Abstract
Endorepellin, a C-terminal fragment of the vascular basement membrane proteoglycan perlecan, inhibits angiogenesis via the α2β1-integrin receptor. Because this integrin is also implicated in platelet-collagen responses and because endorepellin or its fragments are generated in response to injury and inflammation, we hypothesized that endorepellin could also affect platelet biology. We discovered that endorepellin supported α2β1-dependent platelet adhesion, without appreciably activating or aggregating platelets. Notably, endorepellin enhanced collagen-evoked responses in platelets, in a src kinase-dependent fashion, and enhanced the collagen-inhibitory effect of an α2β1-integrin function-blocking antibody. Collectively, these results suggest that endorepellin/α2β1-integrin interaction and effects are specific and dependent on cell type, differ from those emanated by exposure to collagen, and may be due to cellular differences in α2β1-integrin activation/ligand affinity state. These studies also suggest a heretofore unrecognized role for angiostatic basement membrane fragments in platelet biology.
Introduction
Endorepellin, the C-terminal domain of perlecan,1,2 exerts antiangiogenic activity3–5 by interacting with α2β1 integrin and triggering a signaling cascade that leads to disruption of actin cytoskeleton in endothelial cells and ultimately to angiostasis.6,7 The α2β1 integrin also exists in platelets, fibroblasts, and epithelial cells and regulates cell adhesion and signaling.8–12 We hypothesized that endorepellin could affect platelet function via this integrin receptor. This hypothesis is based on the fact that endorepellin or fragments thereof are present in the blood and various body fluids and could interact with platelets at sites of injury, inflammation, and cancer growth. For example, a biologically active fragment of endorepellin (LG3) is present in the urine of patients with end-stage renal disease13 and in the amniotic fluid of pregnant women with premature rupture of fetal membranes.14,15 Perlecan fragments of similar size were found in urinary16 and blood17 proteomes, and LG3 is released by apoptotic endothelial cells.18
Here we show that endorepellin supports α2β1-integrin–mediated platelet adhesion, but does not activate or aggregate platelets. Via an src kinase-dependent mechanism, endorepellin enhances all collagen-evoked platelet responses studied, without directly binding to collagen.3 Our results suggest that endorepellin/α2β1 interactions are cell specific and differ from collagen-α2β1 binding. Generation of endorepellin at sites of injury might enhance initial platelet adhesion and in combination with newly exposed collagen matrix could hasten in vivo platelet responses.
Materials and methods
Informed consent was provided according to the Declaration of Helsinki and Institutional Review Board approval was obtained from Thomas Jefferson University.
Endorepellin, platelets, and materials
Reagents are listed in the Supplemental Materials (available on Blood website; see the Supplemental Materials link at the top of the online article).
Platelet adhesion, activation, and aggregation assays
Methods are detailed in the Supplemental Materials. All experiments were performed 4 times. Data were analyzed with SPSS software (SPSS, Chicago, IL), and statistical significance was determined by the unpaired Student t test.
Results and discussion
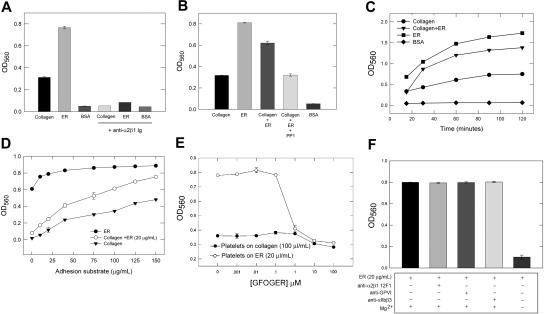
Endorepellin supported platelet adhesion via α2β1 integrin as shown by specific function-blocking antibodies (Figure 1A). Endorepellin enhanced (P < .001) platelet adhesion to collagen, which could be blocked by the src kinase inhibitor PP1 (10 μM; Figure 1B), a concentration sufficient to reduce src phosphorylation on its activation loop19 by 75% (P < .005) in unactivated platelets in suspension (Figure S1A). Adhesion kinetic studies demonstrated a significant (P < .001) increase in adhesion rate to endorepellin and collagen plus liquid-phase endorepellin (Figure 1C). Endorepellin increased platelet adhesion proportionally to increasing amounts of immobilized collagen (Figure 1D). Platelet adhesion to increasing amounts of immobilized endorepellin rapidly saturated (Figure 1D), further suggesting high platelet affinity for endorepellin. PP1 inhibited platelet adhesion to type I collagen but surprisingly enhanced platelet adhesion to endorepellin (Figure S1B), suggesting a differential effect of src in mediating the effects of endorepellin and collagen. As increased platelet src kinase activation beyond constitutive levels20 activates α2β1 via inside-out signalling,21 PP1 may decrease α2β1 activation. If endorepellin has preferential affinity for the inactive α2β1, as reported to occur for other α2β1 ligands,10 this could explain the PP1 differential effects.
