Abstract
Aims
Cisplatin and carboplatin are often used in combination with etoposide. In a randomized cross-over study, the potential interaction between the two platinum drugs and the metabolism of etoposide was explored. In vitro investigations using human liver microsomes were also performed.
Methods
Etoposide was administered to 15 patients over 3 days, with the platinum drug administered on day 2. The alternate platinum drug was administered on the second course. The pharmacokinetics of etoposide were determined on all 3 days of each cycle. The effect of platinum drugs on etoposide metabolism by human liver enzymes was explored in vitro.
Results
Neither cisplatin nor carboplatin coadministration affected the pharmacokinetics of etoposide during cycle 1. When carboplatin was administered on course 2, etoposide AUC was 8% higher on day 2 compared with day 1 or day 3 (for day 2 vs day 3, 95% CI: −0.72, −0.08 mg ml−1 min). In contrast, cisplatin on course 2 increased the AUC of etoposide (28%) on day 3 (day 3 vs day 1, 95% CI: 0.67, 2.09 mg ml−1 min), with no effect on day 2. In vitro carboplatin and cisplatin (10–100 µm) inhibited the metabolism of etoposide, if rat liver microsomes were preincubated (30 min) with NADPH and the platinum complexes. With human liver microsomes a small effect on etoposide metabolism, but not on catechol formation, was observed.
Conclusions
The interaction between etoposide and platinum drugs is small and, given the pharmacokinetic variability seen with etoposide, the clinical impact is unlikely to be significant.
Keywords: carboplatin, cisplatin, etoposide, interaction, pharmacokinetics
Introduction
The combination of etoposide with either cisplatin or carboplatin is a common module in many chemotherapy regimens. For example, in paediatric patients, etoposide/carboplatin or etoposide/cisplatin are part of multidrug treatments for neuroblastoma, rhabdomyosarcoma and brain tumours. Similarly, adult malignancies which respond to platinum/etoposide combinations include lung carcinoma, testicular teratoma and soft tissue sarcomas. Etoposide is thought to act by inhibition of topoisomerase II enzymes, which are involved in uncoiling of DNA and so are important for both DNA repair and replication. The platinum agents react with DNA to form a variety of adducts, including monofunctional and bifunctional intra- and interstrand cross-links. In vitro studies have suggested possible synergy between etoposide and platinum complexes [1, 2], although this has been disputed [3].
The toxic and therapeutic effects of both etoposide [4] and the platinum complexes [5] are related to the plasma drug concentrations achieved in individual patients. Therapeutic drug monitoring to achieve or maintain target plasma drug concentrations has been employed for etoposide [4, 6, 7] and carboplatin [8], and has been suggested for cisplatin [9] in an attempt to optimize drug exposure in each individual patient. The plasma concentration profile of any drug is determined by the balance between the dose administered and the rate of elimination.
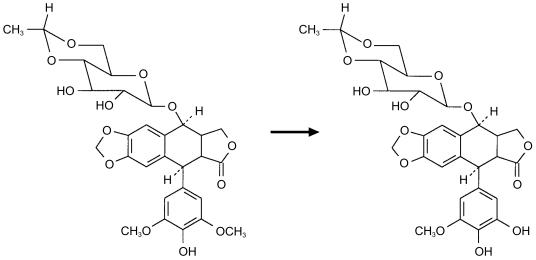
The elimination of etoposide is governed by a combination of renal excretion and oxidative metabolism (Figure 1). In patients with renal or hepatic impairment, the dose of etoposide is usually reduced [10] and anephric paediatric patients may safely be given 50% of the usual dose of etoposide [11]. Elimination of carboplatin is primarily by glomerular filtration, with only 20% of a dose being eliminated by nonrenal mechanisms in patients with normal renal function [12]. Cisplatin, by contrast, is primarily cleared by the reaction of platinum species with suitable nucleophiles, mostly protein or DNA [13], although renal elimination also contributes to the elimination of free cisplatin [14].
Figure 1.
Metabolism of etoposide to the catechol metabolite.
