Abstract
Aims
St John's Wort (SJW) is widely used in the treatment of depression but concerns have been raised about its potential to interact with other drugs. Co-administration with SJW has resulted in significant reductions in trough plasma concentrations of indinavir and cyclosporin [1, 2]. Induction of cytochrome P450 3A4 (CYP3A4) has been implicated as the most likely interaction mechanism. However, the magnitude of the interaction seen in clinical practice is greater than that predicted by in vitro studies suggesting additional interaction mechanisms may exist. As indinavir and cyclosporin are substrates for both CYP3A4 and the multi drug transporter P-glycoprotein we hypothesized that modulation of P-glycoprotein expression and function by SJW may contribute to the development of potentially harmful drug–drug interactions.
Methods
Healthy volunteers were randomized to either SJW (0.15%) 600 mg three times daily for 16 days (n = 15) or placebo (n = 7). Blood samples were obtained for P-glycoprotein expression and function at baseline, 16 and 32 days post treatment. Peripheral blood lymphocytes (PBMCs) were isolated by Ficoll density gradient centrifugation, fixed and permeabilized. Cells were stained with a P-glycoprotein specific antibody, quantified by flow cytometry and median fluorescence intensity (MFI) values obtained. Vimentin and IE (nonsense antibody) were used as controls. The presence of the MDR 1 gene product was confirmed by RT-PCR. P-glycoprotein mediated drug efflux was determined as a function of rhodamine efflux in the absence and presence of ritonavir. Data are expressed as mean±s.d. and were subjected to nonparametric analysis.
Results
P-glycoprotein expression increased 4.2 fold from baseline in subjects treated with SJW (7.0 ± 1.9 vs 29.5 ± 14.3 (MFI); P < 0.05). There was no effect with placebo (5.1 ± 1.3 vs 6.0 ± 1.9 MFI). SJW increased P-glycoprotein mediated rhodamine efflux (reduced ratio) compared with baseline (0.12 ± 0.04 vs 0.24 ± 0.18 P < 0.05). There was no change with placebo. Ritonavir (5 µm) inhibited P-glycoprotein mediated efflux in both groups producing greater intracellular accumulation of rhodamine. However, this effect was attenuated following treatment with SJW (23.9 ± 15.3% vs 75.4 ± 16.4% P < 0.05).
Conclusions
SJW increased expression and enhanced the drug efflux function of the multi drug transporter P-glycoprotein in PBMCs of healthy volunteers. This may represent a second mechanism for the drug–herb interactions seen in clinical practice and account for the discrepancies between in vitro and in vivo data. Since P-glycoprotein and CYP3A4 have distinct though overlapping substrates, patients receiving drugs, which are P-glycoprotein substrates should be warned against self-medication with SJW as clinically significant drug interactions may occur.
Keywords: flow cytometry, P-glycoprotein, ritonavir, St John's Wort
Introduction
St John's Wort (Hypericum perforatum) has been used for centuries as a herbal remedy [3]. Galen (A.D. 150–200) was known to have prescribed it for menstrual disorders and it was also used in the Middle Ages to treat depression, acquiring its popular name due to the fact that flowering occurs on the birthday of St John the Baptist (June 24th). St John's Wort (SJW) has recently gained popularity as ‘nature’s Prozac' with at least five randomized controlled clinical trials providing evidence that it is as effective as conventional antidepressant therapy [4–6]. In Germany, it is currently the most commonly prescribed antidepressant with more than 2.7 million prescriptions written each year [7]. Despite the widespread use of SJW little is known about its pharmacokinetic properties and since it is usually sold as an over the counter herbal remedy it has not been subjected to the rigorous clinical testing required of other compounds [8]. In addition, the public perception that whatever is ‘Natural’ is safe has resulted in the interaction potential of SJW being largely ignored until recently [9]. Given that 18.4% of U.S. adults surveyed reported concurrent use of at least one herbal product or high dose vitamin with regular prescription medications, but over 60% did not disclose such use to their physicians, the potential for SJW to interact with coadministered medications should be viewed with concern [10]. Several recent reports suggest that SJW may produce clinically relevant interactions by promoting the metabolism of coadministered drugs such as warfarin and the oral contraceptive pill [8]. Chronic coadministration of SJW reduced plasma cyclosporin concentrations by approximately 80%, resulting in the development of acute transplanted organ rejection [2]. Similarly, administration with the HIV 1 protease inhibitor indinavir produced an 