Abstract
Aims
Convulsions are a common complication of severe malaria in children and are associated with poor outcome. Diazepam is used to terminate convulsions but its pharmacokinetics and pharmacodynamics have not been studied in this group. Accordingly, we carried out a comparative study of the pharmacokinetics of intravenous (i.v.) and rectal (p.r.) diazepam.
Methods
Twenty-five children with severe malaria and a convulsion lasting >5 min were studied. Sixteen children received diazepam intravenously (i.v.; 0.3 mg kg−1) and nine rectally (p.r.; 0.5 mg kg−1). Plasma diazepam concentrations were measured by reversed phase high-performance liquid chromatography. The duration of convulsions, depth of coma, respiratory and cardiovascular parameters were monitored.
Results
Median maximum plasma diazepam concentrations of 634 (range 402–1507) ng ml−1 and 423 (range 112–1953) ng ml−1 were achieved at 5 and 25 min following i.v. and p.r. administration, respectively. All patients except three (one i.v. and two p.r.) achieved plasma diazepam concentration >200 ng ml−1 within 5 min. Following p.r. administration, plasma diazepam concentrations were more variable than i.v. administration. A single dose of i.v. diazepam terminated convulsions in all children but in only 6/9 after p.r. administration. However, nine children treated with i.v. and all those treated with p.r. diazepam had a recurrence of convulsions occurring at median plasma diazepam concentrations of 157 (range: 67–169) and 172 (range: 74–393) ng ml−1, respectively. All the children in the i.v. and four in the PR diazepam group who had recurrence of convulsions required treatment. None of the children developed respiratory depression or hypotension.
Conclusions
Administration of diazepam i.v. or p.r. resulted in achievement of therapeutic concentrations of diazepam rapidly, without significant cardio-respiratory adverse effects. However, following p.r. administration, diazepam did not terminate all convulsions and plasma drug concentrations were more variable.
Keywords: children, convulsions, diazepam, malaria, pharmacokinetics
Introduction
Convulsions are common in children admitted to hospital with falciparum malaria. About 30% of children with malaria will present with convulsions at some point in the course of the disease [1–3]. Prolonged and repetitive convulsions are associated with death and neurological sequelae in cerebral malaria (CM) [4]. Moreover, in CM, over 80% of these children have a history of convulsions during the present illness and in over 60%, seizures are witnessed after admission to hospital [5, 6]. Children with CM have a mortality rate of 18.6% and a 10.9% chance of developing neurological sequelae if they survive [4]. Thus, rapid termination and prevention of seizures are a priority and may improve the outcome in children with severe malaria.
Benzodiazepines are the drug of choice for rapid termination of seizures. In resource-poor countries (RPC), diazepam is most widely used, since it is widely available, cheap, rapidly acting, effective and can be administered either i.v. or p.r. Although diazepam rectubes are effective in the management of febrile seizures and epilepsy [7], they are expensive (US$3.5 per 5 mg rectube). Thus, in RPC the cheaper i.v. preparation (US$0.07 per vial) is administered p.r. routinely to terminate convulsions.
Recently there has been increasing concern regarding the use of diazepam, especially p.r., to terminate convulsions in children, since it is associated with respiratory depression [8]. Moreover, treatment of breakthrough convulsions with multiple doses of diazepam after phenobarbitone prophylaxis is associated with an increased mortality in children with CM [9]. The pharmacokinetics and pharmacodynamics of diazepam have not been studied in children with severe malaria. While the suggested therapeutic range of plasma diazepam concentrations (200–600 ng ml−1) [10, 11] is based on relatively few studies, values in this range have been associated with decreased blood pressure [12]. There are no reports that relate plasma concentrations of diazepam to clinical outcome in children with severe malaria, who may have compromised respiratory function and hypotension due to associated metabolic acidosis [13] and intracranial hypertension [14]. These effects can be worsened by diazepam.
We have studied the pharmacokinetics and pharmacodynamics of diazepam administered i.v. and p.r. in children with severe falciparum malaria and convulsions in order to (a) establish whether the current doses are appropriate for termination of convulsions and (b) examine the relationship between plasma diazepam concentrations and termination and recurrence of convulsions, respiratory and cardiovascular parameters and (c) evaluate further the suitability of the i.v. preparation administered p.r.
Methods
Subjects and study design
This open label nonrandomized study was approved by the Kenyan National Ethics Committee. Children admitted to the Kenya Medical Research Institute (KEMRI) high dependency unit at Kilifi District Hospital were recruited if they (i) were aged between 6 months and 13 years, (ii) had signs of severe malaria (prostration, deep breathing and coma) [15], (iii) had a convulsion lasting 5 or more min and (iv) if the parent/guardian gave written informed consent. The latter was obtained on admission and children were recruited with intent to treat. They were excluded if they had received diazepam prior to admission, if consent was withdrawn during the study or if the baseline sample had detectable diazepam. The children who received paraldehyde before diazepam were also not recruited.