A potential pharmacokinetic interaction between etoposide and the platinum agents might be expected as both cisplatin, and very rarely, carboplatin are associated with nephrotoxicity. Previous investigations comparing the pharmacokinetics of etoposide in testicular teratoma patients receiving etoposide alone, compared with those also receiving carboplatin, indicated no significant interaction [15]. In contrast, in paediatric patients, the clearance of etoposide was reduced by 20% on courses where cisplatin was coadministered, relative to courses where etoposide was administered without cisplatin [16]. Furthermore the combination of cisplatin and etoposide was more toxic. Although only limited data were available, it was suggested that decreased renal elimination was not responsible for the reduction in etoposide clearance when given in combination with cisplatin. An alternative possibility that platinum drugs, which are not themselves substrates for oxidative metabolism, might interfere with the metabolic elimination of drugs (e.g. etoposide) by cytochrome P450 enzymes has been investigated, but with inconclusive results [17, 18].
In order to investigate possible interactions between etoposide and platinum drugs in a systematic manner, a randomized cross-over comparison of etoposide pharmacokinetics following administration alone, and in combination with both carboplatin or cisplatin, was undertaken. In addition, the possibility of an interaction at the level of etoposide oxidative metabolism was investigated by examining the loss of parent compound and the formation of the catechol metabolite in vitro with rat and human liver microsomes.
Methods
A total of 16 patients were recruited to the clinical study. The majority of patients were diagnosed with small cell lung cancer. Patient details are given in Table 1. Patients were required to have received no more than two previous chemotherapy regimens or radiotherapy, the last completed at least 6 weeks prior to study, and no prior platinum treatment. Patients were required to be younger than 70 years, WHO performance status less than 2 and to have normal blood counts and biochemistry. Elevated liver function tests (greater than twice upper normal limit) were allowed if directly related to hepatic tumour deposits. Patients with brain metastases were excluded and all patients had to be capable of giving written informed consent, which was obtained prior to enrolment in the study. The study was approved by the Joint Ethics Committee of the Newcastle Hospital Trust and the University of Newcastle, Newcastle upon Tyne, UK.
Table 1.
Patient characteristics.
| Patient characteristic | Median | Min | Max |
|---|---|---|---|
| Number studied | 16 | ||
| Number with complete data | 10 | ||
| Age (years) | 52 | 30 | 69 |
| Weight (kg) | 66 | 40 | 83 |
| Surface area (m2) | 1.78 | 1.32 | 1.99 |
| GFR (ml min−1) | 103 | 59 | 153 |
| Carboplatin dose (mg) | 598 | 370 | 890 |
Etoposide, 100 mg m−2 each day for 3 days, was administered for two consecutive courses as part of the study. Patients were randomized to receive either carboplatin or cisplatin on day 2 of the first course, crossing over to the other platinum complex on day 2 of the second course of etoposide. Courses were administered every 28 days. After the first two courses, treatment was to continue with either platinum agent at the discretion of the investigator.
Carboplatin doses were calculated to achieve a target AUC of 5 mg ml−1 min, using 51Cr-EDTA clearance to estimate glomerular filtration rate (GFR), according to the Calvert formula:
Dose = AUC(GFR+25)
where Dose is in mg, AUC is the target AUC in mg ml−1 min and GFR is glomerular filtration rate in ml min−1 and 25 (ml min−1) is a constant that accounts for nonrenal clearance [12]. Carboplatin was administered as a 30 min infusion immediately prior to the dose of etoposide. The dose of cisplatin was 100 mg m−2, which was administered as a short infusion, again immediately prior to the dose of etoposide.
Etoposide was administered as a 60 min infusion. Blood samples were collected into tubes containing EDTA as an anticoagulant immediately prior to etoposide administration, and at 30, 60, 240, 360 and 480 min after the start of infusion. After the third day of administration a further sample was collected 24 h after administration. Plasma was separated by centrifugation at 2000 g for 15 min at room temperature and frozen at −20° C prior to analysis. Urine data were not obtained due to difficulties in obtaining reliable urine collections and of interpreting carry-over effects when comparing data from consecutive days.
Concentrations of etoposide in plasma were determined as described previously [6]. Pharmacokinetic analyses were performed using WinNonlin (Pharsight Corp, California, USA), using a combination of trapezoidal and log-trapezoidal methods with extrapolation to infinity to calculate AUC (less than 5% of total AUC). Values of AUC for days 2 and 3 were corrected by subtraction of the AUC contribution from the previous dose. Comparison of AUC between days of treatment and between courses was performed using a cross-over analysis [19] to account for carry-over and period effects relating to differences in AUC between courses. For example, the possible carryover effect of prior platinum treatment on day 1 etoposide AUC was compared between courses, for patients whose course 1 platinum was either cisplatin or carboplatin. A paired Student's t-test was used to compare AUC values between days in the same course.