Figure 1.
Endorepellin supports platelet adhesion. (A) Human platelets with or without anti-α2β1 antibody (10 μg/mL) were added to wells coated with collagen (100 μg/mL), endorepellin (ER, 20 μg/mL) or 1% BSA for 90 minutes at 37°C followed by fixation with 4% paraformaldehyde and crystal violet colorimetric analysis. Mean OD560 ± SE from 4 separate experiments shown (A-D). (B) Experiment similar to that shown in panel A with the addition of liquid phase ER (20 μg/mL) with or without PP1 (10 μM) to collagen-coated (100 μg/mL) wells. (C) Experiment similar to that shown in panel A (coating with collagen, 100 μg/mL, or ER, 20 μg/mL, repeated with OD560 analyzed at different incubation time points. (D) Experiment similar to that shown in panel A with different coating concentrations (1-150 μg/mL) of collagen with or without liquid-phase ER (20 μg/mL) or different coating concentrations of ER (1-150 μg/mL). (E) Effects of different concentrations of the α2β1-integrin–specific triple-helical collagen peptide GFOGER on platelet adhesion to collagen or ER. (F) Effects of different platelet receptor function blocking antibodies or nonfunction-blocking anti-α2β1 antibody 12F1 (each tested at 10 μg/mL) or magnesium-free conditions on platelet adhesion to endorepellin.
The α2β1-specific collagen I triple-helical peptide GFOGER inhibited endorepellin- and collagen-evoked platelet adhesion (Figure 1E; 1-100 μM; P < .001). Specificity was confirmed by blocking in magnesium-free conditions (Figure 1F), necessary for α2β1-integrin–mediated adhesion,22 but not with antibodies against other platelet receptors or a nonfunction blocking antibody to α2β1 (Figure 1F). Endorepellin (20 μg/mL) inhibited platelet adhesion to GFOGER-coated wells (10 μg/mL) by 33% (not shown). Because GFOGER has high affinity for α2β1 and does not require activated α2β1 for adhesion,21 these results suggest a similar high-affinity endorepellin/α2β1 interaction that potentially competes with GFOGER, but enhances collagen/α2β1 interaction.
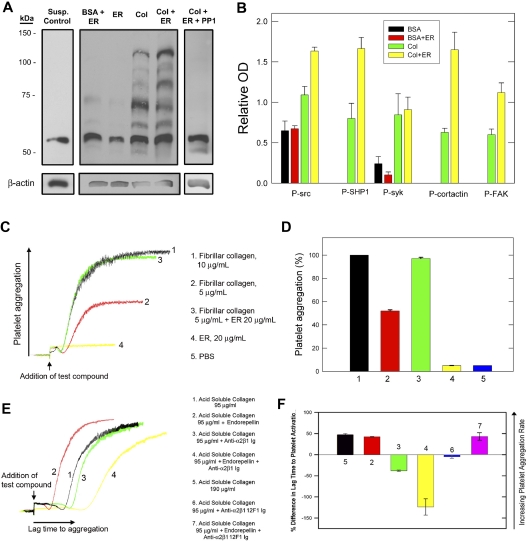
Next, we investigated the effects of endorepellin on platelet activation. Platelet adhesion to endorepellin or BSA plus liquid-phase endorepellin, unlike collagen or GFOGER,21 failed to activate platelets (Figure 2A). These conditions demonstrated phosphorylation of a prominent 60-kDa band that was also present in control platelets (Figure 2A) and very faint bands at 65 and 72 kDa, compared to multiple phosphorylated bands in activated collagen-adherent platelets. Addition of liquid-phase endorepellin to collagen-exposed platelets resulted in the appearance of a single additional band at 95 kDa and quantitative changes in the other bands (Figure 2A). By immunoblotting, we identified the 125-kDa band as focal adhesion kinase (FAK), also phosphorylated in endothelial cells by endorepellin,5 the 65-kDa band as SHP1, and the 85-kDa band as cortactin (not shown). Endorepellin caused an increase (P < .005) in Tyr-phosphorylation of 60-, 65-, 85-, and 125-kDa bands (Figure 2B). Preincubation with 10 μM PP1 with or without endorepellin (without endorepellin not shown) prevented the phosphorylation of all other bands (Figure 2A) except the 60-kDa band (Figure 2B). We hypothesized that the 60-kDa band was src20 and assayed src activation by immunoblotting for src pTyr418 (Figure S2A). Collagen-adherent platelets had more src pTyr418 compared to BSA-adherent platelets (P < .005) demonstrating that collagen activates src. Addition of liquid-phase endorepellin showed a further increase (P < .005) in src pTyr418 that could be blocked by PP1 (pTyr418 level not significantly different, P > .05, from that obtained with collagen alone, Figure S2B). Endorepellin alone had no effect on src pTyr418 and identical results were obtained with platelets in suspension (not shown). The PP1 results agree with our platelet-adhesion studies (Figure 1B) where PP1 suppressed endorepellin effects on collagen-platelet adhesion, but did not further inhibit adhesion. Endorepellin enhanced the rate of collagen-mediated FAK phosphorylation in suspended platelets (Figure S3), demonstrating that endorepellin enhances the rate and extent of collagen platelet activation, which also parallels the endorepellin rate enhancement of platelet adhesion to collagen.