Clinical care
All children had a clinical history and complete physical examination performed on admission. Venous access was obtained by fixing Teflon cannulae (Jelco™, Ethicon S.p.A, Italy), one for i.v. fluids and medication and another in the opposite arm for blood sampling. A single blood sample (8 ml) was drawn for quantitative parasite count, blood culture, measurement of blood glucose, blood gases, electrolytes, total and differential blood count and plasma proteins. A portion of the blood was centrifuged (1500 g; 3 min) to obtain plasma and stored at −20 ° C until assayed for baseline diazepam concentrations.
Malaria was treated with quinine dihydrochloride (Lincoln Pharmaceuticals Ltd, India) and sulfadoxine/pyrimethamine (Cosmos Pharmaceuticals, Nairobi, Kenya) as previously described [16]. Children with hypoglycaemia (blood glucose ≤2.2 mmol l−1) were given 2 ml kg−1 of 25% w/v dextrose, and then maintained on 4.3% w/v dextrose/0.18% w/v saline solution (4 ml kg−1 h−1). Those with acidosis (base excess ≤8) were given normal saline (20 ml kg−1 over 30–60 min) then maintained on 4.3% w/v dextrose/0.18% w/v saline solution (4 ml kg−1 h−1). Severe anaemia (Hb ≤ 5 g dl−1) was corrected with a blood transfusion [17], and all children were treated with chloramphenicol and benzylpenicillin for possible bacteraemia or meningitis [9] until cerebro-spinal fluid (CSF) and blood culture results were available. Children with temperatures >38.5 ° C had all of their clothing removed and were treated with p.r. paracetamol (10 mg kg−1) and tepid sponging. Hypoxia (transcutaneous oxygen saturation < 96%) was corrected with 100% oxygen administered by nasal prongs.
All the children admitted to the unit had a seizure-monitoring chart that detailed the characteristics of the convulsion (type, duration, lowest oxygen saturation and blood glucose during the seizure) together with a 5 min postictal evaluation of the level of consciousness (Blantyre coma score: minimum summated score is 0 and maximum is 5), respiratory rate and transcutaneous oxygen saturation.
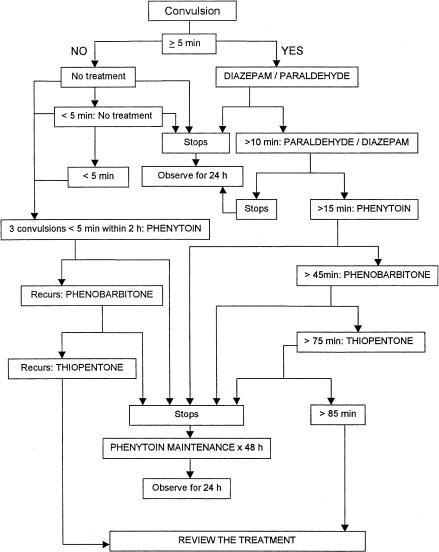
Convulsions were treated as shown in Figure 1. Children with convulsions lasting 5 or more min were treated with diazepam (Laboratory & Allied Ltd, Nairobi, Kenya) 0.3 mg kg−1 i.v. or 0.5 mg kg−1 p.r. (infused over 1 min to avoid inducing respiratory depression or spillage in the case of the rectal route). Paraldehyde (99.5% w/v; Acros Organics, New Jersey, USA) diluted to 10% w/v in olive oil was administered at 0.2 ml kg−1 rectally. A repeat dose of diazepam was administered only when paraldehyde was not available or in case of intractable convulsions. When paraldehyde was available, children who required treatment after diazepam were given the former. The second line anticonvulsant, phenytoin (Faulding Pharmaceuticals Plc., UK; 18 mg kg−1 loading dose) was infused i.v. over 20 min in normal saline with a maintenance dose of 2.5 mg kg 1–2 hourly. Phenobarbitone (Laboratory & Allied Ltd, Nairobi, Kenya) was administered i.v. as a loading dose (15 mg kg−1) infused over 20 min, and if there was a recurrence of convulsions within the 24 h observation period after stopping phenytoin therapy. All patients receiving phenytoin and phenobarbitone were put on an ECG monitor and vital signs monitored for 6 h from the start of infusion. Thiopentone (Rotexmedica GmbH, Germany) was administered as a 4 mg kg−1 bolus over 30 s and a maintenance dose of 4 mg kg−1 h−1 infused over 2 h under close supervision of a doctor. All the children who required second line anticonvulsant were followed up at least once within 3 months after discharge from hospital.
Figure 1.
Convulsion treatment protocol.
