Abstract
Activation of the immune system against protozoan infections relies particularly on two specific signals provided by cognate interaction of T cells with antigen presenting cells (APCs). The first signal is attributed to binding of the T-cell receptor (TCR) to peptide/MHC complexes on the surface of APCs, whereas the second signal is triggered through binding of several costimulatory molecules on the surface of APCs with their corresponding receptors on T cells. Among these costimulatory signallings, CD40/CD40L interactions have been particularly investigated in protozoan infection models with regard to their potential to amplify cell-mediated immunity against intracellular parasites. This article reviews current studies of the potential role of CD40/CD40L interaction in the modulation of immune responses against some protozoan parasites and highlights recent developments regarding manipulation of this interaction for promoting control of parasite infections.
1. INTRODUCTION
A rational design of new approaches aiming to control protozoan infections depends not only on advances in our knowledge of virulent factors, molecular pathogenesis, and immune responses involved in the host defence, but also on our understanding of finely tuned co-stimulatory signallings that play a key role in immunity against infections. CD40-CD40L interaction has emerged in the last decade as an essential system that regulates host immune defence against infectious and noninfectious diseases. CD40, a surface glycoprotein receptor belonging to TNF receptor family is expressed by a number of cells including immunocompetent cells such as professional APCs, B lymphocytes, activated CD4+ and CD8+ T lymphocytes as well as nonhematopoetic cells [1–5]. Its ligand, CD40L, is a co-stimultory molecule that can be expressed on various cells such as CD4+ T lymphocytes, B lymphocytes, natural killer (NK) cells, dendritic cells (DCs), monocytes and macrophages [6–9]. CD40-CD40L interactions play an important role in the regulation of thymus-dependant humoral immune responses through cognate interaction of B and T cells which promotes B cell proliferation, Ig class switching, and generation of B cell memory [10, 11]. On the other hand, CD40-CD40L interactions drive also cell-mediated immune responses. Engagement of CD40 present on APCs with CD40L on T cells is crucial for the priming and expansion of antigen-specific CD4+ T cells and for the induction of costimulatory molecules on APCs [12]. It is well known that triggering of CD40 on the surface of APCs leads to the production of cytokines, particularly IL-12 which plays a central role in the activation of T cells to produce IFN-γ, thereby directing cell-mediated immunity towards Th1 subset that is required for effective immunity against intracellular pathogens [13–15]. Naturally occurring mutation in human CD40L gene results in a defect in CD40 signalling and leads to hyper IgM syndrome. Theses patients not only are defective in humoral immunity but also exhibit impaired T cell-mediated immunity and therefore are susceptible to infections [16, 17]. In this review, we focus on insights provided by different studies arguing that cell-mediated immunity against intracellular parasites depends upon CD40-CD40L interactions.
2. CD40/CD40L INTERACTION PROMOTES CELL-MEDIATED IMMUNITY AGAINST PROTOZOAN INFECTIONS
2.1. Leishmania
Major insights gained into paradigms of Th-subset came from studies on immunity to Leishmania infection. Resistance to Leishmania which multiplies as an amastigote within macrophages depends on polarization of the immune response towards Th1 type in which IL-12 and IFN-γ play a pivotal role, while disease progression is linked to development of Th2 type response [18]. Since CD40-CD40L interaction is crucial for promoting Th1 immune response, various studies were focused on the impact of such interaction on the outcome of experimental infection. The first evidence for a direct role of these molecule pairs in promoting immune responses against protozoan infections came from studies showing that CD40- and CD40L-deficient mice are susceptible to Leishmania infections [19–21]. T cells from these mice fail to produce IFN-γ suggesting a defect in Th1 response. Conversely, administration of IL-12 in these deficient mice prevents disease progression. These deficient mice were unable to mount an effective immunity against parasite infection due to a defect in T cell mediated activation of macrophages. Beside their role in Th1 immune response, CD40-CD40L interactions were shown also to stimulate macrophages to produce number of cytokines and inflammatory mediators among which Nitric Oxide (NO) plays a key role in parasite killing [22]. Theses basic studies on Leishmania infections clearly pointed towards a major role of CD40-CD40L interactions in skewing immune response to Th1 type that is required for antiparasite host defence.