In vitro metabolism studies
Rat liver microsomes were obtained from animals pretreated with phenobarbitone (80 mg kg−1 i.p. × 4 days) or dexamethasone (100 mg kg−1 i.p. × 4 days) and prepared according to standard procedures [20]. Human liver microsomes (pooled from four individuals) were obtained from IIAM, Leicester UK. IIAM state that informed consent for use of tissue in research was explicitly obtained from donors or donors’ next of kin. Etoposide and carboplatin were gifts from Bristol Myers Squibb (New Jersey, USA). Cisplatin was obtained from Sigma. The h.p.l.c. method, as described above for etoposide, was used for rat liver microsome experiments. For experiments with human liver microsomes, etoposide and its catechol metabolite were detected by liquid chromatography-mass spectrometry (LC-MS) using a PE Biosystems API2000 instrument (Warrington, UK). Briefly, the incubation was stopped and etoposide and metabolites extracted by the addition of ethyl acetate, which was then separated and evaporated under nitrogen. Samples were reconstituted in mobile phase (methanol 55%/water 45%) and injected into the LC-MS. The column was a Phenomenex 50 × 2 mm Luna 3µ C8 and the flow rate 200 µl min−1. The instrument was optimised for the detection of the catechol using an authentic standard kindly supplied by Dr M. V. Relling, St Judes Hospital, Tennessee. The identity of the catechol metabolite was verified by the mass of the molecular ion (M-H = 573) and by multiple reaction monitoring (MRM) analysis of samples, with the characteristic fragment at mass 367 (loss of glucopyranoside and OH) [21]. Stability of catechol was assessed by repeated extraction of equivalent samples and repeated injection from the same sample.
For both analytical methods, standard curves (0.1–5.0 µg ml−1) were run on the day of analysis, together with independently prepared quality assurance (QA) samples. All values for standards and QAs were within 15% of the nominal values. Inter and intraday variation of the assays were less than 10% across the range of the standard curve.
Results
Of the 16 patients recruited to the study, 10 completed two cycles of treatment with 3 full days of etoposide pharmacokinetics. In addition, two patients had full data for the first cycle, but only 1 or 2 days for cycle 2. Failure to acquire full pharmacokinetic data from these two individuals was due to the patients withdrawing from the study. Three patients did not complete the crossover to the second cycle of treatment due to progressive disease and one patient was withdrawn prior to any treatment for the same reason. No patient experienced any significant toxicity during the two courses of treatment studied, and there were no differences between the treatment arms.
Patient characteristics were comparable between the two arms of the study (i.e. first course treatment with cisplatin/etoposide vs carboplatin/etoposide). There was no clinically significant change in serum creatinine or in liver function tests between cycles of treatment. No other drugs which might potentially interact with either etoposide or the platinum complexes were administered during the two cycles studied. Two patients received carboplatin doses which were not consistent with their target AUC (5 mg ml−1 min) and GFR. Patient 1 had a dose reduction to 250 mg m−2 due to the presence of renal disease and patient 2 was dosed to a target AUC of 4 mg ml−1 min in error.