Figure 2.
Endorepellin enhances collagen platelet activation and aggregation. (A) Representative immunoblotting (n = 4 experiments) using anti-PTyr antibody (PY20) of total platelet lysate under various conditions as indicated. Washed human platelets were added to wells coated with BSA, ER, or collagen (same concentrations as in Figure 1A) with or without liquid-phase ER (20 μg/mL) or with or without PP1 (10 μM) for 60 minutes at 37°C, followed by removal of nonadherent platelets, lysis of adherent platelets with ice-cold RIPA buffer, and protein separation by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Control, unplated platelets in suspension are also shown. (B) Optical density quantification (ImageJ software, mean OD ± SE, n = 4) of several phosphorylated proteins as preliminarily identified with specific antibodies (not shown) and indicated in the bottom. (C) Representative superimposed platelet aggregation traces in response to fibrillar collagen with or without ER, or ER. (D) Quantification of percent of maximal aggregation response (as seen with fibrillar collagen 10 μg/mL). Mean ± SE, n = 4. (E) Representative superimposed platelet aggregation traces in response to acid-soluble collagen with or without ER, or with or without anti-α2β1-integrin antibody. (F) Quantification of percent difference in lag time to platelet activation (as compared to the lag time to aggregation obtained with acid-soluble collagen, trace ‘1′ in (E). Effects of a nonfunctional blocking α2β1 antibody (12F1) are also shown.
These results demonstrate that, in the absence of collagen, endorepellin supports adhesion of unactivated platelets and does not subsequently activate them, whereas in the presence of collagen, endorepellin enhances platelet adhesion and activation. The ability of PP1 to diminish platelet/src activation, enhance platelet adhesion to endorepellin, and inhibit endorepellin-enhanced collagen responses demonstrates the central role of src in mediating the effects of endorepellin.
Endorepellin alone did not cause platelet aggregation but significantly (P < .001) enhanced platelet aggregation induced by fibrillar collagen I (Figure 2C-D), but not by PAF or ADP (not shown). When we used acid-soluble collagen, which binds and activates platelets specifically via α2β1 integrin,23,24 endorepellin shortened (P < .001) the lag time to acid-soluble collagen platelet aggregation (Figure 2E-F). In contrast, function-blocking α2β1 antibody increased it and nonfunction-blocking α2β1 antibody (12F1) had no effect. Unexpectedly, the combination of acid-soluble collagen, endorepellin, and function-blocking α2β1 antibody (but not 12F1) resulted in a significant increase in lag time (Figure 2E-F), suggesting that endorepellin potentiates antibody inhibition.
Perlecan, a widely expressed vascular basement membrane constituent, likely provides endorepellin at sites of injury and inflammation by proteolytic processing. We demonstrate that endorepellin binds to platelets and enhances collagen-mediated platelet responses that could promote clot formation and healing. Although both collagen and endorepellin interact with the platelet α2β1 receptor, our data suggest they interact differently. Because the α2β1 receptor exists in an inactive and 2 active conformational states with low or high collagen affinities, respectively,25 endorepellin could possibly function as a high-affinity α2β1 ligand, preferentially binding to inactive α2β1 and converting it into a high-affinity state; this would enhance α2β1-ligand–mediated responses. The ability of endorepellin to partially inhibit platelet adhesion to GFOGER is further evidence of a similar, possibly competitive, α2β1 interaction. Endorepellin does not directly bind to collagen3 and unlikely binds GFOGER, the major binding site for the α2 I domain within collagen I. GFOGER sequence is present at a higher frequency in the GFOGER peptide (∼17%, 1 copy/36 residues) than in a collagen I molecule (0.4%)21; so, adding higher concentrations of GFOGER could compete against collagen I for adhesion and enhance its own adhesion to platelets over collagen I. Unlike GFOGER,21 endorepellin does not activate platelets suggesting that GFOGER and endorepellin interact differently with α2β1. Furthermore, inhibition of platelet src kinase may inhibit inside-out activation of platelet α2β1 integrin21 effectively increasing α2β1- mediated adhesion to endorepellin and suppressing endorepellin ligand enhancement effects.