81% reduction in indinavir trough plasma concentrations [1]. In contrast, coadministration with digoxin produced a 30% reduction in plasma digoxin concentrations [11, 12]. Induction of the hepatic CYP 3A4 by SJW has been implicated as the most likely mechanism for these interactions and in vitro studies demonstrate that SJW approximately doubles CYP3A4 expression [13, 14, 15]. In addition, hyperforin (the postulated active ingredient of SJW) is a potent ligand for the pregnane X receptor, an orphan nuclear receptor which regulates expression of CYP3A4 [16, 17]. Interestingly, the magnitude of the interaction seen in clinical reports (80%) is greater than that predicted by in vitro data (50%) suggesting a second interaction mechanism may exist, and since digoxin undergoes primarily renal clearance with metabolism by CYP3A4 providing a minor metabolic pathway, induction of CYP3A4 is unlikely to explain this interaction completely. Cyclosporin, indinavir and digoxin in addition to being substrates for CYP3A4 are also recognized as substrates for the multi drug transporter P-glycoprotein which functions as a transmembrane drug efflux pump [18–21]. We postulate that SJW may alter expression and/or function of P-glycoprotein reducing tissue or cellular concentrations of certain drugs thereby contributing to its potential for drug–drug interactions. Since lymphocytes, express functional P-glycoprotein but not functional CYP3A4 we have chosen the peripheral blood lymphocytes (PBMCs) of healthy volunteers as the model for this study.
Methods
Study design
Twenty-two healthy volunteers (13 female) participated in a single blind randomized placebo controlled trial. Following informed consent, 15 volunteers were randomized to SJW (Good n’ Natural 0.15% standardized extract 600 mg three times daily) for 16 days and seven received placebo for 16 days. Smoking and concomitant medication use (including the oral contraceptive pill) were prohibited throughout the study. All subjects were monitored for the development of adverse effects commonly associated with SJW such as dry mouth, gastro-intestinal upset and constipation.
Venous blood samples were drawn for P-glycoprotein expression and function at baseline, 16 days and 32 days (16 days post discontinuation of treatment). In addition, each batch of SJW used in the study was assayed for hypericin content by a standard h.p.l.c. method described later.
Lymphocyte isolation
Peripheral blood mononuclear cells (PBMCs), were isolated from venous blood (within 2 h) by Ficoll density gradient centrifugation. In brief whole blood was layered onto Lymphoprep (Gibco USA) and centrifuged at 1200 rev min−1, 4° C for 25 min. The buffy coat was removed, resuspended and washed twice (2000 rev min−1 × 5 min) with Hanks balanced salt solution without calcium and magnesium (Life Technology, Paisley U.K.). Cells were finally resuspended in culture medium (RPMI plus glutamine; Life Technology, Paisley U.K.), supplemented with 10% fetal calf serum (FCS; Sigma, Poole, Dorset). Cell count and viability were quantified in a Neubauer's chamber following staining with ethidium bromide/acridine orange. Cells were aliquoted (1 × 106 cell ml−1) and reserved for P-glycoprotein expression and function and for RNA isolation.
P-glycoprotein expression
In accordance with the St Jude consensus guidelines [22] regarding the methodology and interpretation of P-glycoprotein expression and function, the presence of P-glycoprotein was detected by flow cytometry and confirmed by RT-PCR. Cells were fixed in 2% paraformaldehyde (Cellfix, Becton Dickinson, Mountain View C.A.) for 20 min at room temperature and permeabilized with 0.05% (w/v) saponin (Sigma). Cells were labelled with a JSB1 monoclonal antibody (Sanbio, Uden, Netherlands) directed towards a highly conserved intracellular epitope of P-glycoprotein (30 min, at 37° C). A negative fluorescence control was performed using mouse monoclonal IE immunoglobulin (Ig)G derived from a murine hybridoma supernatant (ATCC, Manasses V.A.) HB179, 30 min, at 37° C, whereas antibody to the cytoskeletal component vimentin (Dako AS, Glostrup, Denmark) 30 min, at 37° C was used as a control for the permeabilization technique. After removal of excess antibody by washing with 0.05% (w/v) saponin, cells were incubated with 100 µl rabbit antimouse fluorescein labelled isothiocyanate (FITC)-conjugated F(ab)2 (1:50 dilution; Dako AS, Glostrup) for 30 min, at 37° C. Finally cells were washed free of excess antibody and re-suspended in 0.5 ml of paraformaldehyde before being subjected to flow cytometric analysis, and median fluorescence intensity values (MFI; a measure of expression) obtained. The presence of MDR1 mRNA in each sample was confirmed by RT PCR as previously described [23].
