Abstract
Aims
To examine the effects of an oral contraceptive containing ethinyloestradiol and norgestrel on intestinal and hepatic CYP3A activity using midazolam as a probe substrate.
Methods
In a nonblinded sequential study, nine healthy women received simultaneous doses of intravenous midazolam (0.05 mg kg−1) and oral 15N3-midazolam (3 mg) on days 0, 4, 6, 8, and 14. On study day 5, Ovral™ (50 µg ethinyloestradiol/500 µg norgestrel) was administered for 10 days. Serum and urine samples were assayed for midazolam, 15N3-midazolam and metabolites by liquid chromatography-mass spectrometry. A Digit Symbol Substitution Test (DSST) was used to assess changes in the pharmacodynamic activity of midazolam.
Results
Moderate (% CV 26–46) interindividual variability in the pharmacokinetics of midazolam were observed. Compared with baseline, AUC(0,∞)iv ratios (95% CIs) after 2, 4, and 10 days treatment with OC were 89% (79, 101), 96% (85, 109), and 88% (77, 99), respectively. The AUC(0,∞)oral ratios (95% CIs) were 101% (82, 125), 105% (85, 130), and 114% (92, 141), respectively, after 2, 4, and 10 days OC treatment compared with baseline. Concomitant administration of the oral contraceptive, Ovral™ for 2, 4 or 10 days did not significantly alter the area under the curve, clearance, or half-life of midazolam after either oral or intravenous administration. No alterations in pharmacodynamic effects of midazolam were observed between treatment days. Mean DSST scores strongly correlated with mean total midazolam blood concentrations (r = −0.936).
Conclusions
Administration of Ovral™ for 10 days had no impact on intestinal or hepatic CYP3A activity as determined by midazolam metabolism.
Keywords: CYP3A, ethinyloestradiol, midazolam, norgestrel
Introduction
The cytochrome P450 3A (CYP3A) subfamily is comprised of CYP3A4, CYP3A5, CYP3A7, and CYP3A43. As the most abundant cytochrome P450 in the liver and small intestines, CYP3A4 participates in the metabolism of approximately 50% of drugs currently on the market [1]. CYP3A5 protein is detected in only 30% of human livers [2, 3] and is the principal CYP3A isoform expressed in renal tissue [4]. CYP3A7 is expressed almost exclusively in fetal liver and is thought to play an important role in the 16-α-hydroxylation of dehydroepiandrosterone-sulphate [5]. The newest member of the CYP3A family to be identified, CYP3A43 is expressed in the liver, kidney, pancreas and prostate [6]. At this point, the catalytic activity and relative abundance of CYP3A43 have not been defined. The CYP3A subfamily is known to metabolize a large number of structurally diverse compounds including nifedipine, diltiazem, lignocaine, lovastatin, erythromycin, troleandomycin, cyclosporin and steroids such as oral contraceptives (OC) [7].
Approximately 60–70 million women worldwide take an OC. The major oestrogenic component of OC is 17α-ethinyloestradiol (EE2). About 65% of EE2 undergoes first-pass metabolism in the gut wall to EE2-sulphate [8]. In humans, the dominant oxidative reaction of EE2 is aromatic hydroxylation at the 2-position with 29–64% of an oral dose being converted to 2-hydroxy-ethinyloestradiol [8]. The 2-hydroxylation of EE2 is mediated primarily by CYP3A [9]. This metabolite can undergo further oxidation with subsequent sulphation and glucuronidation prior to excretion [9]. Not only is EE2 hydroxylated at the 2-position by CYP3A, but it also can undergo oxidation at the 17α-acetylenic bond resulting in mechanism-based inhibition of CYP3A [10]. In addition, studies in human liver microsomes have shown that progestins containing the 17α-acetylenic moiety, which are commonly used with EE2 in OC formulations, are also mechanism-based inactivators of CYP3A in vitro [9]. Thus as a result of mechanism-based inhibition, women on OCs would appear to have an elevated risk for experiencing drug–drug interactions with CYP3A substrates. In fact, a number of case reports describe an increase in cyclosporin concentrations after the initiation of OC therapy suggesting an inhibition of cyclosporin metabolism [11, 12]. In addition, women are known to have higher CYP3A activity compared with men [13]. However as demonstrated by a recent study, women taking OCs had midazolam clearance values similar to those obtained in men [14]. The extent of CYP3A modulation by OCs is unclear and thus additional studies examining this issue are warranted.