Blood sampling
Blood samples (0.4 ml) for measurement of plasma diazepam concentrations were withdrawn at 5, 10, 20, 30, 40, 60 min, and 2, 4, 6, 8, 12, 24, 36 and 48 h after diazepam administration. The cannula was flushed with sterile heparinized normal saline solution (1.0 ml; 20 i.u ml−1). Residual saline was removed before every sample was withdrawn. The blood was mixed in lithium heparinized tubes, and centrifuged (1500 g; 3 min) at room temperature. Plasma was transferred into polyvinyl vials and stored at −20 ° C until analysis for diazepam and desmethyldiazepam.
Clinical measurements
All children had heart rate, blood pressure, respiratory rate, breathing pattern, transcutaneous oxygen saturation and level of consciousness recorded at every sampling interval. In addition, the presence or absence, duration and pattern of convulsions were recorded. Children had 4 hourly measurements of temperature, pulse, Blantyre coma score [18] and pupillary light reaction. Hypotension was defined as a mean blood pressure of <50 mmHg, bradycardia as a heart rate of < 80 beats min−1, and respiratory depression as a respiratory rate < 20 breaths min−1 and a transcutaneous oxygen saturation < 96%.
Analytical procedures, pharmacokinetic and statistical analysis
Plasma concentrations of diazepam and desmethyldiazepam were determined using reversed-phase high performance liquid chromatography [19]. The method is selective for diazepam and desmethyldiazepam, with no interference from drugs commonly administered in these children (antimalarials, antibiotics, other anticonvulsants and analgesics). The limit of detection was 5 ng ml−1, recovery was > 80% and inter- and intra-assay coefficients of variation (CV) were <10%. The maximum plasma drug concentrations (Cmax), the corresponding times (tmax), the times for plasma diazepam to exceed 200 ng ml−1 (t> 200) and decline to below 200 ng ml−1 (t<200) were the experimentally observed values. It was necessary to determine the initial distribution half-life (t1/2,λ1) of diazepam following i.v. administration, since this is the parameter most closely related to its anticonvulsant effect. This was achieved by using a weighted least squares nonlinear regression analysis program (TopFit) [20] to fit the plasma diazepam concentration–time data for the i.v. group with a two-compartment model. The apparent volume of distribution at steady state (Vss), terminal elimination half-life (t1/2,λz)and clearance (CL) were obtained by noncompartmental analysis using the same programme. Area under the plasma drug concentration-time curve (AUC) for i.v. and p.r. routes of administration was estimated using the trapezoidal method [21]. AUC was not extrapolated beyond 48 h since in the management of convulsions in severe malaria, the critical period is the first 2–3 days. The model of best fit (two compartment in this case) was selected on the basis of Akaike information criterion [22]. The Mann–Whitney U-test was used to compare demographic and pharmacokinetic parameters between the p.r. treatment and i.v. treatment groups. A value of P < 0.05 was considered statistically significant. The 95% confidence interval (CI) was estimated using a commercially available computer programme CIA [23].
Results
Subjects
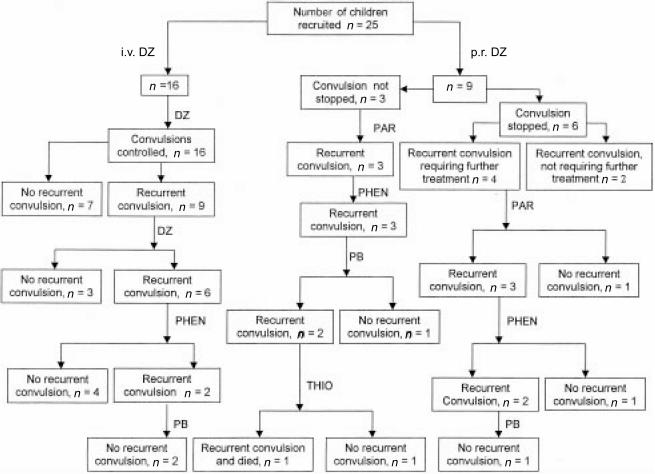
Twenty-five patients were recruited into the study (Figure 2). All baseline parameters are as per Table 1. Total protein, albumin, plasma albumin and blood glucose were within normal ranges for this child population. Most of the children had mild or moderate anaemia, which is common in children with malaria [18]. All the children had a history of prior antimalarial treatment before admission during their current illness. One child had hypoglycaemia on admission and seven children had an episode of hypoglycaemia after admission, although none of the episodes occurred during a convulsion (Table 2).
Figure 2.
Response to anticonvulsant treatment: DZ – diazepam; PAR – paraldehyde; PHEN – phenytoin; PB – phenobarbitone and THIO – thiopentone.
Table 1.
