCTTAGACCTCTATGACCGCCTGC-3' and 5'-GGAATTCAAGAGGTCCAGATAATCTTCC-3', respectively (HindIII and EcoRI sites underlined). Amplified DNA was cloned into HindIII and EcoRI sites of pGEM-3Zf (+) (Promega), and the resultant vector was used as a template to construct the probe, after confirming the correct sequence using an ABI3100 DNA sequencer (Applied Biosystems). A DIG-labeled antisense RNA probe was obtained from HindIII-cut template and T7 RNA polymerase with a DIG RNA Labeling Mixture (Roche), as described [18]. Similarly, control sense probes were generated using EcoRI-cut template and SP6 RNA polymerase. Linearized probes were precipitated with LiCl-ethanol, dissolved in DEPC-water, and stored at −80°C until use.
ISH
Hybridization and detection of the probes were carried out as described [12, 18]. Briefly, 7 µm-thick tissue sections prepared from the same tissue blocks containing colorectal cancer analyzed by real-time RT-PCR were deparaffinized in Hemo De (FALMA), hydrated with ethanol, neutralized with 0.2 M HCl for 20 min, treated with 50 µg/ml proteinase K (Amresco, Solon, OH, USA) at 37°C for 30 min, and then postfixed with 4% paraformaldehyde. Tissue slides were rinsed in 0.2% glycine for quenching and acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0). Hydrated slides were defatted with chloroform and air-dried. Sections were prehybridized with 50% deionized formamide/2×SSC at 45°C for 1 hr and then hybridized with 1 µg/ml of GalNAc4S-6ST antisense or sense probe in 50% deionized formamide, 2.5 mM EDTA (pH 8.0), 0.3 M NaCl, 1×Denhardt’s solution, 10% dextran sulfate, and 1 mg/ml brewer’s yeast tRNA at 45°C for 36 hr.
After hybridization, slides were washed in 50% formamide/2×SSC for 1 hr at 45°C and digested with 10 µg/ml RNase A (Amresco) in RNase buffer (0.5 M NaCl, 10 mM Tris-HCl) at 37°C for 30 min, followed by washing in 50% formamide/2×SSC at 37°C for 1 hr and then in 50% formamide/1×SSC at 37°C for 1 hr. After washing, sections were subjected to immunohistochemistry to detect hybridized probes with an alkaline phosphatase-conjugated anti-DIG antibody (Roche). The alkaline phosphatase reaction was carried out with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium in the presence of 10% polyvinyl alcohol at 4°C overnight. Sense probe controls showed no specific reactivity.
Statistical analysis
Statistical analysis was carried out using JMP 6 software (SAS, Cary, NC, USA). Significance with non-parametric distribution was evaluated by the Mann-Whitney U test for unpaired observations. Comparison of more than two groups was analyzed using the Kruskal-Wallis test. P values <0.05 were considered to be statistically significant.
III. Results
Expression levels of GalNAc4S-6ST mRNA determined by real-time RT-PCR
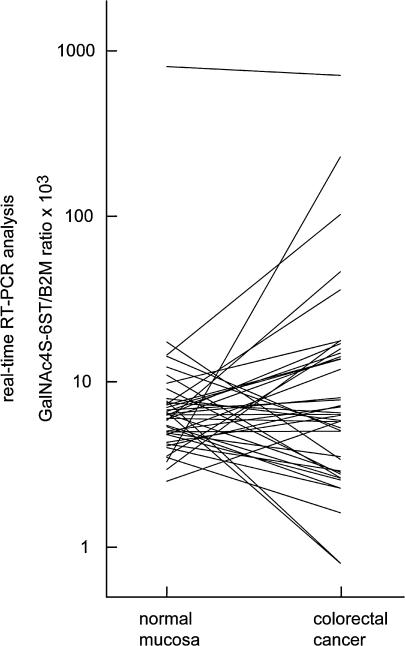
GalNAc4S-6ST mRNA expression levels in formalin-fixed and paraffin-embedded sections of colorectal cancer tissues from 40 patients and normal counterparts were determined using real-time RT-PCR. Based on the standard curve for B2M, amounts of the reverse-transcribed B2M cDNA in the samples were found ranging from 1.43 fg to 947.95 fg (median (25–75 percentile)=91.38 (43.73–139.90)), indicating that RNA retention in the tissue blocks was variable. GalNAc4S-6ST transcripts were expressed at a much lower level than B2M transcripts, whether from cancer or normal mucosa, consistent with previous observations that glycogene expression is generally low [3]. The expression level of GalNAc4S-6ST mRNA in cancer tissues normalized by B2M, which was defined by GalNAc4S-6ST mRNA/B2M mRNA ratios multiplied by 1×103, ranged from 0.80 to 709.68 (6.30 (2.85–14.68)), while that in normal mucosa ranged from 2.50 to 802.10 (6.29 (4.78–7.59)). When compared to paired normal colorectal mucosa, up- and down-regulations of GalNAc4S-6ST in cancer tissues were found in 57.5% and 42.5% of the patients, respectively (Fig. 2).