The goal of the current study was to evaluate the effects of OC dosing on hepatic and intestinal CYP3A catalytic activity. Midazolam is selectively hydroxylated at the 1′ and 4-positions by CYP3A and was employed in the current study as an in vivo probe for CYP3A hepatic and intestinal activity [15].
Methods
This was a nonblinded, sequential study comprised of five periods evaluating the effect of an OC Ovral™ (50 µg EE2 and 500 µg norgestrel) on CYP3A activity. Ovral™ was chosen because of its high EE2 content (50 µg) and because EE2 and norgestrel (levonorgestrel is the active isomer of norgestrel) combination products are the most commonly prescribed OC [16]. The study was conducted at the Lilly Laboratory for Clinical Research in Indianapolis, Indiana. The protocol and informed consent documents were approved by Indiana University – Purdue University at Indianapolis Institutional Review Board. After giving written informed consent, participants were enrolled into the study. Volunteers were determined to be in good health on the basis of medical histories, physical examinations, vital signs, electrocardiograms and laboratory evaluations. Exclusion criteria included any clinically relevant abnormality identified at the physical examination or laboratory screening, the use of any medication within 14 days before the first drug administration, blood donation within 60 days before the start of the study and a documented history of drug allergy.
Starting on day 1, participants were served a restricted diet avoiding foods such as grapefruit juice, oranges, orange juice, limes, mandarin oranges, ethanol containing beverages, charbroiled meat and cruciferous vegetables, which may alter cytochrome P450 activity. During the inpatient study days, subjects were not allowed to drink caffeine containing beverages or smoke tobacco. On each dosing day, subjects fasted from midnight until approximately four hours after dosing. Participants were simultaneously administered 0.05 mg kg−1 intravenous midazolam (infused over 30 min) and 3 mg of 15N3-midazolam oral solution on days 0, 4, 6, 8, and 14. On day 5, Ovral™ therapy was initiated. Serial blood samples (7 ml) were collected before and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, and 10 h following midazolam and 15N3-midazolam administration. All blood samples were drawn into heparinized tubes. Plasma was separated by centrifugation, transferred to polypropylene tubes and stored at −70 ° C until analysis. Each participant emptied her bladder just before the start of the urine collection period. Urine was collected over the following intervals 0–10 and 10–24 h post dose and frozen at −70 ° C until assayed for 1′-hydroxymidazolam.
Plasma samples were processed with the use of a liquid-liquid extraction technique [14]. The internal standard, desmethyldiazepam, was added to each 0.5 ml plasma sample before extraction. The sample residue was reconstituted with 200 µl mobile phase (acetonitrile, methanol and 20 mm ammonium acetate, pH 7.4; 40:20:40) and a portion was injected onto a h.p.l.c. column. Chromatographic separation of the analytes and internal standard was accomplished with a Phenomenex Luna C-18 column (5 µm × 4.6 mm i.d. × 150 mm) and a Brownlee RP-18 guard column. The mobile phase was delivered isocratically at a flow rate of 1 ml min−1. The effluent was delivered to a mass spectrometer (Navigator™, Finnigan, San Jose, CA) interfaced with a Hewlett Packard 1100 binary pump equipped with a HP1100 autosampler. The APCI probe was run in the positive ion mode with source and probe temperatures of 200 ° C and 550 ° C, respectively. Midazolam, 15N3-midazolam, 1′-hydroxymidazolam, 1′-hydroxy-15N3-midazolam and desmethyldiazepam were detected in the selected ion recording mode at m/z 326, 331, 342, 347, and 271, respectively. The limit of quantification was 0.25 ng ml−1 for midazolam, 15N3-midazolam, 1′-hydroxymidazolam and 1′-hydroxy-15N3-midazolam. The accuracy and precision for midazolam and 15N3-midazolam at 1.4, 10, and 40 ng ml−1 was less than 5% and 6%, respectively. In the case of 1′-hydroxymidazolam the accuracy and precision at 1.5 ng ml−1 and 3 ng ml−1 was less than 2% and 6%, respectively. The same analytical procedure was used to determine the 1′-hydroxymidazolam and 1′-hydroxy-15N3-midazolam in urine after treatment of the samples with β-glucuronidase. The lower limit of quantification for 1′-hydroxymidazolam in the urine was 0.075 µg ml−1. The accuracy and precision for 1′-hydroxymidazolam in urine was less than 10% and 9% at concentrations of 0.4 and 8 µg ml−1, respectively. The midazolam concentration in the infusate was determined by h.p.l.c. [17].