Demographic and biochemical parameters. Values are mean (95% confidence interval, CI).
| Diazepam i.v. (n =16) | Diazepam p.r. (n =9) | 95% CI for difference between means | |
|---|---|---|---|
| Sex (M/F) | 6:10 | 5:4 | |
| Age (months) | 27.8 (20.6, 34.9) | 30.2 (16.3, 44) | −15.6, 10.6 |
| Weight (kg) | 10.4 (9.3, 11.5) | 10.7 (8.5, 12.9) | −2.38, 1.8 |
| Rectal temperature (°C) | 38.6 (37.6, 39.6) | 38.8 (37.8, 39.8) | −1.45, 1.2 |
| WAZ (weight for age) score | −2 (−4.1,−0.09) | −1.7 (−4.0, 0.4) | – |
| Laboratory measurements | |||
| Haemoglobin (g dl−1) | 6.4 (5.1, 7.8) | 8.4 (6.9, 9.9) | −3.7, 0.2 |
| WBC (×106); l−1 | 16.4 (8.4, 24.5) | 20.4 (10, 30.9) | −16.1, 8.1 |
| Parasite count (µl−1)†, ‡ | 61898 (17152, 223379) | 15779 (2472, 1007210) | 0.45, 23.4 |
| Sodium (mmol l−1)‡ | 134 (130.3, 136.9) | 138 (135.3, 141.6) | −9.3, −0.46 |
| Potassium (mmol l−1) | 4.4 (3.9, 4.8) | 4.7 (4.1, 5.4) | −1.1, 0.34 |
| Creatinine (µmol l−1) | 60.5 (28.2, 92.8) | 80.9 (41.7, 120) | −66.9, 26.1 |
| Total plasma proteins (g dl−1) | 57.4 (51.2, 63.6) | 66 (57.9, 74) | −18, 0.85 |
| Plasma albumin (g dl−1)‡ | 31.5 (27.2, 35.9) | 39 (33.5, 44.5) | −14, −0.84 |
| Blood glucose (mmol l−1) | 9.3 (5.9, 12.7) | 7.6 (4.1, 11) | −2.9, 6.3 |
| pH | 7.3 (7.1, 7.4) | 7.2 (7.1, 7.3) | −0.1, 0.3 |
| Base excess | −7.5 (−13.8, −1.2) | −12.3 (−14.8, −9.7) | −2.3, 12.3 |
Geometric mean (95% CI).
P < 0.05 (all other comparisons were not statistically significant).
Table 2.
Clinical progress and outcome. Values are expressed as †median (range).
| Diazepam i.v. (n = 16) | Diazepam p.r. (n = 9) | |
|---|---|---|
| Admission Blantyre coma score† | 1 0 0 (0 0 0–2 2 1) | 0 1 0 (0 0 0–2 1 0) |
| 4 h Blantyre Coma Score (BCS)† | 1 1 0 (0 0 0–2 2 1) | 2 1 0 (0 0 0–2 2 1) |
| Decline in BCS at 4 h | 4 | 2 |
| Episode of hypoglycaemia | 2 | 3 |
| Transfusion | 3 | 2 |
| Convulsions before admission† | 3 (0–99) | 1 (0–99) |
| Prolonged convulsion (> 30 min) | 6 | 6 |
| Convulsions after admission† | 1 (0–28) | 3 (0–44) |
| Convulsion terminated with first DZ dose | 16 | 4 |
| Convulsions after treatment with first DZ† | 1 (1–28) | 3 (3–43) |
| 2nd dose of DZ or PAR | 9 | 7 |
| 3rd line treatment (PHEN) | 6 | 6 |
| Convulsion >30 min after treatment | 6 | 3 |
| Deaths | 0 | 1 |
| Gross neurological sequelae on discharge | 2 | 1 |
Pharmacokinetics
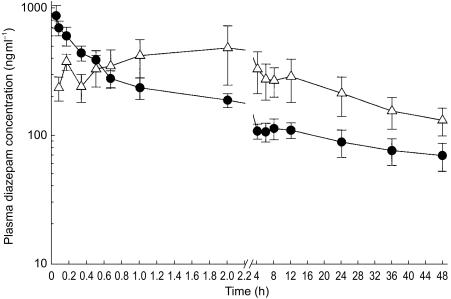
Plasma diazepam peak concentrations were within the reported therapeutic range (200–600 ng ml−1) and were achieved within 5 min by both routes of administration in 88% of the children, although interindividual variability was greater in the p.r. group (Figure 3). P.r. administration was also characterized by a more gradual decline in plasma diazepam concentrations. Although the median t<200 ng ml−1 was similar for both routes of administration, the range was wider in the p.r. group (0.17–36 h) compared with the i.v. group (0.42–3.13 h). A summary of the other estimated pharmacokinetic parameters is shown on Table 3. One child had a plasma diazepam concentration below 200 ng ml−1 5 min following i.v. administration. Even after a repeat dose of diazepam 30 min later, plasma diazepam concentrations in this child declined rapidly from 414 ng ml−1 at 5 min after the repeat dose to less than 200 ng ml−1 within 20 min. Two children who received diazepam p.r. also failed to achieve a plasma concentration of 200 ng ml−1. Despite not achieving this target, all three children stopped convulsing. Although convulsions recurred within 30 min in each child.