Functional studies of rhodamine 123 transport: efflux and inhibition
Methods: PBMCs (1 × 106) cells were loaded with rhodamine 123 (1.25 µg ml−1; Sigma) for 25 min at 37° C in RPMI 1640 supplemented with 10% FCS. Cells were washed twice in ice-cold media and incubated at 37° C for 3 h in 3 ml of dye free media to allow dye efflux. At the time points baseline, t = 0 (maximum loading) and three hours later t = 180 min (maximum efflux) an aliquot was removed and washed twice in ice-cold media (3 min × 2000 rev min−1) before being fixed in ice-cold paraformaldehyde (Cellfix). Cellular fluorescence was determined by flow cytometric analysis and expressed as median fluorescence intensity.
Flow cytometry: Lymphocytes shown in forward scatter and side scatter were electronically gated and acquired (10000 events) through the FL1 channel (expression) or the FL2 channel (function). The amount of fluorescence was plotted as a histogram of FL1 or FL2 staining within the gate. Data acquisition was performed using Cellquest software (WINMDI version 2.6) to determine median fluorescence intensity values.
Parallel experiments were performed in the presence of ritonavir (5 µm), a known P-glycoprotein inhibitor. This concentration was chosen from a previously derived dose response curve for the inhibition of P-glycoprotein mediated rhodamine efflux by ritonavir in the PBMCs of healthy volunteers (unpublished data). Ritonavir was a gift from Abbott laboratories.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was isolated from PBMCs by a modification of the method of Chomczynski & Sacchi [24] using Tri Reagent (Sigma). 1st Strand cDNA was synthesized from 2 µg total RNA using random decamers and M-MLV reverse transcriptase (Reverse-iT kit, Abgene). The resultant cDNA was amplified by PCR using gene specific primers (Table 1) as described by Egashira et al. [23], and a standard PCR protocol. All PCR reactions were performed in duplicate. The PCR products were electrophoresed on a 2.0% (w/v) agarose gel and stained with ethidium bromide. Gels were visualized under ultraviolet illumination, photographed and analysed on a PC using Gene Tools analysis software (Syngene).
Table 1.
Sequences of upstream and downstream oligonucleotide primers.
| mRNA | Forward | Reverse | Tm | Number of cycles |
|---|---|---|---|---|
| β2M | ACCCCCACTGAAAAAGATGA | ATCTTCAAACCTCCATGATG | 55° C | 32 |
| MDR-1 | CCCATCATTGCAATAGCAGG | GTTCAAACTTCTGCTCCTGA | 55° C | 34 |
Hypericin assay
The h.p.l.c. method was based upon that of Chi & Franklin [25, 26]. In brief, hypericin (Fluka Chemie AG, Buchs Germany) was assayed by using a C-8 column (15 × 4.6 mm, 5 µm; column temperature: 60° C) with a Shimadzu LC-10AS pump (flow rate: 1.5 ml min−1) and a Shimadzu RF-10AXL fluorescence detector (Ex 390 nm; Em 620 nm). A standard curve was constructed using serial dilutions of a stock solution of hypericin (1 mg ml−1) prepared in DMSO and diluted accordingly with the h.p.l.c mobile phase (0.03 m phosphate buffer (pH 7) and methanol (30:70, v/v)). A 10 mg aliquot of each St John's Wort capsule (0.15% extract) was dissolved in DMSO (1 ml) and centrifuged at 2000 rev min−1 for 5 min. The supernatant was removed and diluted (1:100) with mobile phase prior to injection.