Blood concentrations were determined as previously described [14]. The pharmacokinetic evaluation of midazolam and 15N3-midazolam blood concentration-time data employed noncompartmental methods of analysis using WinNonlin (Version 3, Pharsight Corp., Mountain View, CA). The maximum blood concentration (Cmax) and the corresponding time of the maximum concentration (tmax) were identified by visual inspection of the data. The elimination rate constant (λz) was determined as the slope of the linear regression for the terminal log-linear portion of the concentration-time curve. A terminal half-life (t½) was calculated as 0.693/λz. The area under the blood concentration-time curve (AUC from zero to the final detectable midazolam plasma concentration) after intravenous and oral administration was determined by a combination of linear and logarithmic trapezoidal methods with extrapolation to infinity (AUC(0,∞)). The percent area extrapolated after intravenous administration ranged from 3.58 to 29.0% with a mean value of 12.9 ± 7.21% for all treatment periods. For oral administration, the percent area extrapolated ranged from 1.81 to 25.9% with a mean value of 12.6 ± 5.81% for all treatment periods. Volume of distribution (Vd), systemic clearance (CLiv), oral clearance (CLoral) and oral availability (Foral) were determined using standard methods. The intravenous dose of midazolam was estimated as the infused solution concentration multiplied by the rate of infusion and the duration of infusion.
Psychomotor performance was assessed using the Digit Symbol Substitution Test (DSST) [18]. Participants were presented with a code in which the numbers 1 through 9 were matched with symbols. Prior to the administration of midazolam a DSST was administered to subjects for a minimum of three 90 s practice sessions in order to minimize the effect of learning. The DSST was administered before and at 0.5, 1, 2, 3, 4, and 24 h after midazolam and 15N3-midazolam dosing. The pharmacodynamic end-point was the number of correctly completed matches in 90 s. A decrease in DSST score is indicative of psychomotor impairment. During periods of complete sedation, a DSST score of zero was recorded.
Summary statistics (arithmetic mean and percent CV) were calculated for the derived pharmacokinetic parameters. The pharmacokinetic parameters were subjected to statistical analysis by using a cross-over analysis of variance (anova) model. The effects due to subject and treatment were extracted. The Cmax and AUC parameters were evaluated based on log-transformed data and were expressed as the ratio of the mean parameter of the treatment (days 4, 6, 8, and 12) to the control (day 0). Ninety-five percent confidence intervals (95% CI) for the Cmax and AUC ratios were calculated using the pooled residual error and associated degrees of freedom from the anova.
Results
Pharmacokinetics
Nine women ranging from 24 to 39 years of age participated in the study. Eight women completed all aspects of the study. After finishing four of the five study periods, one subject withdrew from the study for personal reasons. For all study days a higher percentage of the administered dose was excreted into the urine as 1′-hydroxymidazolam after oral administration compared to the amount of 1′-hydroxymidazolam recovered after intravenous administration (P < 0.05) indicating complete intestinal absorption of 15N3-midazolam (Tables 1 and 2).
Table 1.
Arithmetic mean (% CV) pharmacokinetic parameters of intravenous midazolam in eight healthy females following a restricted diet and oral contraceptive (OC) therapy.
| Day 0 (Control) | Day 4 (Diet) | Day 6 (2 days OC) | Day 8 (4 days OC) | Day 14 (10 days OC) | |
|---|---|---|---|---|---|
| Intravenous dose (mg) | 3.26 (24) | 3.29 (24) | 3.26 (24) | 3.25 (24) | 3.26 (24) |
| AUC(0,∞)iv (µg l−1 h) | 90.8 (28) | 86.5 (29) | 81.7 (29) | 87.4 (28) | 80.8 (34) |
| Ratio; 95% CIa | – | 95%; 84, 107 | 89%; 79, 101 | 96%; 85, 109 | 88%; 77, 99 |
| t½,iv (h) | 3.96 (31) | 3.65 (39) | 3.82 (31) | 4.01 (28) | 3.99 (37) |
| Vd (l) | 208 (33) | 207 (42) | 225 (31) | 218 (30) | 242 (38) |
| CLiv (l h−1 kg−1) | 0.588 (27) | 0.627 (28) | 0.663 (32) | 0.608 (27) | 0.675 (32) |
| % dose excreted in urine as 1′-hydroxymidazolam | 71.4 (8) | 68.0 (13) | 60.3 (26) | 58.4 (29) | 55.1 (27) |
AUC(0,∞)iv, intravenous area under the concentration-time curve; t½,iv, elimination half-life after intravenous administration; Vd, volume of distribution; CLiv, total blood clearance after intravenous administration.
Ratio and 95% confidence intervals based on log-transformed data.
Table 2.