Figure 3.
Mean (± s.e.mean) plasma diazepam concentrations (ng ml−1) following i.v. (• 0.3 mg kg−1, n=6) and p.r. (▵; 0.5 mg kg−1, n = 8) administration of diazepam in children.
Table 3.
Pharmacokinetic parameters of diazepam following i.v. and p.r. administration. Values are expressed as mean (95% confidence interval, CI) or
| Parameter | i.v. | p.r. |
|---|---|---|
| t½, λ1 (h) | 0.5 (0.28, 0.73) [n = 11] | – |
| t½, λz (h) | 60 (38, 82) [n = 11] | – |
| Vss (l kg−1) | 3.5 (1.3, 5.7) [n = 11] | – |
| CL (ml min−1 kg−1) | 0.73 (0.4, 1.03) [n = 11] | – |
| tmax (min)† | 5 (5–10) [n = 16] [n = 16] | 25 (10–120) [n = 7] |
| Cmax (ng ml−1)† | 634 (402–1507) [n = 16] | 423 (112–1953) [n = 7] |
| t > 200 ng ml−1 (min)† | 5 (5–5) [n=16] | 5 (5–10) [n=9] |
| AUC(0,12 h) (ng ml−1 h) | 2367 (1661, 3073) [n = 5] | 4082 (970, 5872) [n = 7] |
| AUC(12,24 h) (ng ml−1 h) | 1717 (1107, 2327) [n = 5] | 3247 (623, 5872) [n = 7] |
| AUC(24,48 h) (ng ml−1 h) | 2663 (1090, 4634) [n = 5] | 5604 (833, 10 374) [n = 7] |
In four children who received a repeat dose of diazepam in the i.v. group, the median plasma diazepam concentrations determined 5 min after the second dose was 456 ng ml−1 (range: 414–622 ng ml−1).
Desmethyldiazepam concentrations were below the limit of quantification in all the children who received diazepam i.v., but it was detected in three children who received p.r. diazepam (range: 50–177 ng ml−1).
Drug administration and convulsion control
Control of convulsions following the administration of various anticonvulsants is summarized in Figure 2. Following diazepam i.v. and p.r. administration, median times to convulsion recurrence were 3.25 h (range: 0.5–6 h) and 1.6 h (range: 0.2–7 h), respectively. The corresponding plasma diazepam concentrations at the time of convulsion recurrence were 157 (range: 67–169) and 172 (range: 74–393) ng ml−1, following i.v. and p.r. administration, respectively.
Respiratory, cardiovascular and CNS effects of diazepam
Intravenous diazepam
Mean blood pressure and respiratory rate decreased as the children improved clinically. Ten children had a summated Blantyre score of ≤2 and two children of ≥3 on admission and by 4 h after admission, eight had a total score of ≤2 and 4 of ≥3. Five children showed a decline in the Blantyre coma score 5 min after diazepam administration. Four children did not have their Blantyre score determined at admission and at 4 h. None of the children developed hypotension, bradycardia and respiratory depression even after the second dose of diazepam.
Rectal diazepam
The changes in respiratory rate and BP following p.r. administration were similar to those after i.v. administration. Seven children had a summated Blantyre score of ≤2 and two children of ≥3 on admission, and by 4 h after admission, five had a total score of ≤2 and four of ≥3. Seven children had a decline in Blantyre coma score or no improvement by 30 min after diazepam administration. None of the children suffered bradycardia, hypotension and respiratory depression.
Of the 24 children who survived, three had neurological sequelae at the time of discharge from the hospital. All the three children suffered prolonged and/or repeated convulsions before and after admission. In addition, they were all acidotic with base excesses ranging from −19 to −15 and pHs between 7.1 and 7.2. One of the children in the i.v. group also had a hyperparasitaemia of 1573 840 malaria parasites µl−1 (41% parasitaemia) on admission, which was fully cleared by the time of discharge. Two children who developed paraparesis and speech problems, were in the i.v. group, while the other child who developed tremors and blindness was in the p.r. group.
Discussion
The plasma concentrations of diazepam needed to stop convulsions are not well established although a range of 200–600 ng ml−1 has been suggested [10, 11, 24]. In the present study this was achieved in 94% of children treated with 0.3 mg kg−1 i.v. and all children stopped convulsing without suffering significant haemodynamic effects. Of the children who were given diazepam rectally only six had their convulsions terminated within 5 min and three had a Cmax of <200 ng ml−1.