Fig. 2.
Comparison of expression levels of GalNAc4S-6ST mRNA between cancer and paired normal colorectal mucosa tissue in 40 patients with colorectal cancer. GalNAc4S-6ST expression was determined by real-time RT-PCR using archived tissue blocks, and the expression level of GalNAc4S-6ST was defined by GalNAc4S-6ST/B2M ratios multiplied by 1×103. Y-axis indicates logarithmic scale.
Detection of GalNAc4S-6ST mRNA in colorectal cancer cells by ISH
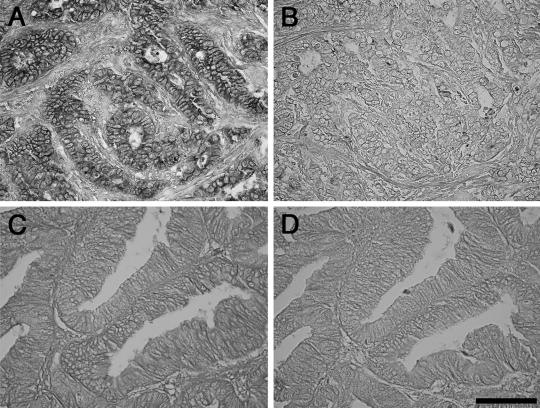
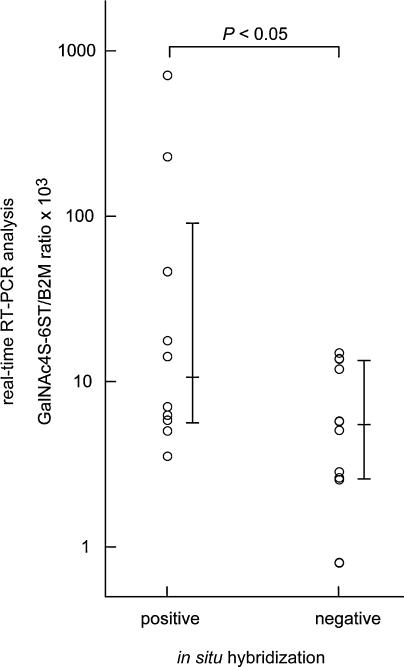
Analysis using real-time RT-PCR cannot determine which cell type(s) express GalNAc4S-6ST mRNA in cancer tissues. Thus, cancer tissues from 27 patients randomly selected from among the 40 patients analyzed by real-time RT-PCR were also examined by ISH using a DIG-labeled GalNAc4S-6ST RNA probe. Because the signal intensity for GalNAc4S-6ST mRNA was generally low, we classified the ISH results into 2 categories, “positive” or “negative”. The “positive” result was defined only when the signals were detected in the cytoplasm of cells using the antisense probe, regardless of the signal intensity. Accordingly 5 patients were excluded from the evaluation because nuclear staining was found in the tissue specimens. Based on the criteria, the positive signals for GalNAc4S-6ST were detected in colorectal cancer cells in 12 patients, whereas such signals were not seen in the remaining 10 (Fig. 3). Other cells such as stromal cells and inflammatory cells were negative for GalNAc4S-6ST mRNA. When expression of GalNAc4S-6ST mRNA determined by real-time RT-PCR in cancer tissues was compared between patients positive or negative for GalNAc4S-6ST mRNA based on ISH analysis, expression of GalNAc4S-6ST mRNA was significantly higher in positive compared to negative patients (P<0.05) (Fig. 4), indicating the validity of the real-time RT-PCR assay for GalNAc4S-6ST mRNA using archived tissue sections.