2.2. Trypanosoma cruzi
T. cruzi is an obligate intracellular parasite that invades several types of cells in vertebrate hosts. Development of a Th1-like immune response was sown to be associated with the control of infection in mice [23]. In particular, IL-12, IFN-γ, and TNFα play a crucial role in the development of cell-mediated immunity against the parasite [24]. Indeed, treatment of T. cruzi-infected mice with anti-IL-12 MAb increases parasitemia and mortality while an exogenous supply of IL-12 confers protection against infection [25, 26]. In view of the important role of IL-12, it seemed likely that CD40 ligation is important for induction of effector phases of immune response. The potent effect of the CD40-CD40L pathway in T. cruzi infection was first assessed by using CD40L-transfected 3T3 fibroblasts to monitor parasitological and immunological parameters in infected mice [27]. This study indicated that supernatants of murine spleen cells stimulated with CD40L-transfected cells prevent infection of macrophages in vitro and this phenomenon depends on de novo production of nitric oxide (NO). Anti-IL-12, anti-IFN-γ, and anti-TNFα MAb neutralize the effect of supernatants suggesting the importance of these cytokines in the prevention of macrophage infection. This in vitro data were further supported by in vivo experiments showing that coinoculation of CD40L-transfected 3T3 fibroblasts and T. cruzi into mice leads to reduced parasitemia and mortality and this effect is abolished by injection of anti-IL-12 MAb. Recently, we examined further the role of CD40 ligation in T. cruzi infection by using a new approach based on generation of CD40L-transfected parasite strain [28]. Mice inoculated with this recombinant strain exhibit a very low parasitemia and no mortality associated with preserved production of IFN-γ by spleen cells compared to wild-type strain. These findings highlight the potent role of CD40-CD40L interaction in the stimulation of an effective immunity against T. cruzi.
2.3. Toxoplasma gondii
Tachyzoites of T. gondii can disseminate in the host because of its ability to infect many nucleated cells. Since IFN-γ is the major cytokine required for the activation of a cell-mediated immunity against T. gondii [29], and giving the importance of IL-12 in the stimulation of early IFN-γ synthesis [30], one may suspect a critical role of CD40-CD40L interaction in the control of Toxoplasma infections. Studies performed in human with hyper IgM syndrome due to a natural CD40L mutation revealed that these patients exhibited a defect in IFN-γ secretion in response to T. gondii [31]. The lack of IFN-γ production was linked to impaired IL-12 secretion, indicating that CD40-CD40L signalling was required for an optimal T cell activation and production of IFN-γ. On the other hand, Toxoplasma infection was also investigated in CD40L-deficient mice [32]. This study showed that these mice produced less IL-12 than wild type when infected with T. gondii. Moreover, CD40L-deficient mice succumbed to toxoplasmic encephalitis indicating that these mice were not able to control parasite replication in the brain and suggesting an important role of the CD40-CD40L interaction in this process. Furthermore, CD40 signalling was shown to regulate IFN-γ-independent host protection against Toxoplasma infection through TNF-α-dependant induction of macrophage antimicrobial activity [33]. Susceptibility of both patients with hyper IgM syndrome and CD40L-deficient mice to Toxoplasma infection argues for the requirement of CD40/CD40L signalling for resistance to parasite infection.