A two compartment open model adequately described diazepam pharmacokinetics following i.v. administration. This is in accordance with a previous report [25], although a three-compartment model has also been used to describe diazepam disposition [26]. In the two-compartment model, the brain is usually considered as part of the central compartment. The high plasma diazepam concentrations achieved during the initial distribution phase fall within the therapeutic range, and are associated with strong sedative properties of diazepam [27]. In the present study, this phase was characterized by termination of convulsions and decline in the level of consciousness in 41% of children given diazepam i.v. The latter may also be caused by the seizure. The initial rapid decline in plasma diazepam concentrations has been reported to reflect a parallel decline in its CNS effects [10, 11, 28–30]. In the present study, this decline in concentration was reflected by the recurrence of convulsions at times corresponding to the completion of the redistribution phase. The mean clearance and volume of distribution estimated in the present study are comparable with values previously reported in healthy adults [31] and children [32]. Furthermore, in a previous study [33], we showed that common antimalarial drugs do not inhibit the metabolism of diazepam in vitro. Thus, concomitant administration of other drugs used in the management of malaria and associated complications does not appear to have an effect on the pharmacokinetics of diazepam. In three of the five children who received a second dose of diazepam i.v., the plasma concentrations exceeded the upper limit of the reported therapeutic range (600 ng ml−1), although they were not associated with any adverse effects. In one patient who had received p.r. diazepam 33 h earlier, a second dose i.v. increased the plasma diazepam concentrations from 539 to 1152 ng ml−1. Multiple doses of diazepam are associated with cardiorespiratory complications. Therefore, repeated doses or continuous infusions of the drug should be discouraged unless inotropic and respiratory support is on hand. This is because in most rural health facilities where records may not be available some children will present after having been given diazepam.
Rapid termination of convulsions and prevention of prolonged and repetitive convulsions is necessary to prevent exacerbation of hypoxia, hypoglycaemia and intracranial hypertension in children with severe malaria [14, 34]. These complications can result in neuronal damage such as mesial sclerosis in the hippocampal area [35], cortical infarction and atrophy [6, 36]. Half of the children in this study had recurrence of convulsions requiring additional anticonvulsant drugs. This suggests that diazepam has little prophylactic effect, even though a previous report [37] indicated that p.r. diazepam provided short-term prophylaxis against febrile seizures in infants and children. In children with severe malaria and convulsions, diazepam can be used to terminate convulsions, but long acting anticonvulsants such as phenytoin or phenobarbitone are likely to be required to prevent the recurrence of further convulsions [38].
In this study, we did not detect any major adverse effects on respiratory or cardiovascular function similar to those reported in previous studies [8, 39]. This may be related to the effect of convulsions on cardiorespiratory parameters; increasing respiratory rate and blood pressure. However, the number of children in the present study was too small for any conclusions to be made regarding diazepam-induced respiratory depression. Nevertheless, a lack of respiratory depression has been previously reported, despite very high diazepam plasma concentrations (range: 5775–10800 ng ml−1) in neonates [11], and also in a population of older children with epilepsy [40].
Diazepam is mainly metabolized by N-demethylation in the liver to a one-third equipotent metabolite, N-desmethyldiazepam [32]. Desmethyldiazepam was only detected in three children in this study. Because of the slow and variable rate of desmethyldiazepam formation, it only accounts approximately for half the total rate of diazepam elimination [25] and does not contribute significantly to the pharmacological effect in the emergency treatment of convulsions [11, 41, 42].
In children with severe malaria and convulsions, p.r. administration of the i.v. solution of diazepam would be a cheap alternative route of administration provided it resulted in adequate seizure control. The intravenous diazepam solution is much cheaper than rectubes, as a branded 10 mg vial costs US$1 and an equally effective generic formulation as little as US$0.07. However, owing to the high variability in plasma diazepam concentrations and unpredictable desired clinical outcome, the rectal route of administration should not be used unless in unavoidable circumstances.
In conclusion, we have shown that i.v. and p.r. administration of diazepam results in the rapid achievement of therapeutic plasma diazepam concentrations with few cardio-respiratory side-effects in children with severe malaria. The p.r. route is easy to use, but the systemic absorption is more variable and does not terminate all convulsions. Both routes of administration did not prevent the recurrence of convulsions. In resource poor countries, a single dose of diazepam can be used to terminate convulsions in children with severe malaria. We recommend that diazepam (0.3 mg kg−1) should be administered i.v., but in health units with no facilities for i.v. cannulation the diazepam p.r. (0.5 mg kg−1) can be used cautiously as an alternative. Ideally, diazepam should be administered together with a long acting anticonvulsant in children with severe malaria and convulsions.