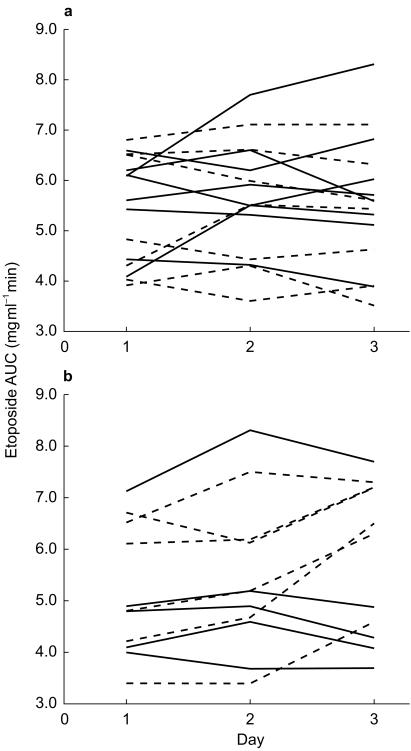
Etoposide AUC values for each patient on the two cycles of treatment are shown in Figure 2a,b. Etoposide plasma concentration-time data for representative patients on each arm of the study are shown in Figure 3. Initial cross-over analysis showed no carryover or treatment effects relating to day 1 of etoposide. However, an interaction or carry-over effect on AUC changes between days 2 and 3 of cycle 2, dependent on platinum treatment in cycle 1, was observed. Considering the AUC values for each treatment day on cycle 1, there was no significant effect of carboplatin or cisplatin on etoposide AUC comparing day 1 and day 2 of treatment (Figure 2, Table 2 carboplatin; mean difference in etoposide AUC = 0.31 mg ml−1 min, 95% CI [−0.36, 0.99], cisplatin; 0.10 mg ml−1 min, 95% CI [−0.46, 0.66]). Likewise there was no difference between day 1 and day 3 or day 2 and day 3 on this cycle. However, on cycle 2, treatment either with cisplatin or with carboplatin did result in an alteration in etoposide pharmacokinetics, although the effect of the two platinum drugs was different (Table 2). Patients who had received cisplatin in the first cycle showed an increase in etoposide AUC on day 2 compared with day 1 (5.34 vs 4.98 mg ml−1 min, mean difference in AUC = 0.36 mg ml−1 min, 95% CI [−0.33, 1.05]). Although this difference was not significant, an increase was seen in four of the five patients for whom paired data were available. Moreover, a decrease in AUC was seen between day 2 and day 3 (5.34 vs 4.94 mg ml−1 min, mean difference in AUC = −0.40 mg ml−1 min, 95% CI [−0.72, −0.08]), whereas there was no difference between day 1 and day 3. Patients who had received carboplatin on cycle 1, and so were treated with cisplatin on cycle 2, did not have an increase in AUC on day 2 of the second cycle (Figure 2b), but did show an increase in the etoposide AUC on day 3 compared with either day 1 (mean difference in AUC = 1.38 mg ml−1 min, 95% CI [0.67, 2.09]) or day 2 (mean difference in AUC = 0.98 mg ml−1 min, 95% CI [0.07, 1.89]) (Table 2).
Figure 2.
Etoposide AUC values for cycle 1 (a) and cycle 2 (b) Each line represents an individual patient, grouped according to which platinum drug was given on day 2 of the cycle. Solid line: carboplatin; dotted line: cisplatin.
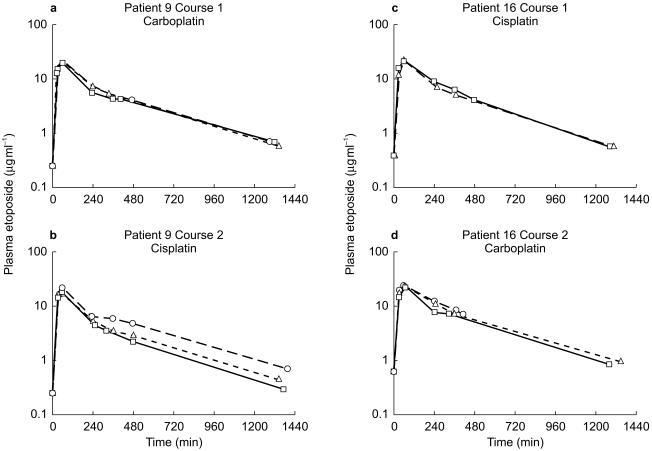
Figure 3.
Representative plasma concentration-time data for a patient receiving carboplatin on cycle 1 (a) and cisplatin on cycle 2 (b) and for a patient receiving cisplatin on cycle 1 (c) and carboplatin on cycle 2 (d). □ Day 1; ▵ Day 2; ○ Day 3.
Table 2.
Mean difference in etoposide AUC (mg ml−1 min) with 95% confidence intervals (CIs for those shown in italic type exclude 0).