Fig. 3.
Detection of GalNAc4S-6ST mRNA in colorectal cancer cells by ISH. Positive GalNAc4S-6ST mRNA signals detected in cancer cells in which GalNAc4S-6ST mRNA expression as determined by real-time RT-PCR assay was high (GalNAc4S-6ST/B2M×103=228.28) (A). By contrast, GalNAc4S-6ST mRNA is not detectable in cancer cells in which expression of GalNAc4S-6ST mRNA was low (GalNAc4S-6ST/B2M×103=2.843) (C). A and C; antisense probe. B and D; sense probe. Bar=100 µm. (×400)
Fig. 4.
Association between expression levels of GalNAc4S-6ST mRNA and results obtained by ISH. Expression of GalNAc4S-6ST mRNA is significantly increased in patients positive for ISH (n=10) compared with patients negative for ISH (n=12) (P<0.05). The expression level of GalNAc4S-6ST was defined by GalNAc4S-6ST/B2M ratios multiplied by 1×103. Vertical bars indicate median and 25–75 percentiles, and Y-axis indicates logarithmic scale.
These data establish that GalNAc4S-6ST is transcribed by colorectal cancer cells and that real-time RT-PCR combined with ISH is a useful technique to investigate GalNAc4S-6ST mRNA expression quantitatively in formalin-fixed and paraffin-embedded tissue sections.
Clinicopathological significance of GalNAc4S-6ST mRNA on tumor progression
CS-E is a known to have high affinity to P-selectin [11]. Thus, it was possible that GalNAc4S-6ST expressed by colorectal cancer cells was associated with tumor progression. To test this hypothesis, a retrospective analysis was carried out to determine the relationship between GalNAc4S-6ST expression in colorectal cancer tissues as determined by real-time RT-PCR and clinicopathological variables such as histopathology of tumor, depth of invasion, and tumor stage. Among the variables examined, GalNAc4S-6ST was most positively associated with lymphatic vessel invasion and venous invasion of tumor cells, but this relationship was not statistically significant (P=0.125 for lymphatic vessel invasion; P=0.242 for venous invasion) (Table 1). P values of other valuables such as tumor stage were greater than 0.3. These results suggest that GalNAc4S-6ST plays a minor role in progression of colorectal cancer.
Table 1.
Comparison of expression levels of GalNAc4S-6ST mRNA with colorectal cancer according to clinicopathological parameters
| Parameters | Patients | GalNAc4S-6STa | P value |
|---|---|---|---|
| Locationb | |||
| Proximal | 15 | 5.73 (2.62–13.64) | 0.349f |
| Distal | 25 | 7.04 (3.46–16.37) | |
| Histopathologyc | |||
| Well | 27 | 5.77 (2.84–13.76) | 0.305f |
| Moderate and poor | 13 | 7.76 (3.89–17.36) | |
| Depth of invasiond | |||
| sm and mp | 9 | 7.76 (1.94–17.69) | 0.961f |
| ss, se, si, and a | 31 | 6.25 (2.89–14.15) | |
| Duke’s classificatione | |||
| A | 4 | 1.94 (1.00–13.80) | 0.334g |
| B | 11 | 5.77 (3.39–15.76) | |
| C | 11 | 6.48 (2.62–13.76) | |
| D | 14 | 7.18 (4.49–15.39) | |
| Lymph node metastasis | |||
| Negative | 20 | 6.01 (2.98–15.36) | 0.871f |
| Positive | 20 | 6.42 (2.80–14.58) | |
| Lymphatic vessel invasion | |||
| Negative | 4 | 2.50 (1.00–12.67) | 0.125f |
| Positive | 36 | 6.42 (3.05–14.68) | |
| Venous invasion | |||
| Negative | 1 | 17.64 (17.64–17.64) | 0.242f |
| Positive | 39 | 6.25 (2.84–14.15) | |
GalNAc4S-6ST/B2M mRNA ratios multiplied by 1×103 are indicated as median (25–75 percentile).
“Proximal” includes cecum, ascending colon, and transverse colon; “distal” includes descending colon, sigmoid colon, and rectum.