Arithmetic mean (% CV) pharmacokinetic parameters of oral 15N3-midazolam in eight healthy females following a restricted diet and oral contraceptive (OC) therapy.
| Day 0 (Control) | Day 4 (Diet) | Day 6 (2 days OC) | Day 8 (4 days OC) | Day 14 (10 days OC) | |
|---|---|---|---|---|---|
| Oral dose (mg) | 3.03 (3) | 2.97 (3) | 3.06 (3) | 2.94 (3) | 3.02 (3) |
| AUC(0,∞)oral (µg l−1 h) | 25.5 (46) | 25.6 (32) | 24.3 (27) | 25.7 (29) | 28.1 (30) |
| Ratio; 95% CIa | – | 104%; 84–129 | 101%; 82–125 | 105%; 85–130 | 114%; 92–141 |
| Cmax (ng ml−1) | 8.67 (42) | 9.24 (45) | 7.32 (32) | 7.98 (40) | 9.81 (26) |
| Ratio; 95% CIa | – | 105%; 80, 139 | 87%; 66, 115 | 93%; 70, 122 | 118%; 90, 156 |
| tmax (h) | 1.53 (57) | 1.44 (77) | 1.66 (70) | 1.63 (54) | 1.13 (56) |
| t½,oral (h) | 1.85 (17) | 2.04 (35) | 2.62 (93) | 1.91 (25) | 1.81 (33) |
| CLoral (l h−1 kg−1) | 2.15 (37) | 2.01 (32) | 2.04 (13) | 1.92 (26) | 1.85 (34) |
| Foral | 0.310 (51) | 0.336 (39) | 0.329 (32) | 0.325 (26) | 0.380 (29) |
| % dose excreted in urine as 1′-hydroxymidazolam | 93.5 (15) | 90.7 (10) | 73.4 (28) | 72.1 (31) | 73.0 (29) |
AUC(0,∞)oral, oral area under the concentration-time curve; Cmax, maximum blood concentration; tmax, time of the maximum concentration; t½,oral, elimination half-life after oral administration; CLoral, oral clearance; Foral, oral availability.
Ratio and 95% confidence intervals based on log-transformed data.
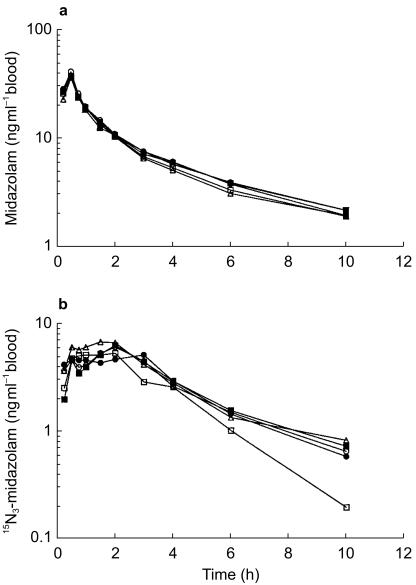
Mean midazolam and 15N3-midazolam blood concentration-time profiles for study days 0, 4, 6, 8, and 14 are presented in Figure 1. To examine potential confounding dietary influences on CYP3A activity, the results from day 0 (regular diet) and day 4 (restricted diet) were compared. The midazolam and 15N3-midazolam blood concentration-time profiles for study days 0 and 4 showed comparable characteristics. Mean midazolam and 15N3-midazolam pharmacokinetic parameters for days 0 and 4 are summarized in Tables 1 and 2. For both routes of administration, these parameters were not significantly changed with diet. When comparing diet effects (day 4 to day 0) on midazolam's pharmacokinetics, the AUC(0,∞)iv ratio was 95% (95% CI: 84, 107); AUC(0,∞)oral ratio was 104% (95% CI: 84, 129); the Cmax ratio was 105% (95% CI: 80, 139). The mean tmax value following oral administration on day 4 (1.44 h) was similar to that on day 0 (1.53 h). The values of t½, Vd, CLiv, CLoral and Foral for intravenous or oral midazolam were essentially unchanged by diet treatment. Overall, the mean pharmacokinetic parameters suggest that the diets of the participants on days 0 and 4 had a comparable effect on the hepatic or intestinal CYP3A. As a result, day 0 was employed as baseline.
Figure 1.
Mean blood concentration vs time curve of (a) midazolam and (b) 15N3-midazolam after simultaneous intravenous and oral administration. ○ represents day 0 (control); • represents day 4 after a restricted diet; □ represents day 6 after oral administration of oral contraceptive for 2 days; ▪ represents day 8 after oral administration of oral contraceptive for 4 days; ▵ represents day 14 after oral administration of oral contraceptive for 10 days.