| Order and cycle | Day 1 vs Day 2 | Day 1 vs Day 3 | Day 2 vs Day 3 |
|---|---|---|---|
| Car/Cis Cycle 1 | 0.31 (−0.36, 0.99) | 0.28 (−0.53, 1.08) | −0.04 (−0.51, 0.44) |
| Car/Cis Cycle 2 | 0.23 (−0.33, 0.80) | 1.38 (0.67, 2.09) | 0.98 (0.07, 1.89) |
| Cis/Car Cycle 1 | 0.10 (−0.46, 0.66) | −0.06 (−0.64, 0.52) | −0.16 (−0.51, 0.19) |
| Cis/Car Cycle 2 | 0.36 (−0.33, 1.05) | −0.04 (−0.56, 0.48) | −0.40 (−0.72, −0.08) |
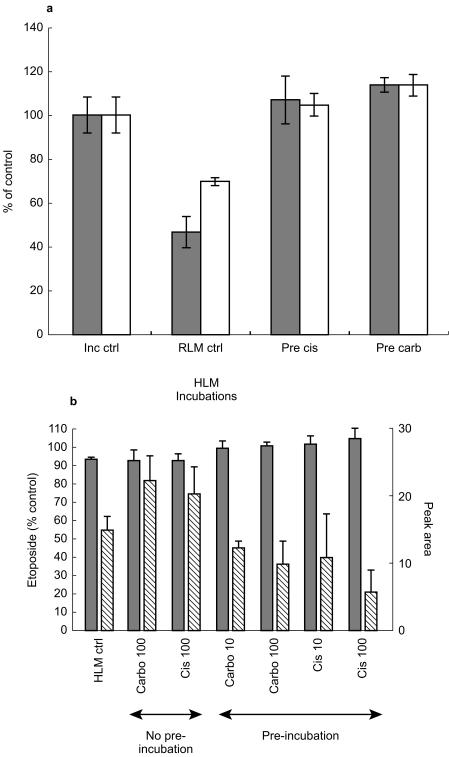
In an attempt to investigate the nature of this apparent delayed interaction further, in vitro studies were undertaken, in which etoposide was incubated with either rat or human liver microsomes and metabolism was monitored by both the loss of etoposide, using h.p.l.c. with u.v. detection, and the formation of the catechol metabolite of etoposide by LC-MS. Inhibition of etoposide metabolism could not be demonstrated at concentrations of up to 100 µm of either cisplatin or carboplatin when the reaction was initiated by addition of either microsomes or NADPH. However, preincubation of microsomes with either carboplatin or cisplatin at 10 or 100 µm for 30 min completely blocked the metabolism of etoposide. This phenomenon could be demonstrated with rat liver microsomes obtained from animals induced with either phenobarbitone or dexamethasone (Figure 4a). Similarly, inhibition of etoposide metabolism was observed using human liver microsomes following preincubation of the platinum drugs (10 µm) with microsomes and NADPH (Figure 4b). Although formation of the catechol could be demonstrated by LCMS, no consistent effect of platinum agents on the formation of this metabolite was observed (Figure 4b), either with concurrent or preincubation of platinum complexes with the microsomal system.
Figure 4.
a) Metabolism of etoposide by rat liver microsomes (dexamethasone (□) or phenobarbitone (dotted box) induced, pooled from two animals each) and inhibition by pre-incubation with cisplatin or carboplatin. Data shown for control experiment in the absence of microsomes (Inc ctrl), metabolism of etoposide in the absence of platinum drugs (Mic ctrl) and the inhibition of metabolism caused by pre-incubation with 10 µm cisplatin (Pre cis) or carboplatin (Pre carb). Data shown are mean ± s.d. (n=4 incubations). b) Metabolism of etoposide by human liver microsomes (HLM) and inhibition by pre-incubation with cisplatin or carboplatin. When platinum drugs were not pre-incubated with HLM there was no inhibition of etoposide metabolism at 100 µm. Preincubation of platinum drugs (at 10 or 100 µm) inhibited the metabolism of etoposide (P<0.05, Student's t-test), but had no significant effect on catechol formation (P>0.05). Left axis disappearance of etoposide (% of control with no HLM, dotted box). Catechol peak area (diagonal box). Data shown are mean ± s.d. (n=4 incubations).
Discussion
The aim of this study was to determine the nature and magnitude of any pharmacokinetic interaction between etoposide and the platinum complexes cisplatin and carboplatin. A number of clinical observations have suggested such interactions and a lack of therapeutic equivalence of cisplatin/etoposide and carboplatin/etoposide combinations. For example, the combination of etoposide with cisplatin is more effective than that with carboplatin in germ cell tumours [22]. Similarly, administration of etoposide after cisplatin administration has been found to be more effective than the opposite order [23]. The combination of etoposide and carboplatin, compared with the combination with cisplatin, has also been associated with less toxicity [24]. These clinical observations may suggest a pharmacokinetic interaction.