Well: well differentiated adenocarcinoma; moderate: moderately differentiated adenocarcinoma; poor: poorly differentiated adenocarcinoma.
sm, submucosa; mp, muscularis propria; ss, subserosa; se, exposure on serosa; si, invasion to neighboring tissue; a, adventitia.
A, confined to the intestinal wall; B, complete penetration of the intestinal wall; C, presence of nodal involvement; D, presence of distant metastasis.
Analyzed by Mann-Whitney U test.
Analyzed by Kruskal-Wallis test.
IV. Discussion
GalNAc4S-6ST is a key sulfotransferase functioning in the biosynthesis of CS-E, a highly sulfated form of CS. GalNAc4S-6ST was originally purified from squid cartilage [9], and cDNA encoding human GalNAc4S-6ST has been cloned [20]. Although CS-E plays important roles in various biological events [2], the expression profile of GalNAc4S-6ST mRNA in tumors has not been reported. Here for the first time, we analyzed expression of GalNAc4S-6ST mRNA in colorectal cancer tissues using archived formalin-fixed and paraffin-embedded tissue blocks, combining both quantitative real-time RT-PCR and ISH. Our studies reveal that GalNAc4S-6ST is transcribed in colorectal cancer cells at a low but significant level. These results indicate that combining these techniques can detect mRNA of glycogenes, which are generally transcribed at low levels, in tissue sections prepared in this manner. Since GalNAc4S-6ST mRNA is also detected in some colon cancer cell lines, such as Caco2, SW480, and Colo26 (Ito and Nakayama, personal communication), the role of GalNAc4S-6ST expressed by colorectal cancer is of great interest. In addition, it is also noteworthy that the expression level of GalNAc4S-6ST mRNA in normal colorectal mucosa is restricted to a narrow range, whereas that in colorectal cancer tissues is apparently distributed diversely, indicating that the GalNAc4S-6ST gene is variably transcribed in malignant cells. It would be important to reveal the mechanism how the GalNAc4S-6ST gene is regulated in the tumor.
In the present study, we have clearly shown that GalNAc4S-6ST is transcribed in normal colorectal mucosa by using quantitative RT-PCR (see Fig. 2). Because our preliminary study using ISH indicated that GalNAc4S-6ST was transcribed in absorptive cells and goblet cells located in the lower portion of normal colorectal mucosa (unpublished results), it will be of great significance to determine the physiological role(s) of GalNAc4S-6ST in these cells.
For ISH, an RNA probe covering the entire coding region of a cDNA can be utilized after limited alkaline degradation. However, it is well known that some glycogenes show a structural relationship in having different functions as found in the case of GalNAc4S-6ST and heparin sulfate 3-O-sulfotransferase 1 (3OST-1) [20]. To avoid cross-hybridization with 3OST-1 or other related sulfotransferases, we designed the RNA probe to hybridize with a short segment (150 bp) of the cDNA, specific for GalNAc4S-6ST. Indeed, significant homology was not seen between the RNA probe for GalNAc4S-6ST used in this study and other related genes. We showed here that a significant association is present between the results of real-time RT-PCR and those of ISH, indicating that the positive signal detected by ISH should represent GalNAc4S-6ST mRNA itself.
Our results of ISH were negative in 5 patients even though the expression level of GalNAc4S-6ST mRNA was higher than the lowest expression level of this transcript found in the patients positive for ISH (see Fig. 4). In the present study, the length of the nucleotide sequence of GalNAc4S-6ST detected by ISH was 150 base pairs, while that detected by the real-time RT-PCR assay was 72 base pairs. Thus, it might be possible that limited degradation of mRNA occurred in the tissue blocks during preservation would work against ISH rather than real-time RT-PCR, because ISH required longer nucleotide sequence than real-time RT-PCR.