The intra-day coefficients of variation (n = 4) for 10 µg ml−1 and 50 µg ml−1 were 3.1% and 2.2%, respectively. The inter-day coefficients of variation (n = 8) at these concentrations were 4.3% and 3.5%, respectively.
Data analysis
P-glycoprotein expression was calculated as the difference between the median fluorescence intensities following labelling with JSB1 and IE for each sample.
P-glycoprotein function was expressed as the ratio t180/t0 (maximum efflux/maximum loading), therefore a reduced ratio indicates enhanced drug efflux function. The percentage of reversible efflux in the presence of ritonavir (an inhibitor) was calculated as:
Statistical analysis
Data were subjected to nonparametric analysis for paired (Wilcoxon), unpaired (Mann Whitney U) t-tests or a one-way analysis of variance followed by multiple comparison (Dunn's test) where appropriate. Data are expressed as mean±s.d. P < 0.05 indicates statistical significance.
Results
Duplicate analyses were performed on two capsules from each of the three batches of SJW. They were found to contain 0.15, 0.14 and 0.15% (w/w) hypericin, which is in agreement with the content quoted by the manufacturers.
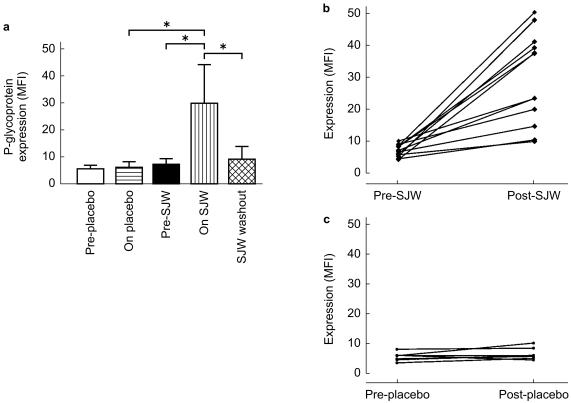
Fifteen subjects were initially randomized to receive SJW and of these 12 completed the study. One subject discontinued SJW due to adverse effects (nausea, dry mouth), and two subjects were withdrawn because of the need for a potentially interacting medication. All seven healthy volunteers randomized to receive placebo completed the study. We have previously established that in resting peripheral blood lymphocytes of healthy individuals and under identical experimental conditions (i.e. nonsmoking and non medicated) that P-glycoprotein expression in vivo is stable over 2 months (baseline vs 2 months: 6.02 ± 1.5 vs 6.4 ± 2.7 MFI; 95% CI: −2.57, 3.3) and normally distributed (KS value=0.07; n = 77; mean±s.d.; 7.82 ± 3.2, unpublished data). In the present study, there was a mean 4.2 fold increase in P-glycoprotein expression in subjects treated with SJW for 16 days compared with baseline values (29.5 ± 14.3 vs 7.0 ± 1.9 MFI; P < 0.05; Figure 1a; 95% CI: 13.5, 31.6). In contrast there was no change in P-glycoprotein expression in the PBMCs of those treated with placebo (5.1 ± 1.3 vs 6.0 ± 1.9 MFI; Figure 1a, c; 95% CI: −0.6, 2.4). There was large individual variability with respect to increased P-glycoprotein expression following SJW (Figure 1b; range: 1.09–9.06 MFI). P-glycoprotein expression had returned to baseline 16 days post discontinuation of SJW (8.5 ± 4.8 MFI; Figure 1a).
Figure 1.
P-glycoprotein expression in peripheral blood lymphocytes of (a) subjects pre- and post- 16 days treatment with either St John's Wort (600 mg 0.15%; n = 12) or placebo (n = 7) and in individual subjects before and after 16 days treatment with either (b) St John's Wort or (c) placebo. Data are expressed as net MFI (median fluorescence intensity) following labelling with JSB1 and IE for each sample. Data are shown as mean±s.d. *P < 0.05.