Previous investigations have suggested a pharmacokinetic interaction between etoposide and the platinum drugs. Most notably, decreased etoposide clearance was reported in children receiving multiagent chemotherapy in cycles where etoposide was administered 2 days after cisplatin, compared with cycles where the gap between administration of the two drugs was 21 days [16]. No change in the renal clearance of etoposide or in the excretion of the catechol metabolite was observed. The observed increase in etoposide AUC, with a two day interval between the administration of cisplatin and etoposide may be consistent with the delayed effect seen in day 3, cycle 2 of the current study.
Other studies of etoposide pharmacokinetics indicated that there was no effect of carboplatin on etoposide pharmacokinetics [15]. The design of this study was very similar to that reported here, except that carboplatin was administered on both courses. Conversely, in a study of high-dose carboplatin and etoposide the clearance of etoposide was reported to be less than expected and a pharmacokinetic interaction, either via renal elimination or hepatic metabolism was suggested [25]. Interestingly, a high proportion (83%) of the patients had received prior cisplatin treatment. Prior exposure to cisplatin has been noted as a factor reducing the clearance of etoposide in the absence of concurrent platinum administration [26, 27], although no carryover effect from course 1 treatment with cisplatin or carboplatin was observed for day 1 etoposide pharmacokinetics in the patients studied here. A clinically significant sequence effect with the administration of paclitaxel and cisplatin has also been reported, suggesting a possible effect of the latter drug on the metabolic elimination of paclitaxel [28].
Platinum drugs have been noted to have a number of effects on drug elimination, and more particularly metabolism, both in vivo and in vitro. Effects on hormone metabolism have been noted in both rats and humans, with cisplatin, but not carboplatin, reducing the activity of the cytochrome P450 enzyme CYP2C11 in male rats [18]. This effect was associated with a reduced concentration of the enzyme itself, suggesting some change in the regulation of enzyme expression or inactivation [18]. A decrease in rat testicular cytochrome P450 enzymes following cisplatin administration has been reported and a specific interaction with the heme element of cytochrome P450 enzymes suggested [17]. Interestingly, treatment with cisplatin was associated with an increase of free iron content of LLC-PK renal cells. It was suggested that cytochrome P450 enzymes were the source of the free iron and that the release of iron, and cisplatin nephrotoxicity, could be blocked by inhibitors of cytochrome P450 enzymes [29]. The inactive, but toxic, cisplatin isomer transplatin, has also been found to displace iron from cytochrome C [30]. Inactivation of cytochrome P450 enzymes by displacement of iron may account for the delayed effect in vivo and the need for preincubation in vitro in the studies presented here.
Etoposide is eliminated predominantly by metabolism, with at most 30–40% of the dose excreted unchanged in urine [31]. The most widely studied metabolic pathway for etoposide is O-demethylation to yield the catechol metabolite [32], a molecule which is likely to be short lived and subject to further oxidation or conjugation reactions. Formation of etoposide catechol is mediated by CYP3A4 [33]. Although in the present work etoposide metabolism by liver microsomes was monitored both by loss of etoposide and formation of the catechol metabolite, no effect of the platinum drugs could be demonstrated unless the microsomes were incubated with the platinum drugs prior to initiation of the metabolic reaction. In a previous investigation of potential inhibitors of etoposide demethylation, cisplatin was found to be noninhibitory at a concentration of 100 µm [34], although the order of addition of substrate, enzyme and inhibitor were not specified. Although there is not complete agreement between the clinical study and the results of these in vitro experiments, this is likely to be due to difficulties in matching the precise in vivo scenario of prior platinum exposure. These possible explanations do not account completely for the observed effects, particularly the disparity between course 1 and course 2 etoposide pharmacokinetics. Further studies would be necessary to shed more light on this phenomenon.
The impact of platinum drugs on etoposide elimination observed in this paper is modest, particularly in the context of inter and intrasubject pharmacokinetic variation, and is therefore unlikely to be of clinical significance. However, interactions between platinum agents and other drugs are also possible and may be more significant for agents whose elimination is totally dependent on cytochrome P450 enzymes.
Acknowledgments
We should like to acknowledge the work of the nurses (Madeline Proctor, Dorothy Simmons, Fiona Chapman and Avril Oakey) and data managers (Lesley Robson and Kevin Fishwick) at the Northern centre for Cancer Treatment. This work was supported by the Cancer Research Campaign.