As demonstrated in Figure 1, the midazolam and 15N3-midazolam blood concentration-time profiles for study days 0, 6, 8, and 14 were nearly superimposable, indicating no differences in the pharmacokinetics of midazolam and 15N3-midazolam, with or without coadministration of Ovral™. The effects of Ovral™ administration on midazolam pharmacokinetics following intravenous and oral dosing are presented in Tables 1 and 2. No mean significant differences were observed in the pharamacokinetic parameters for midazolam and 15N3-midazolam between the four treatment periods. Midazolam AUC(0,∞)iv ratios for days 6, 8, and 14 relative to day 0 were 89% (95% CI: 79, 101), 96% (95% CI: 85, 109), and 88% (95% CI: 77, 99), respectively (Table 1). For 15N3-midazolam, AUC(0,∞)oral ratios for days 6, 8, and 14 relative to day 0 were 101% (95% CI: 82, 125), 105% (95% CI: 85, 130), and 114% (95% CI: 92, 141), respectively; Cmax ratios for 87% (95% CI: 66, 115), 93% (95% CI: 70, 122), and 118% (95% CI: 90, 156), respectively (Table 2). The wide 95% CI for AUC(0,∞)iv, AUC(0,∞)oral, and Cmax suggests that there may be individuals with differences between treatments. The mean tmax values following oral administration of 15N3-midazolam with OC therapy (day 6 h, 1.66; day 8, 1.63 h; day 14, 1.13 h) were similar to control (day 0, 1.53 h) (Table 2). Plasma elimination half-life was calculated from λz after both routes of administration. Among the four treatments, mean t½ for midazolam ranged from 3.65 to 4.01 h and for 15N3-midazolam from 1.81 to 2.62 h. As expected, the mean calculated values for Vd, CLiv, CLoral, and Foral were unchanged by OC therapy (Tables 1 and 2). These results indicate that the doses of EE2 and norgestrel found in Ovral™ do not alter intestinal or hepatic CYP3A activity.
Interestingly, double-peaks in 15N3-midazolam blood concentration-time profiles were observed in six of the eight individuals for day 8, which is indicative of delayed absorption. This phenomena has been described with other benzodiazepines and is thought to be a result of reduced gastric motility caused by muscle relaxant effect of benzodiazepines [19].
Pharmacodynamics
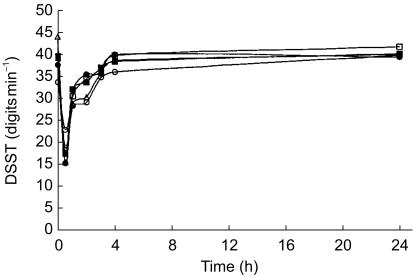
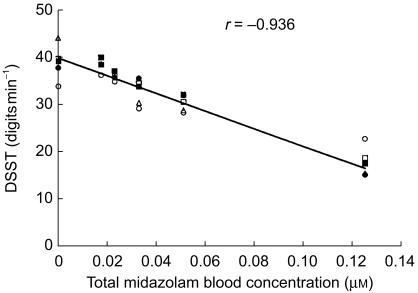
Co-treatment with Ovral™ did not alter the pharmacodynamics of midazolam. The mean DSST score-time profiles for the 5 treatment days are illustrated in Figure 2. The lowest DSST score achieved by subjects corresponded well with the respective tmax for intravenous administration of midazolam. Maximum pharmacodynamic response occurred 30 min after dosing with DSST scores returning to baseline values by 4 h. In all groups, a strong negative correlation (r = −0.936) between DSST scores and total blood midazolam (midazolam plus 15N3-midazolam) concentrations was observed (Figure 3).
Figure 2.
Mean digit symbol substitution test (DSST) scores vs time curve after simultaneous administration of intravenous and oral midazolam. ○ represents day 0 (control); • represents day 4 after a restricted diet; □ represents day 6 after oral administration of oral contraceptive for 2 days; ▪ represents day 8 after oral administration of oral contraceptive for 4 days; ▵ represents day 14 after oral administration of oral contraceptive for 10 days.
Figure 3.
Relationship of total blood midazolam concentration (µm) to the digit symbol substitution test (DSST) score. ○ represents day 0 (control); • represents day 4 after a restricted diet; □ represents day 6 after oral administration of oral contraceptive for 2 days; ▪ represent day 8 after oral administration of oral contraceptive for 4 days; ▵ represents day 14 after oral administration of oral contraceptive for 10 days. Each point represents the mean value.