In the present study, we found that GalNAc4S-6ST mRNA was expressed in colorectal cancer cells. Recently, Kawashima et al. reported that CS-E units could bind to P-selectin with high affinity [11]. In addition, CS-E units inhibited P-selectin binding to a human breast cancer cell line [17]. Those reports indicate that CS-E may serve as a P-selectin ligand. Thus it is possible that CS-E on the tumor cells facilitate the metastasis by binding to P-selectin expressed in the endothelial cells and/or platelets. In addition, it is also likely that the negative charge of CS-E may attenuate the adhesion of tumor cells, thus allowing them to migrate from the primary site, similar to polysialic acid expression in non-small cell lung cancer [24] and astrocytoma [23]. To determine if any relationship is present between the expression of GalANc4S-6ST and progression of colorectal cancer, we compared the expression level of GalNAc4S-6ST in the cancer tissue with clinicopathological variables, since GalNAc4S-6ST is a key enzyme required to form CS-E. However, GalNAc4S-6ST expression was only slightly associated with vessel invasion. Previously we showed that sialyl Lea [Siaα2,3Galβ1,3(Fucα1,4)GlcNAcβ1→R] and sialyl Lex [Siaα2,3Galβ1,4(Fucα1,3)GlcNAcβ1→R] on core2-branched O-glycans displayed on the surface of colorectal cancer cells, an established ligand for E- and P-selectins, was significantly correlated with vessel invasion [22]. These results combined together indicate that CS-E plays a role in tumor progression of colorectal cancer, but its effects are more minor than those of sialyl Lea and sialyl Lex expressed on core2-branched O-glycans. Further study is needed to establish the effects of CS-E on the tumor progression with a larger number of colorectal cancer patients.
V. Acknowledgments
We wish to thank Drs Minoru Fukuda and Hiroyoshi Ota for encouragement and helpful suggestions during this study, and Dr Elise Lamar for critical reading of the manuscript. This work was supported by a Grant-in-Aid for Scientific Research B-18390113 from the Japan Society for the Promotion of Science (to J. N.). Y. I. is a recipient of a Research Fellowship of the Japan Society for the Promotion of Science.
VI. References
- 1.Akimoto Y., Yamamoto K., Munetomo E., Wells L., Vosseller K., Hart G. W., Hayato K., Hirano H. Elevated post-translational modification of proteins by O-linked N-acetylglucosamine in various tissues of diabetic Goto-Kakizaki rats accompanied by diabetic complications. Acta Histochem. Cytochem. 2005;38:131–142. [Google Scholar]
- 2.Clement A. M., Sugahara K., Faissner A. Chondroitin sulfate E promotes neurite outgrowth of rat embryonic day 18 hippocampal neurons. Neurosci. Lett. 1999;269:125–128. doi: 10.1016/s0304-3940(99)00432-2. [DOI] [PubMed] [Google Scholar]
- 3.Comelli E. M., Head S. R., Gilmartin T., Whisenant T., Haslam S. M., North S. J., Wong N. K., Kudo T., Narimatsu H., Esko J. D., Drickamer K., Dell A., Paulson J. C. A focused microarray approach to functional glycomics: transcriptional regulation of the glycome. Glycobiology. 2006;16:117–131. doi: 10.1093/glycob/cwj048. [DOI] [PubMed] [Google Scholar]
- 4.Deepa S. S., Umehara Y., Higashiyama S., Itoh N., Sugahara K. Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J. Biol. Chem. 2002;277:43707–43716. doi: 10.1074/jbc.M207105200. [DOI] [PubMed] [Google Scholar]
- 5.Eliakim R., Gilead L., Ligumsky M., Okon E., Rachmilewitz D., Razin E. Histamine and chondroitin sulfate E proteoglycan released by cultured human colonic mucosa: indication for possible presence of E mast cells. Proc. Natl. Acad. Sci. U S A. 1986;83:461–464. doi: 10.1073/pnas.83.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56:2237–2244. [PubMed] [Google Scholar]
- 7.Hakomori S., Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J. Natl. Cancer Inst. 1983;71:231–251. [PubMed] [Google Scholar]
- 8.Honke K., Taniguchi N. Sulfotransferases and sulfated oligosaccharides. Med. Res. Rev. 2002;22:637–654. doi: 10.1002/med.10020. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y., Habuchi O. Purification and characterization of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase from the squid cartilage. J. Biol. Chem. 2000;275:34728–34736. doi: 10.1074/jbc.M909633199. [DOI] [PubMed] [Google Scholar]