References
- 1.Durand RE, Goldie JH. Interaction of etoposide and cisplatin in an in vitro tumor-model. Cancer Treat Rep. 1987;71:673–679. [PubMed] [Google Scholar]
- 2.Kanzawa F, Nishio K, Fukuoka K, et al. Evaluation of synergism by a novel three-dimensional model for the combined action of cisplatin and etoposide on the growth of a human small-cell lung-cancer cell line, SBC-3. Int J Cancer. 1997;71:311–319. doi: 10.1002/(sici)1097-0215(19970502)71:3<311::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Tsai CM, Gazdar AF, Venzon DJ, et al. Lack of in vitro synergy between etoposide and cis-diammine-dichloroplatinum (Ii) Cancer Res. 1989;49:2390–2397. [PubMed] [Google Scholar]
- 4.Joel SP, Ellis P, O'Byrne K, et al. Therapeutic monitoring of continuous infusion etoposide in small-cell lung cancer. J Clin Oncol. 1996;14:1903–1912. doi: 10.1200/JCO.1996.14.6.1903. [DOI] [PubMed] [Google Scholar]
- 5.Jodrell DI, Egorin MJ, Canetta RM, et al. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol. 1992;10:520–528. doi: 10.1200/JCO.1992.10.4.520. [DOI] [PubMed] [Google Scholar]
- 6.Porter D, Boddy A, Thomas H, et al. Etoposide phosphate infusion with therapeutic drug monitoring in combination with carboplatin dosed by area under the curve: a cancer research campaign phase I/II committee study. Semin Oncol. 1996;23:34–44. [PubMed] [Google Scholar]
- 7.Joel SP, Oakley P, Slevin ML, et al. Further investigation of the therapeutic window with 15-day infusional etoposide phosphate in small cell lung cancer. Br Cancer Res Meeting Edinburgh. 1999;80(Suppl 2):94. [Google Scholar]
- 8.Veal GJ, Boddy AV, Thomas HD, et al. Real-time monitoring of carboplatin pharmacokinetics in paediatric patients receiving high dose chemotherapy. Br Cancer Res Meeting Edinburgh. 1999;80(Suppl 2):93. P258. [Google Scholar]
- 9.Bonetti A, Franceschi T, Apostoli P, et al. Cisplatin pharmacokinetics in elderly patients. Ther Drug Monit. 1994;16:477–482. doi: 10.1097/00007691-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Stewart C. Use of etoposide in patients with organ dysfunction: Pharmacokinetic and pharmacodynamic considerations. Cancer Chemother Pharmacol. 1994;34:S76–S83. doi: 10.1007/BF00684868. [DOI] [PubMed] [Google Scholar]
- 11.English MW, Lowis SP, Peng B, et al. Pharmacokinetically guided dosing of carboplatin and etoposide during peritoneal dialysis and haemodialysis. Br J Cancer. 1996;73:776–780. doi: 10.1038/bjc.1996.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 13.Drobnik J. Antitumor activity of platinum complexes. Cancer Chemother Pharmacol. 1983;10:145–149. doi: 10.1007/BF00255749. [DOI] [PubMed] [Google Scholar]
- 14.Reece Pa, Stafford I, Davy M, Freeman S. Disposition of unchanged cisplatin in patients with ovarian cancer. Clin Pharmacol Ther. 1987;42:320–325. doi: 10.1038/clpt.1987.155. [DOI] [PubMed] [Google Scholar]
- 15.Newell DR, Eeles RA, Gumbrell LA, et al. Carboplatin and etoposide pharmacokinetics in patients with testicular teratoma. Cancer Chemother Pharmacol. 1989;23:367–372. doi: 10.1007/BF00435838. [DOI] [PubMed] [Google Scholar]
- 16.Relling M, McLeod H, Bowman L, Santana V. Etoposide pharmacokinetics and pharmacodynamics after acute and chronic exposure to cisplatin. Clin Pharmacol Ther. 1994;56:503–511. doi: 10.1038/clpt.1994.171. [DOI] [PubMed] [Google Scholar]
- 17.Maines MD, Mayer RD. Inhibition of testicular cytochrome p450-dependent steroid biosynthesis by cis-platinum. J Biol Chem. 1985;260:6063–6068. [PubMed] [Google Scholar]