Discussion
The liver contains high levels of CYP3A and thus it was thought that the relatively small amounts of norgestrel and EE2 present in birth control pills may have little effect on the systemic clearance of substrates of CYP3A. Although CYP3A4 represents a large portion of the CYP present in the small intestine, its absolute levels are small compared with the liver [20]. Yet, the intestinal CYP3A4 has been shown to play an important role in the first pass metabolism of a number of compounds, including midazolam [21, 22]. Therefore the consequence of modulating intestinal CYP3A activity may be pronounced and exposure to OC treatment was predicted to effect intestinal CYP3A activity.
The CYP3A isoforms participate in the metabolism of midazolam and EE2 [9, 17]. The biotransformation of midazolam involves oxidation to 1′-hydroxymidazolam, 4-hydroxymidazolam and 1′,4-dihydroxymidazolam with urinary recovery of 1′-hydroxymidazolam accounting for 70% of the administered dose [23]. The main oxidative pathway of metabolism for EE2 is aromatic hydroxylation at the 2-position [9]. Most combination OC preparations contain both EE2 and a progestin, such as norgestrel. Acetylenic steroids such as EE2 and norgestrel have been shown to be mechanism-based inhibitors of CYP3A mediated metabolism in vitro [9, 10, 24]. However since steroids such as these may also induce CYP3A levels in vivo, it is possible that these agents can cause an initial inhibition followed by an offsetting-induction of CYP3A [7].
In the current investigation, short and long-term, repeated dosing of 50 µg EE2 and 500 µg norgestrel produced no effect on the mean pharmacokinetic parameters of intravenous or oral midazolam. The observed disposition characteristics of midazolam were similar to those previously reported in healthy women after intravenous and oral midazolam administration [14, 22]. Several previous in vivo studies evaluating the effect of OC use on the metabolism of CYP3A substrates have produced conflicting results. Repeated dosing of oral EE2 (60 µg) alone did not alter the pharmacokinetic parameters of 13C-EE2 when administered intravenously [25]. Similarly, the OC combinations of 2 mg dienogest plus 30 µg EE2 and 125 µg levonorgestrel plus 30 µg EE2 did not influence the plasma concentration-time curve of the CYP3A substrate nifedipine [26]. In contrast, a recent study demonstrated that OC users (150 µg desogestrel/30 µg EE2) had a 131% increase in prednisolone steady-state plasma concentrations when compared with control [27]. In a long-term study comparing two OC preparations, Jung-Hoffmann & Kuhl noted that the areas under the EE2 serum concentration-time curves were 70% higher in women taking 75 µg gestodene plus 30 µg EE2 compared with women taking 150 µg desogestrel plus 30 µg EE2 [28]. Since in the majority of these studies the EE2 dose was identical, it would appear that the variation might reflect the influence of the different progestins on CYP3A.
Several in vitro studies have addressed the issue of the effects of the different acetylenic steroids on CYP3A activity. Back et al. studied the inhibition of microsomal ethinyl oestradiol 2-hydroxylase activity by coincubation with various progestins [10]. This group demonstrated that with EE2 as the substrate, the Ki values for gestodene, 3-keto desogestrel, desogestrel, levonorgestrel and norgestimate for competitive inhibition were 99, 93, 54, 40, and 74 µm, respectively [10]. Peak plasma concentration after a 30–50 µg dose of EE2 range between 0.6 nm–0.7 nm [8, 29]. Additionally, peak plasma concentrations with 250–500 µg dose of levonorgestrel can range between 0.01 µm and 0.05 µm [30]. Thus, it is unlikely that the small dose of EE2 and norgestrel present in OCs could attain concentrations sufficient to competitively inhibit the metabolism of a second substrate of CYP3A.
Back et al. also performed studies examining 17α-acetylenic progestins as mechanism-based inhibitors of CYP3A using cyclosporin as the substrate and rank ordered the progestins according to their inhibitory potency such that gestodene>3-keto desogestrel>norethisterone>norgestrel [10]. A similar rank order of 17α-acetylenic progestins as mechanism based inhibitors was obtained by Guengerich [24]. In addition, Guengerich found EE2 to be a mechanism-based inhibitor of relatively similar potency to norgestrel [24]. Thus the differing results of the various in vivo studies on the effects of OC on CYP3A activity appear to be related to the choice of progestin. In the current case, norgestrel a relatively poor mechanism-based inhibitor was used with EE2 and no interaction was observed. However, with the use of more potent mechanism-based inhibitors like gestodene, inhibition may occur [10, 24].