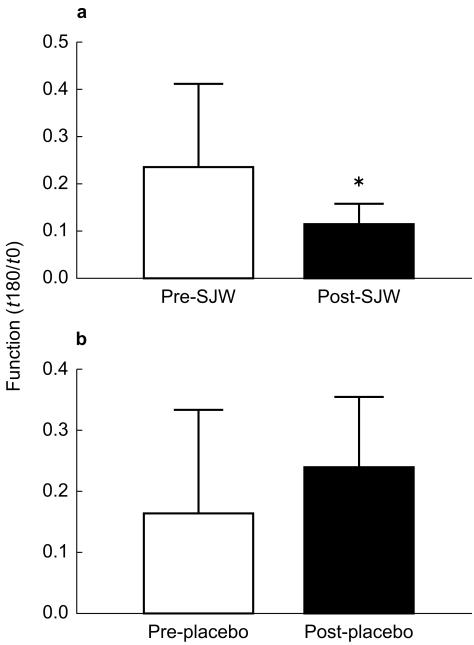
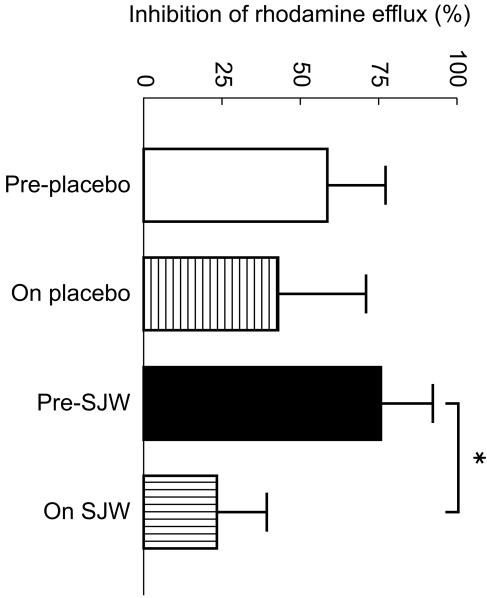
Treatment with SJW increased P-glycoprotein mediated rhodamine efflux (reduced ratio) compared with baseline levels (0.12 ± 0.04 vs 0.24 ± 0.18; P < 0.05; 95% CI: 0.01, 0.29; Figure 2a). There was no change in P-glycoprotein function with placebo (0.16 ± 0.17 vs 0.24 ± 0.11; 95% CI: −0.05, 0.21; Figure 2b). Ritonavir (5 µm) produced significantly greater inhibition of P-glycoprotein mediated rhodamine efflux in the PBMCs of subjects before treatment with SJW compared with post treatment values (75.4 ± 16.4 vs 23.9 ± 15.3%; P < 0.01; 95% CI: 43.7, 70.1; Figure 3). The percentage inhibition by ritonavir was similar before and after treatment with placebo.
Figure 2.
P-glycoprotein function expressed as the ratio of rhodamine efflux at time 0 and 180 min (t180/t0) in peripheral blood lymphocytes of subjects pre- and post- 16 days treatment with either (a) St John's Wort or (b) placebo. Data are expressed as mean±s.d. *P < 0.05 compared with pre- St John's Wort.
Figure 3.
Effect of ritonavir (5 µm) on rhodamine efflux from peripheral blood lymphocytes of subjects pre- and post- 16 days treatment with either St John's Wort or placebo. Data are expressed as mean±s.d. *P < 0.05 compared with pre- St John's Wort.
The presence of MDR1 mRNA was confirmed by non quantitative RT-PCR. Amplification products of the expected size [23] were detected at 167 base pairs for MDR1 and 120 base pairs for β2 microglobulin in PBMCs in every sample collected.
Discussion
P-glycoprotein is an energy dependant membrane-associated multidrug efflux pump encoded by the MDR-1 gene on the long arm of chromosome 7. Increasing multidrug resistance is associated with altered expression of P-glycoprotein, which then actively transports drug substrates out of the cell lowering their intracellular drug concentration and facilitating the development of drug resistance [21, 27–29]. P-glycoprotein is differentially expressed in a variety of normal tissues including gut epithelium and peripheral blood lymphocytes [30]. Many drugs including methotrexate, protease inhibitors, and steroids in addition to some cytotoxic agents are known to be substrates for this drug efflux mechanism. Therefore, their disposition and metabolism will be affected by its expression and activity [18, 28, 31–33]. A number of noncytotoxic compounds are capable of reversing the drug efflux effect including verapamil, cyclosporin and more recently the anti HIV drug ritonavir [29, 34, 35]. Inhibition of the efflux pump may facilitate accumulation of drugs within previously resistant cells, improving drug bioavailability, intracellular concentrations and penetration into sanctuary sites such as the central nervous system [32, 36].