- 18.Leblanc GA, Sundseth SS, Weber GF, Waxman DJ. Platinum anticancer drugs modulate P-450 mRNA levels and differentially alter hepatic drug and steroid hormone metabolism in male and female rats. Cancer Res. 1992;52:540–547. [PubMed] [Google Scholar]
- 19.Armitage P, Berry G. Statistical Methods in Medical Research. 3. Oxford. UK: Blackwell Scientific Publications; 1994. p. p 245. [Google Scholar]
- 20.Schenkman JB, Jansson I. Isolation and purification of constutive forms of microsomal cytochrome P450. In: Phillips IR, Shephard EA, Totowa NJ, editors. Cytochrome P450 Protocols. Humana Press Inc; 1998. [Google Scholar]
- 21.van Maanen JMS, de Vries J, Pappie D, et al. Cytochrome P450-mediated O-demethylation: a route in the metabolic activation of etoposide. Cancer Res. 1987;47:4658–4662. [PubMed] [Google Scholar]
- 22.Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598–606. doi: 10.1200/JCO.1993.11.4.598. [DOI] [PubMed] [Google Scholar]
- 23.Maksymiuk AW, Jett JR, Earle JD, et al. Sequencing and schedule effects of cisplatin plus etoposide in small-cell lung cancer: Results of a North Central Cancer Treatment Group randomized clinical trial. J Clin Oncol. 1994;12:70–76. doi: 10.1200/JCO.1994.12.1.70. [DOI] [PubMed] [Google Scholar]
- 24.Horwich A, Dearnaley DP, Nicholls J, et al. Effectiveness of carboplatin, etoposide, and bleomycin combination chemotherapy in good-prognosis metastatic testicular nonseminomatous germ cell tumors. J Clin Oncol. 1991;9:62–69. doi: 10.1200/JCO.1991.9.1.62. [DOI] [PubMed] [Google Scholar]
- 25.Rodman J, Murry D, Madden T, Santana V. Altered etoposide pharmacokinetics and time to engraftment in pediatric patients undergoing autologous bone marrow transplantation. J Clin Oncol. 1994;12:2390–2397. doi: 10.1200/JCO.1994.12.11.2390. [DOI] [PubMed] [Google Scholar]
- 26.Sinkule JA, Hutson P, Hayes FA, Etcubanas E, Evans W. Pharmacokinetics of etoposide (VP16) in children and adolescents with refractory solid tumors. Cancer Res. 1984;44:3109–3113. [PubMed] [Google Scholar]
- 27.Pflüger K-H, Hahn M, Holz J-B, et al. Pharmacokinetics of etoposide: correlation of pharmacokinetic parameters with clinical conditions. Cancer Chemother Pharmacol. 1993;31:350–356. doi: 10.1007/BF00686147. [DOI] [PubMed] [Google Scholar]
- 28.Rowinsky EK, Gilbert MR, McGuire WP, et al. Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol. 1991;9:1692–1703. doi: 10.1200/JCO.1991.9.9.1692. [DOI] [PubMed] [Google Scholar]
- 29.Baliga R, Zhang Z, Baliga M, Ueda N, Shah S. Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;54:1562–1569. doi: 10.1046/j.1523-1755.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 30.Jiang LJ, Tang GZ, Tang WX. The interaction between cytochrome C and trans-[PtCl2 (NH3) (2) ] J Chem Soc – Dalton Transactions 1996. 1996:2223–2226. [Google Scholar]
- 31.Clarke PI, Slevin ML. The clinical pharmacology of etoposide and teniposide. Clin Pharmacokin. 1987;12:223–252. doi: 10.2165/00003088-198712040-00001. [DOI] [PubMed] [Google Scholar]
- 32.Relling MV, Evans R, Dass C, Desiderio DM, Nemec J. Human cytochrome P450 metabolism of teniposide and etoposide. J Pharmacol Exp Ther. 1992;261:491–496. [PubMed] [Google Scholar]
- 33.Relling MV, Nemec J, Schuetz EG, et al. O-demethylation of epipodophyllotoxins is catalyzed by human cytochrome P450 3A4. Mol Pharmacol. 1994;45:352–358. [PubMed] [Google Scholar]
- 34.Kawashiro T, Yamashita K, Zhao X-J, et al. A study on the metabolism of etoposide and possible interactions with antitumor or supporting agents by human liver microsomes. J Pharmacol Exp Ther. 1998;286:1294–1300. [PubMed] [Google Scholar]