One possible explanation for the lack of CYP3A alteration seen in this study, particularly with EE2, is that metabolism of these steroids at the 17α-acetylenic position is not a major metabolic pathway. To date, most in vitro studies have been performed using EE2 at micromolar concentrations instead of at clinically relevant concentrations, which are in the nanomolar range. A recent study in human hepatocytes in which the investigators used clinically relevant concentrations of EE2 (1 nm), suggests that direct conjugation of EE2 as opposed to EE2 oxidation represents the predominant elimination pathway for this drug [31]. Because of the predominant role of phase II enzymes in the metabolism of these agents, it is possible that the interactions of EE2 and norgestel with CYP3A are minimal at therapeutic concentrations and thus alteration of CYP3A activity would be unlikely.
In conclusion, this study did not find a difference in the pharmacokinetics or the pharmacodynamics of midazolam after intravenous or oral administration in women taking Ovral™. These results indicate that the doses of EE2 and norgestrel contained in this very popular combination OC product did not produce significant inhibition of hepatic or intestinal CYP3A activity. However, drug–drug interaction studies with OCs containing different progestins, such as gestodene, which is a more potent in vitro mechanism-based inhibitor than norgestrel may yield a different response.
Acknowledgments
This work was supported by grants T32GM08425, AG13718 from the National Institutes of Health (Bethesda, MD, USA) and Eli Lilly and Company (Indianapolis, IN, USA).
References
- 1.Wrighton SA, Thummel KE. CYP3A. In: Levy LH, Thummel KE, Trager WF, Hansten PD, Eichelbaum M, editors. Metabolic Drug Interactions. Philadelphia: Lippincott, Williams & Wilkins; 2000. pp. 115–132. [Google Scholar]
- 2.Wrighton SA, Ring BJ, Watkins PB, Vandenbranden M. Identification of a polymorphically expressed member of the human cytochrome P-450III family. Mol Pharmacol. 1989;36:97–105. [PubMed] [Google Scholar]
- 3.Wrighton SA, Brian WR, Sari MA, et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3) Mol Pharmacol. 1990;38:207–213. [PubMed] [Google Scholar]
- 4.Haehner BD, Gorski JC, Vandenbranden M, et al. Bimodal distribution of renal cytochrome P450, 3A activity in humans. Mol Pharmacol. 1996;50:52–59. [PubMed] [Google Scholar]
- 5.Kitada M, Kamataki T, Itahashi K, Rikihisa T, Kanakubo Y. P-450 HFLa, a form of cytochrome P-450 purified from human fetal livers, is the 16 alpha-hydroxylase of dehydroepiandrosterone 3-sulfate. J Biol Chem. 1987;262:13534–13537. [PubMed] [Google Scholar]
- 6.Domanski TL, Finta C, Halpert JR, Zaphiropoulos PG. CDNA cloning and initial characterization of CYP3A43, a novel human cytochrome P450. Mol Pharmacol. 2001;59:386–392. doi: 10.1124/mol.59.2.386. [DOI] [PubMed] [Google Scholar]
- 7.Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 8.Orme ML, Back DJ, Breckenridge AM. Clinical pharmacokinetics of oral contraceptive steroids. Clin Pharmacokinet. 1983;8:95–136. doi: 10.2165/00003088-198308020-00001. [DOI] [PubMed] [Google Scholar]
- 9.Guengerich FP. Oxidation of 17 alpha-ethynylestradiol by human liver cytochrome P-450. Mol Pharmacol. 1988;33:500–508. [PubMed] [Google Scholar]
- 10.Back DJ, Houlgrave R, Tjia JF, Ward S, Orme ML. Effect of the progestogens, gestodene, 3-keto desogestrel, levonorgestrel, norethisterone and norgestimate on the oxidation of ethinyloestradiol and other substrates by human liver microsomes. J Steroid Biochem Mol Biol. 1991;38:219–225. doi: 10.1016/0960-0760(91)90129-s. [DOI] [PubMed] [Google Scholar]
- 11.Deray G, le Hoang P, Cacoub P, Assogba U, Grippon P, Baumelou A. Oral contraceptive interaction with cyclosporin. Lancet. 1987;i:158–159. doi: 10.1016/s0140-6736(87)91988-x. [DOI] [PubMed] [Google Scholar]
- 12.Ross WB, Roberts D, Griffin PJ, Salaman JR. Cyclosporin interaction with danazol and norethisterone. Lancet. 1986;i:330. doi: 10.1016/s0140-6736(86)90867-6. [DOI] [PubMed] [Google Scholar]