Recent reports have documented clinically relevant drug interactions between SJW and coadministered drugs such as indinavir, cyclosporin and digoxin [1, 2, 12], attributing induction of hepatic CYP3A4 as the likely mechanism [14, 15]. However, interactions with digoxin are unlikely to be fully explained by this mechanism, as it is not a CYP3A4 substrate. Furthermore, the discrepancy between in vitro and clinical data suggests that a second interaction mechanism may be involved. It has been postulated that drug interactions with SJW may be mediated through P-glycoprotein [1, 37]. The present study assesses the effects of chronic administration of SJW on P-glycoprotein expression and function in human PBMCs. We found that chronic treatment with SJW produced a greater than 4 fold increase in expression of the multidrug transporter P-glycoprotein in the PBMCs of healthy volunteers. This was associated with enhanced drug efflux function resulting in reduced intracellular accumulation of rhodamine. Furthermore, in the presence of increased P-glycoprotein expression the inhibitory effects of ritonavir (a potent P-glycoprotein inhibitor) [38] were attenuated. Our data supports those of Durr et al. which showed a 1.4 fold increase in intestinal P-glycoprotein/MDR1 expression following chronic oral administration of SJW [37]. Furthermore, Greiner et al. [39] demonsrated a reduction in plasma digoxin concentrations following a similar inductive response to oral treatment with rifampicin suggesting P-glycoprotein induction as an alternative mechanism for drug–drug interactions. Our study provides further evidence of a second mechanism by which SJW may interact with coadministered drugs. Since P-glycoprotein and CYP3A4 and have distinct though overlapping substrates the magnitude of interactions encountered clinically may depend on whether a drug is transported mainly by P-glycoprotein (digoxin, colchicine), or metabolized by CYP3A4 (cimetidine, oral contraceptive pill), or both (indinavir, ritonavir, cyclosporin).
Our data and that of others [14, 40] would suggest that CYP3A4 and P-glycoprotein expression may be coinduced by SJW. Studies indicate that St John's Wort induces hepatic drug metabolism through activation of the pregnane X receptor [16], and have identified PXR response elements in the upstream regulatory regions of these genes [17, 41–44]. In addition Geick et al. have recently identified a distinct DR4 nuclear receptor response element that is essential for MDR1 induction by rifampicin [45]. Whether the inductive response to St John's Wort seen in PBMCs is via an interaction with the PXR receptor was not addressed in the present study.
In recent years the therapeutic potential of P-glycoprotein modulation has been re-examined and at least one specific inhibitor (valspodar) is presently undergoing clinical trials for use in oncology [11, 46]. Similarly low dose ritonavir has been added to some HIV antiretroviral salvage therapies in an effort to enhance efficacy [28, 31, 47, 48]. Our finding that chronic administration of SJW reduces the potential of ritonavir to inhibit P-glycoprotein mediated drug efflux suggests that the clinical use of P-glycoprotein modulators such as ritonavir or valspodar (PSC833) may be limited in the presence of SJW. In conclusion, when prescribing drugs, that are substrates of P-glycoprotein, CYP3A4 or both, patients should be informed about the risk of the chronic coadministration of SJW, which may give rise to clinically significant drug–drug interactions.