Acknowledgments
This publication is made with the permission of the Director of the Kenya Medical Research Institute (KEMRI). We wish to thank all our clinical, nursing and laboratory colleagues in Kilifi and Nairobi. Gilbert O. Kokwaro is supported by a Research Capability Strengthening grant from WHO (TDR/MIM grant no. 980074) and a Collaborative Research Initiative Grant from The Wellcome Trust (grant no. 057978/Z/99/Z). Charles R. J. C. Newton (grant no. 050533) and Kevin Marsh are Wellcome Trust Senior Clinical Research Fellows. Bernhards R. Ogutu is a trainee in clinical pharmacology supported by TDR/MIM and KEMRI.
References
- 1.Asindi AA, Ekanem EE, Ibia EO, Nwangwa MA. Upsurge of malaria-related convulsions in a paediatric emergency room in Nigeria. Consequence of emergence of chloroquine-resistant Plasmodium falciparum. Trop Geogr Med. 1993;45:110–113. [PubMed] [Google Scholar]
- 2.Waruiru CM, Newton CR, Forster D, et al. Epileptic seizures and malaria in Kenyan children. Trans R Soc Trop Med Hyg. 1996;90:152–155. doi: 10.1016/s0035-9203(96)90120-0. [DOI] [PubMed] [Google Scholar]
- 3.Wattanagoon Y, Srivilairit S, Looareesuwan S, White NJ. Convulsions in childhood malaria. Trans R Soc Trop Med Hyg. 1994;88:426–428. doi: 10.1016/0035-9203(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 4.Newton CRJC, Krishna S. Severe falciparum malaria in children; current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79:1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 5.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 6.Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epilepticus in childhood cerebral malaria. Q J Med. 1996;89:591–597. doi: 10.1093/qjmed/89.8.591. [DOI] [PubMed] [Google Scholar]
- 7.Dreifuss FE, Rosman NP, Cloyd JC, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med. 1998;338:1869–1875. doi: 10.1056/NEJM199806253382602. [DOI] [PubMed] [Google Scholar]
- 8.Norris E, Marzouk O, Nunn A, McIntyre J, Choonara I. Respiratory depression in children receiving diazepam for acute seizures: a prospective study. Dev Med Child Neurol. 1999;41:340–343. doi: 10.1017/s0012162299000742. 10.1017/s0012162299000742. [DOI] [PubMed] [Google Scholar]
- 9.Crawley J, Waruiru C, Mithwani S, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000;355:701–706. doi: 10.1016/S0140-6736(99)07148-2. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen FU. Plasma-diazepam in infants after rectal administration in solution and by suppository. Acta Paediatr Scand. 1977;66:563–567. doi: 10.1111/j.1651-2227.1977.tb07947.x. [DOI] [PubMed] [Google Scholar]
- 11.Shorvon S. Status Epilepticus: its Clinical Features and Treatment in Childhood and Adults. Cambridge: Cambridge University Press; 1994. Emergency treatment of status epilepticus. [Google Scholar]
- 12.Sunzel M, Paalzow L, Berggren L, Eriksson I. Respiratory and cardiovascular effects in relation to plasma levels of midazolam and diazepam. Br J Clin Pharmacol. 1988;25:561–569. doi: 10.1111/j.1365-2125.1988.tb03346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.English M, Sauerwein R, Waruiru C, et al. Acidosis in severe childhood malaria. Q J Med. 1997;90:263–270. doi: 10.1093/qjmed/90.4.263. [DOI] [PubMed] [Google Scholar]
- 14.Newton CR, Kirkham FJ, Winstanley PA, et al. Intracranial pressure in African children with cerebral malaria. Lancet. 1991;337:573–576. doi: 10.1016/0140-6736(91)91638-b. [DOI] [PubMed] [Google Scholar]
- 15.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 16.Murphy S, English M, Omar A, et al. The management of severe malaria in children: a review. E Afr Med J. 1995;72:536–539. [PubMed] [Google Scholar]
- 17.Newton CR, Warn PA, Winstanley PA, et al. Severe anaemia in children living in a malaria endemic area of Kenya. Trop Med Int Health. 1997;2:165–178. doi: 10.1046/j.1365-3156.1997.d01-238.x. [DOI] [PubMed] [Google Scholar]
- 18.Newton CR, Chokwe T, Schellenberg JA, et al. Coma scales for children with severe falciparum malaria. Trans R Soc Trop Med Hyg 1997. 2001;91:161–165. doi: 10.1016/s0035-9203(97)90207-8. [DOI] [PubMed] [Google Scholar]
- 19.Muchohi SN, Ogutu BR, Newton CRJC, Kokwaro GO. High performance liquid chromatographic determination of diazepam in plasma of children with severe malaria. J Chromatogr B. 2001;761:255–259. doi: 10.1016/s0378-4347(01)00284-5. [DOI] [PubMed] [Google Scholar]