3. MANIPULATION OF CD40 SIGNALLING AS A POTENTIAL TOOL TO IMPROVE CONTROL OF PROTOZOAN INFECTIONS
As reported above, CD40-CD40L interaction is crucial for the outcome of infection in a number of intracellular parasite models. Stimulation of CD40 on APCs has proved to be useful for amplification of Th1-type response in which IL-12 and IFN-γ play a cardinal role (Figure 1). The first approach used to modulate CD40 signalling was based on agonistic anti-CD40 Ab. Injection of these Ab in mice infected with Leishmania stimulates IL-12 and IFN-γ production and induces killing of the parasites within macrophages [34, 35]. Similarly, administration of anti-CD40L MAb in mice infected with T. cruzi results in a stimulation of IFN-γ-activated macrophages to produce NO and tocontrol parasite infection [27]. Although CD40L exists in nature predominantly as a membrane-anchored molecule, the molecular characterization of CD40L molecule indicated that the extra-cellular carboxy-terminal region can be soluble and biologically active [6, 36]. Therefore, a variety of reports were focused on this agonistic molecule with the aim to assess its stimulatory role in the control of parasite infection as it is the case for other infectious diseases [14, 37]. Studies in patients with hyper IgM syndrome that exhibit deficient secretion of IL-12 showed that in vitro incubation of peripheral blood mononuclear cells (PBMC) with a soluble CD40L resulted in enhanced IL-12-dependant production of IFN-γ in response to Toxoplasma gondii [31]. Soluble CD40L was also shown to activate murine macrophages in vitro to control the replication of T. gondii [32].
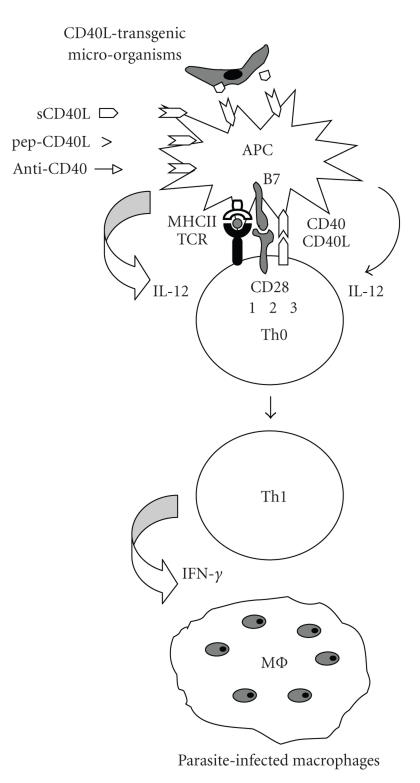
Figure 1.
Schematical representation of enhanced cell-mediated immunity against protozoan parasites through CD40-CD40L interaction. Following the first signal (1) illustrated by the recognition of parasite peptides combined with major histocompatibility complex II (MHC II) on antigen presenting cells (APC), such as dendritic cells, by T cell receptor (TCR) on naïve T helper cells (Th0), and the second signal (2) which is attributed mainly to binding of CD28/B7 molecule pairs, interaction of CD40-CD40L (3) activates APC to produce IL-12 which promotes Th1 cell differentiation and secretion of IFN-γ. This leads to stimulation of macrophages to control parasite replication. This activation process can be amplified by agonistic anti-CD40 antiboy, by soluble CD40L (sCD40L) or by CD40L peptide mimetics (pep-CD40L). Transgenic parasites expressing CD40L can also activate CD40 signalling through membrane-bound or secreted CD40L.
As for many adjuvant proteins, the major obstacles related to stability and issues of in vivo delivery of CD40L had to be faced. In this regard, host cells transfected with CD40L gene were developed as a way of delivery in different parasitic models [27, 38]. Interestingly, Chen et al. described a strategy based on directing CD40L to macrophages by coexpressing the molecule on the surface of a cell line along with gp63 recombinant Leishmania antigen and showed that mice treated with these cotransfected cells produce higher amounts of IL-12 and control the disease progression [38]. Delivery of CD40L expressed by transfected 3T3 fibroblasts was shown to reduce parasitemia in T. cruzi-infected mice [27]. Recently, we developed a new concept based on the use of the pathogenic organism as a vehicle for CD40L delivery [28]. Following transfection of T. cruzi with CD40L gene, the encoded molecule was found properly processed and secreted across the parasite membrane. Notably, CD40L recombinant strain exhibited lower virulence and induced higher INF-γ production when injected into mice. Moreover, surviving mice resisted a challenge infection with wild-type strain, thereby confirming the vaccine adjuvant capacity of CD40L.