References
- 1.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John's Wort. Lancet. 2000;355:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 2.Ruschitzka F, Meier PJ, Turina M, Luscher TF, Noll G. Acute heart transplant rejection due to Saint John's wort. Lancet. 2000;355:548–549. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 3.Pepping J. St John's Wort: Hypericum perforatum. Am J Health-Syst Pharm. 1999;56:329–330. doi: 10.1093/ajhp/56.4.329. [DOI] [PubMed] [Google Scholar]
- 4.Gaster B, Holroyd J. St John's Wort for depression: a systematic review. Arch Intern Med. 2001;160:152–156. doi: 10.1001/archinte.160.2.152. [DOI] [PubMed] [Google Scholar]
- 5.Kim HL, Streltzer J, Goebert D. St John's Wort for depression: a meta-analysis of well defined clinical trials. J Nerv Ment Dis. 1999;187:532–538. doi: 10.1097/00005053-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Schrader E. Equivalence of St John's wort extract (Ze 117) and fluoxetine: a randomised, controlled study in mild–moderate depression. Int Clin Psychopharmacol. 2000;15:61–68. doi: 10.1097/00004850-200015020-00001. [DOI] [PubMed] [Google Scholar]
- 7.Linde K, Ramirez G, Mulrow CD. St John's Wort for depression – an overview and meta-analysis of randomised clinical trials. Br Med J. 1996;313:253–258. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst E. Second thoughts about the safety of St John's Wort. Lancet. 1999;354:2014–2016. doi: 10.1016/S0140-6736(99)00418-3. [DOI] [PubMed] [Google Scholar]
- 9.Fugh-Berman A. Herb–drug interactions. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg DM, Davis RB, Ettner SL. Trends in alternative medicine use in the United States 1990–1997. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 11.Kovariik JM, Rigaudy L, Guerret M, Gerbeau C, Ludwig-Rost K. Longitudinal assessment of a P-glycoprotein–mediated drug interaction of valspodar on digoxin. Clin Pharmacol Ther. 1999;66:391–398. doi: 10.1053/cp.1999.v66.a101462. [DOI] [PubMed] [Google Scholar]
- 12.Johne A, Brockmoller J, Steffen B, Maurer A, Langheinrich MD, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John's Wort (Hypericum perforatum) Clin Pharmacol Ther. 1999;66:338–345. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz JS, De Vane CL, Boulton DW, Carson SW, Nahas Z, Risch SC. Effect of St John's Wort (Hypericum perforatum) on cytochrome P-450 2D6 and 3A4 activity in healthy volunteers. Pharmacol Lett. 2000;66:133–139. doi: 10.1016/s0024-3205(99)00659-1. [DOI] [PubMed] [Google Scholar]
- 14.Roby CA, Anderson GD, Kantor E, Dryer DA, Burstein AH. St John's Wort: effect on CYP3A4 activity. Clin Pharmacol Ther. 2000;67:451–457. doi: 10.1067/mcp.2000.106793. [DOI] [PubMed] [Google Scholar]
- 15.Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St John's Wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther. 2000;294:88–95. [PubMed] [Google Scholar]
- 16.Moore LB, Goodwin B, Jones SA, et al. St. John's Wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertilsson G, Heidrich H, Svensson K, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A4 induction. Proc Natl Acad Sci. 2001;95:12208–12214. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clayette P, Jorjuria S, Dormont D. Significance of P-glycoprotein for the pharmacology and clinical use of HIV protease inhibitors. AIDS. 2000;14:235–236. doi: 10.1097/00002030-200002180-00004. [DOI] [PubMed] [Google Scholar]
- 19.Lee CGL, Gottesman MM, Cardarelli CO, et al. HIV-1 protease inhibitors are substrates for the MDR-1 multidrug transporter. Biochemistry. 1998;37:3594–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 20.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the MDR1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambudkar SV. Biochemical, cellular and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 22.Beck WT, Grogan TM. Methods to detect P-glycoprotein and implications for other drug resistance-associated proteins. Leukemia. 1997;11:1107–1109. doi: 10.1038/sj.leu.2400675. [DOI] [PubMed] [Google Scholar]
- 23.Egashira M, Kawamata N, Sugimoto K, Kaneko T, Oshimi K. P-glycoprotein expression on normal and abnormally expanded natural killer cells and inhibition of P-glycoprotein function by cyclosporin A and its analogue, PSC833. Blood. 1999;93:599–606. [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Ann Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Chi JD, Franklin M. Determination of Hypericin in plasma by high-performance liquid chromatography. J Chromatogr B. 1999;724:195–198. doi: 10.1016/s0378-4347(98)00572-6. [DOI] [PubMed] [Google Scholar]