- 10.Kawai Y., Seno N., Anno K. Chondroitin polysulfate of squid cartilage. J. Biochem. 1966;60:317–321. doi: 10.1093/oxfordjournals.jbchem.a128438. [DOI] [PubMed] [Google Scholar]
- 11.Kawashima H., Atarashi K., Hirose M., Hirose J., Yamada S., Sugahara K., Miyasaka M. Oversulfated chondroitin/dermatan sulfates containing GlcAβ1/Idoα1-3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J. Biol. Chem. 2002;277:12921–12930. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- 12.Kitazawa R., Kitazawa S. In situ hybridization on calcified tissue: detection of RANKL mRNA in mouse osteolytic bone lesions. Acta Histochem. Cytochem. 2005;38:143–149. [Google Scholar]
- 13.Koshiishi I., Takenouchi M., Imanari T. Structural characteristics of oversulfated chondroitin/dermatan sulfates in the fibrous lesions of the liver with cirrhosis. Arch. Biochem. Biophys. 1999;370:151–155. doi: 10.1006/abbi.1999.1396. [DOI] [PubMed] [Google Scholar]
- 14.Kusche-Gullberg M., Kjellen L. Sulfotransferases in glycosaminoglycan biosynthesis. Curr. Opin. Struct. Biol. 2003;13:605–611. doi: 10.1016/j.sbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 15.McEver R. P., Beckstead J. H., Moore K. L., Marshall-Carlson L., Bainton D. F. GMP-140, a platelet α-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J. Clin. Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGee M. P., Teuschler H., Parthasarathy N., Wagner W. D. Specific regulation of procoagulant activity on monocytes. Intrinsic pathway inhibition by chondroitin 4,6-disulfate. J. Biol. Chem. 1995;270:26109–26115. doi: 10.1074/jbc.270.44.26109. [DOI] [PubMed] [Google Scholar]
- 17.Monzavi-Karbassi B., Stanley J. S., Hennings L., Jousheghany F., Artaud C., Shaaf S., Kieber-Emmons T. Chondroitin sulfate glycosaminoglycans as major P-selectin ligands on metastatic breast cancer cell lines. Int. J. Cancer. 2007;120:1179–1191. doi: 10.1002/ijc.22424. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama J., Suzuki M., Suzuki M., Fukuda M. Expression profiling of glycosyltransferases and related enzymes using in situ hybridization. Methods Enzymol. 2006;416:120–129. doi: 10.1016/S0076-6879(06)16008-5. [DOI] [PubMed] [Google Scholar]
- 19.Narimatsu H. Human glycogene cloning: focus on β3-glycosyltransferase and β4-glycosyltransferase families. Curr. Opin. Struct. Biol. 2006;16:567–575. doi: 10.1016/j.sbi.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Ohtake S., Ito Y., Fukuta M., Habuchi O. Human N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase cDNA is related to human B cell recombination activating gene-associated gene. J. Biol. Chem. 2001;276:43894–43900. doi: 10.1074/jbc.M104922200. [DOI] [PubMed] [Google Scholar]
- 21.Rosen S. D. Endothelial ligands for L-selectin: from lymphocyte recirculation to allograft rejection. Am. J. Pathol. 1999;155:1013–1020. doi: 10.1016/S0002-9440(10)65201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimodaira K., Nakayama J., Nakamura N., Hasebe O., Katsuyama T., Fukuda M. Carcinoma-associated expression of core 2 β-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 1997;57:5201–5206. [PubMed] [Google Scholar]
- 23.Suzuki M., Suzuki M., Nakayama J., Suzuki A., Angata K., Chen S., Sakai K., Hagihara K., Yamaguchi Y., Fukuda M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology. 2005;15:887–894. doi: 10.1093/glycob/cwi071. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka F., Otake Y., Nakagawa T., Kawano Y., Miyahara R., Li M., Yanagihara K., Inui K., Oyanagi H., Yamada T., Nakayama J., Fujimoto I., Ikenaka K., Wada H. Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer Res. 2000;60:3072–3080. [PubMed] [Google Scholar]
- 25.Wegrowski Y., Maquart F. X. Involvement of stromal proteoglycans in tumour progression. Crit. Rev. Oncol. Hematol. 2004;49:259–268. doi: 10.1016/j.critrevonc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Zou P., Zou K., Muramatsu H., Ichihara-Tanaka K., Habuchi O., Ohtake S., Ikematsu S., Sakuma S., Muramatsu T. Glycosaminoglycan structures required for strong binding to midkine, a heparin-binding growth factor. Glycobiology. 2003;13:35–42. doi: 10.1093/glycob/cwg001. [DOI] [PubMed] [Google Scholar]