- 13.Hunt CM, Westerkam WR, Stave GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992;44:275–283. doi: 10.1016/0006-2952(92)90010-g. [DOI] [PubMed] [Google Scholar]
- 14.Gorski JC, Jones DJ, Haehner-Daniel BD, Hammon MA, O'Mara EM, Hall SD. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 15.Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450, 3A probe: I. In vitro–in vivo correlations in liver transplant patients. J Pharmacol Exp Ther. 1994;271:549–556. [PubMed] [Google Scholar]
- 16.Top 200 brand-name drugs by prescriptions in 1999. Drug Topics. 2000;144:69. [Google Scholar]
- 17.Gorski JC, Hall SD, Jones DR, Vandenbranden M, Wrighton SA. Regioselective biotransformation of midazolam by members of the human cytochromes P450, 3A (CYP3A) subfamily. Biochem Pharmacol. 1994;47:1643–1653. doi: 10.1016/0006-2952(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhi J, Massarella JW, Melia AT, et al. The pharmacokinetic-pharmacodynamic (digit symbol substitution test) relationship of flumazenil in a midazolam steady-state model in healthy volunteers. Clin Pharmacol Ther. 1994;56:530–536. doi: 10.1038/clpt.1994.174. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Roy A, Sun L, Lau CE. A double-peak phenomenon in the pharmacokinetics of alprazolam after oral administration. Drug Metab Dispos. 1999;27:855–859. [PubMed] [Google Scholar]
- 20.Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolars JC, Awni WM, Merion RM, Watkins PB. First-pass metabolism of cyclosporin by the gut. Lancet. 1991;338:1488–1490. doi: 10.1016/0140-6736(91)92302-i. [DOI] [PubMed] [Google Scholar]
- 22.Thummel KE, O'Shea D, Paine MF, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 23.Dundee JW, Halliday NJ, Harper KW, Brogden RN. Midazolam. A review of its pharmacologic properties and therapeutic use. Drugs. 1984;28:519–543. doi: 10.2165/00003495-198428060-00002. [DOI] [PubMed] [Google Scholar]
- 24.Guengerich FP. Mechanism-based inactivation of human liver microsomal cytochrome P-450 IIIA4 by gestodene. Chem Res Toxicol. 1990;3:363–371. doi: 10.1021/tx00016a015. [DOI] [PubMed] [Google Scholar]
- 25.Kuhnz W. Hümpel, Biere H, Gross D. Influence of repeated oral doses of ethinyloestradiol on the metabolic dispositon of [13C2]-ethinyloestradiol in young women. Eur J Clin Pharmacol. 1996;50:231–235. doi: 10.1007/s002280050098. [DOI] [PubMed] [Google Scholar]
- 26.Balogh A, Gessinger S, Svarovsky U, et al. Can oral contraceptive steroids influence the elimination of nifedipine and its primary pryidine metabolite in humans? Eur J Clin Pharmacol. 1998;54:729–734. doi: 10.1007/s002280050543. [DOI] [PubMed] [Google Scholar]
- 27.Seidegard J, Simonsson M, Edsbäcker S. Effect of an oral contraceptive on the plasma levels of budesonide and prednisolone and the influence on plasma cortisol. Clin Pharmacol Ther. 2000;67:373–381. doi: 10.1067/mcp.2000.105762. [DOI] [PubMed] [Google Scholar]
- 28.Jung-Hoffman C, Kuhl H. Interaction with the pharmacokinetics of ethinylestradiol and prosestogens contained in oral contraceptives. Contraception. 1989;40:299–312. doi: 10.1016/0010-7824(89)90094-2. [DOI] [PubMed] [Google Scholar]
- 29.Brody SA, Turkes A, Goldzieher JW. Pharmacokinetics of three bioequivalent norethindrone/mestranol-50 micrograms and three norethindrone/ethinyl estradiol-35 micrograms OC formulations: are ‘low-dose’ pills really lower? Contraception. 1989;40:269–284. doi: 10.1016/0010-7824(89)90092-9. [DOI] [PubMed] [Google Scholar]
- 30.Fotherby K. Levonorgestrel. Clinical pharmacokinetics. Clin Pharmacokinet. 1995;28:203–215. doi: 10.2165/00003088-199528030-00003. [DOI] [PubMed] [Google Scholar]
- 31.Li AP, Hartman NR, Lu C, Collins JM, Strong JM. Effects of cytochrome P450 inducers on 17alpha-ethinyloestradiol (EE2) conjugation by primary human hepatocytes. Br J Clin Pharmacol. 1999;48:733–742. doi: 10.1046/j.1365-2125.1999.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]