- 26.Micali G, Lanuzza F, Curro P. High-performance liquid chromatographic determination of biologically active hypericin in vegetable extracts and alcoholic beverages. J Chromatogr A. 1996;731:336–339. doi: 10.1016/0021-9673(95)01222-2. [DOI] [PubMed] [Google Scholar]
- 27.Debouck C. The HIV 1 protease as a therapeutic target for AIDS. AIDS Res Hum Retroviruses. 1992;8:153–164. doi: 10.1089/aid.1992.8.153. [DOI] [PubMed] [Google Scholar]
- 28.Merry C, Barry M, Mulcahy F. Saquinavir pharmacokinetics alone and in combination with ritonavir in patients with HIV disease. AIDS. 1996;10:13. doi: 10.1097/00002030-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Nascimbeni M, Lamotte C, Peytavin G, Farinotti R, Clavel F. Kinetics of antiviral activity and intracellular pharmacokinetics of human immunodeficiency virus type 1 protease inhibitors in tissue culture. Antimicrob Agents Chemother. 2000;43:2629–2634. doi: 10.1128/aac.43.11.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illmer T, Schaich M, Oelschlagel U, et al. A new PCR MIMIC strategy to quantify low MDR1 mRNA levels in drug resistant cell lines and AML blast samples. Leuk Res. 1999;23:653–663. doi: 10.1016/s0145-2126(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 31.Hsu A, Granneman GR, Bertz RJ. Ritonavir: Clinical pharmacokinetics and interactions with other HIV agents. Clin Pharmacokinet. 2000;35:275–291. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 32.Huisman T, Smit JW, Schinkel AH. Significance of P-glycoprotein for the pharmacology and clinical use of HIV protease inhibitors. AIDS. 2000;14:237–242. doi: 10.1097/00002030-200002180-00005. 10.1097/00002030-200002180-00005. [DOI] [PubMed] [Google Scholar]
- 33.Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 34.Durant J, Clevenbergh P, Halfon P, et al. Drug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trial. Lancet. 1999;353:2195–2199. doi: 10.1016/s0140-6736(98)12291-2. [DOI] [PubMed] [Google Scholar]
- 35.Gulick RM, Mellors JW, Havlir D, et al. 3-year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann Intern Med. 2000;133:35–39. doi: 10.7326/0003-4819-133-1-200007040-00007. [DOI] [PubMed] [Google Scholar]
- 36.Eagling VA, Profit L, Back DJ. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-1 protease inhibitor saquinavir by grapefruit juice components. Br J Clin Pharmacol. 1999;48:543–552. doi: 10.1046/j.1365-2125.1999.00052.x. 10.1046/j.1365-2125.1999.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durr D, Stieger B, Kullak-Ublick GA, et al. St. John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 38.Drewe J, Gutmann H, Fricker G, Torok M, Beglinger C, Huwyler J. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporin analog SDZ PSC 833. Biochem Pharmacol. 1999;57:1147–1152. doi: 10.1016/s0006-2952(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 39.Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampicin. J Clin Invest. 1999;104:147–153. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seree E, Villard PH, Hever H, et al. Modulation of MDR1 and CYP3A expression by dexamehasone: evidence for an inverse regulation in the adrenals. Biochem Biophys Res Commun. 1998;252:392–395. doi: 10.1006/bbrc.1998.9662. [DOI] [PubMed] [Google Scholar]
- 41.Kliewer SA, Moore JT, Wade L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 42.Blumberg B, Sabbagh W, Jr, Juguilon H, et al. SXR. A novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. In the regulatory region of these genes the human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56:1329–1339. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- 45.Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampicin. J Biol Chem. 2001;276:14581–14587. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- 46.Mayer U, Wagenaar E, Dorobek B, Beijnen JH, Borst P, Schinkel AH. Full blockade of intestinal P-glycoprotein and extensive inhibition of blood–brain barrier P×glycoprotein by oral treatment of mice with PSC833. J Clin Invest. 1997;100:2430–2436. doi: 10.1172/JCI119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289–304. doi: 10.2165/00003088-199936040-00004. [DOI] [PubMed] [Google Scholar]
- 48.Carpenter CC, Fischel MA, Hammer SM, et al. Anti retroviral therapy for HIV infection in1998: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]