Supplementary Material
Acknowledgments
This work was supported by funds from the National Institutes of Health (RO1 CA39481 and R01 CA47282).
We thank Dr James San Antonio for valuable discussion and advice.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.B. was responsible for designing and performing experiments and interpreting results, and was the primary author of the manuscript; R.A.I., B.W., M.B., A.M., and S.C. assisted in performing all experiments as well as interpreting the results along with assisting in manuscript preparation; G.B.F. provided the collagen triple-helical peptide and helped in experimental design; and R.V.I. was responsible for supervising all the authors at Thomas Jefferson University, assisting in experimental design, interpreting results, formulating discussions, and editing the manuscript for scientific accuracy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renato V. Iozzo, Department of Pathology, Anatomy and Cell Biology, Rm 249 JAH, Thomas Jefferson University, 1020 Locust St, Philadelphia, PA, 19107; e-mail: iozzo@mail.jci.tju.edu.
References
- 1.Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- 2.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 3.Mongiat M, Sweeney S, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez EM, Reed CC, Bix G, et al. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J Biol Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 5.Bix G, Fu J, Gonzalez E, et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bix G, Iozzo RV. Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends Cell Biol. 2005;15:52–60. doi: 10.1016/j.tcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nature Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 8.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 9.Senger DR, Perruzzi CA, Streit M, et al. The α1β1 and α2β1 integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eble JA. Collagen-binding integrins as pharmaceutical targets. Curr Pharm Design. 2005;11:867–880. doi: 10.2174/1381612053381738. [DOI] [PubMed] [Google Scholar]
- 11.Holtkötter O, Nieswandt B, Smyth N, et al. Integrin α2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZG, Bothe I, Hirche F, et al. Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins: contribution of α2β1 integrin. J Cell Sci. 2006;119:1886–1895. doi: 10.1242/jcs.02921. [DOI] [PubMed] [Google Scholar]
- 13.Oda O, Shinzato T, Ohbayashi K, et al. Purification and characterization of perlecan fragment in urine of end-stage renal failure patients. Clin Chim Acta. 1996;255:119–132. doi: 10.1016/0009-8981(96)06395-4. [DOI] [PubMed] [Google Scholar]
- 14.Vuadens F, Benay C, Crettaz D, et al. Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics. 2003;3:1521–1525. doi: 10.1002/pmic.200300455. [DOI] [PubMed] [Google Scholar]
- 15.Thadikkaran L, Crettaz D, Siegenthaler MA, et al. The role of proteomics in the assessment of premature rupture of fetal membranes. Clinica Chimica Acta. 2005;360:27–36. doi: 10.1016/j.cccn.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Pieper R, Gatlin CL, McGrath AM, et al. Characterization of the human urinary proteome: a method for high-resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1400 distinct protein spots. Proteomics. 2004;4:1159–1174. doi: 10.1002/pmic.200300661. [DOI] [PubMed] [Google Scholar]
- 17.Adkins JN, Varnum SM, Auberry KJ, et al. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteom. 2002;1:947–955. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- 18.Laplante P, Raymond M-A, Labelle A, et al. Perlecan proteolysis induces α2β1 integrin and src-family kinases dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation. J Biol Chem. 2006;281:30383–30392. doi: 10.1074/jbc.M606412200. [DOI] [PubMed] [Google Scholar]
- 19.Arias-Salgado EG, Lizano S, Sarkar S, et al. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13295–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden A, Nemeth SP, Brugge JS. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986;83:852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin α2β1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCγ2. J Cell Biol. 2003;160:769–780. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farndale RW. Collagen-induced platelet activation. Blood Cells Mol Dis. 2006;36:162–165. doi: 10.1016/j.bcmd.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Jung SM, Moroi M. Platelets interact with soluble and insoluble collagens through characteristically different reactions. J Biol Chem. 1998;273:14827–14837. doi: 10.1074/jbc.273.24.14827. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Kahn ML. Reciprocal signaling by integrin and nonintegrin receptors during collagen activation of platelets. Mol Cell Biol. 2003;23:4764–4777. doi: 10.1128/MCB.23.14.4764-4777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van de Walle GR, Vanhoorelbeke K, Majer Z, et al. Two functional active conformations of the integrin α2β1, depending on activation condition and cell type. J Biol Chem. 2005;280:36873–36882. doi: 10.1074/jbc.M508148200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.