- 20.Heinzel G, Woloszczak R, Thomann P. TopFit,Version 2.0: Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. Stuttgart, Germany: Gustav Fischer Schering AG; [Google Scholar]
- 21.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker; 1982. [Google Scholar]
- 22.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- 23.Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with Confidence. 2. BMJ Books; 2000. [Google Scholar]
- 24.Agurell S, Berlin A, Ferngren H, Hellstrom B. Plasma levels of diazepam after parenteral and rectal administration in children. Epilepsia. 1975;16:277–283. doi: 10.1111/j.1528-1157.1975.tb06058.x. [DOI] [PubMed] [Google Scholar]
- 25.Jack ML, Colburn WA. Pharmacokinetic model for diazepam and its major metabolite desmethyldiazepam following diazepam administration. J Pharm Sci. 1983;72:1318–1323. doi: 10.1002/jps.2600721120. [DOI] [PubMed] [Google Scholar]
- 26.Klotz U, Reimann I. Delayed clearance of diazepam due to cimetidine. N Engl J Med. 1980;302:1012–1014. doi: 10.1056/NEJM198005013021807. [DOI] [PubMed] [Google Scholar]
- 27.Amrein RBL. Importance of pharmacokinetic data in clinical practice. In: Priest RG, Filho UV, Amrein R, Skreta M, editors. Benzodiazepines Today and Tomorrow. MTP Press Ltd; 1980. [Google Scholar]
- 28.Ghoneim MM, Korttila K, Chiang CK, et al. Diazepam effects and kinetics in Caucasians and Orientals. Clin Pharmacol Ther. 1981;29:749–756. doi: 10.1038/clpt.1981.106. [DOI] [PubMed] [Google Scholar]
- 29.Alldredge BKGM, Lowenstein DH. The effect of pre-hospital treatment of status epilepticus (SE) on subsequent patient management. Neurology. 1995;42(Suppl 3):400. [Google Scholar]
- 30.Rey E, Treluyer JM, Pons G. Pharmacokinetic optimization of benzodiazepine therapy for acute seizures. Focus on delivery routes. Clin Pharmacokinet. 1999;36:409–424. doi: 10.2165/00003088-199936060-00003. [DOI] [PubMed] [Google Scholar]
- 31.Klotz U, Antonin KH, Bieck PR. Comparison of the pharmacokinetics of diazepam after single and subchronic doses. Eur J Clin Pharmacol. 1976;10:121–126. doi: 10.1007/BF00609470. [DOI] [PubMed] [Google Scholar]
- 32.Morselli PL, Principi N, Tognoni G, et al. Diazepam elimination in premature and full term infants, and children. J Perinat Med. 1973;1:133–141. doi: 10.1515/jpme.1973.1.2.133. [DOI] [PubMed] [Google Scholar]
- 33.Kokwaro GO, Edwards G, Ward SA, Winstanley PA, Marsh K, Watkins WM. Common antimalarial drugs do not affect metabolism of diazepam and desmethyldiazepam by human liver microsomes in vitro. Pharm Sci. 1996;2:243–245. [Google Scholar]
- 34.Verity CM. Do seizures damage the brain? The epidemiological evidence. Arch Dis Child. 1998;78:78–84. doi: 10.1136/adc.78.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton CR, Peshu N, Kendall B, et al. Brain swelling and ischaemia in Kenyans with cerebral malaria. Arch Dis Child. 1994;70:281–287. doi: 10.1136/adc.70.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leppik IE, Derivan AT, Homan RW, Walker J, Ramsay RE, Patrick B. Double-blind study of lorazepam and diazepam in status epilepticus. J Am Med Ass. 1983;249:1452–1454. [PubMed] [Google Scholar]
- 37.Knudsen FU. Effective short-term diazepam prophylaxis in febrile convulsions. J Pediatr. 1985;106:487–490. doi: 10.1016/s0022-3476(85)80688-0. [DOI] [PubMed] [Google Scholar]
- 38.Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38:202–207. doi: 10.1212/wnl.38.2.202. [DOI] [PubMed] [Google Scholar]
- 39.Brown JK, Hussain IH. Status epilepticus. I. Pathogenesis. Dev Med Child Neurol. 1991;33:3–17. doi: 10.1111/j.1469-8749.1991.tb14780.x. [DOI] [PubMed] [Google Scholar]
- 40.Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353:623–626. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 41.Randall LO, Scheker GL, Banziger RF. Pharmacology of the metabolites of chlordiazepoxide and diazepam. Curr Ther Res. 1965;7:590–606. [PubMed] [Google Scholar]
- 42.Marcucci F, Guaitani A, Fanelli R, Mussini E, Garattini S. Metabolism and anticonvulsant activity of diazepam in guinea pigs. Biochem Pharmacol. 1971;20:1711–1713. doi: 10.1016/0006-2952(71)90304-2. [DOI] [PubMed] [Google Scholar]