The molecular characterization of CD40L and the analysis of its binding domains were determinant steps towards the manipulation of CD40 signalling [39]. As for TNF receptors family, the signalling through CD40 depends upon the formation of a CD40L trimer complex that can each bind three CD40 molecules [40]. Incorporation of an isoleucine zipper motif that improves trimerization of the CD40L was shown to enhance its biological activity [41]. Advances in the molecular structures of CD40L binding domains allowed a conception of small CD40L mimetic molecules that could compete with the binding of CD40L homotrimers and induce IL-12 secretion by DCs [42]. Interestingly, recent findings indicate that when these mini-CD40L synthetic peptides were coinjected with T. cruzi into mice, a low parasitemia associated with enhanced CD8+ T cells producing IFN-γ was observed [43]. These recent reports further support the importance of CD40L delivery as an adjuvant that can be used to drive type 1 immune response against intracellular parasite infection (Figure 1).
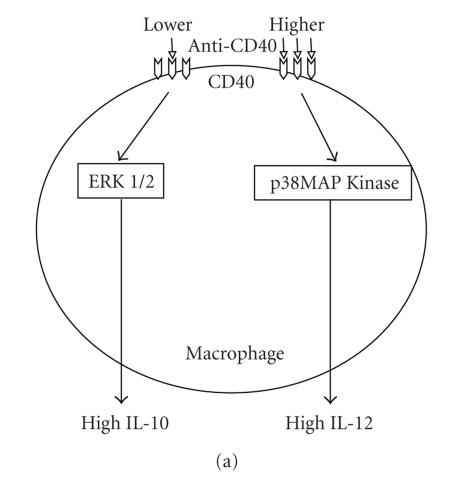
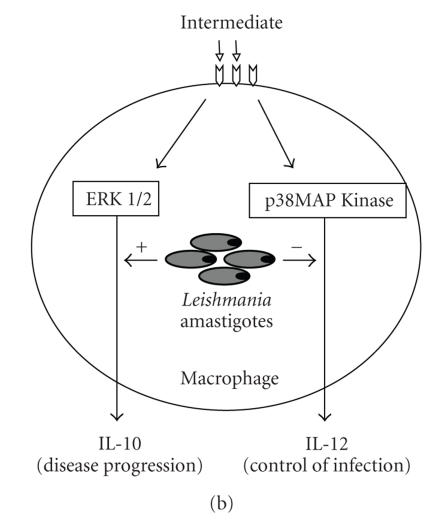
Protozoan parasites have a remarkable ability to adapt to different host microenvironments. To make the host “as safe as possible” for them, they have evolved many devises among which the immunosuppression is considered as a powerful mechanism to subvert the host's immune response [44]. Interfering with CD40 presentation or signalling can be one of the powerful arms used by the parasite. Indeed, CD40 molecules were found to be reduced in the surface of macrophages and DCs following their infection by T. cruzi [45, 46]. Recently, the CD40 was defined as a central molecule through which counteractive immune responses can be triggered [47]. Based on the cross-linking experiments with anti-CD40 Ab on macrophages, this study indicated that the strength of CD40 signalling activates p38-mitogen activated protein kinase (p38MAPK) or extracellular stress-related kinase 1/2 (ERK-1/2) signalling molecules leading to a differential expression of IL-12 or IL-10. CD40 cross-linking at lower doses induces activation of ERK-1/2 and production of IL-10, a cytokine which promotes immunosuppression, whereas at higher doses it induces activation of p38MAPK and secretion of IL-12 (Figure 2(a)). How such strength of CD40 signalling is operating through interaction of the immunocompetent cells and whether it can influence the Th1 and Th2 immunoregulatory processes are still unclear. Interestingly, Leishmania-infected macrophages treated with an intermediate dose of anti-CD40 Ab produced more IL-10 and less IL-12 than uninfected macrophages (Figure 2(b)), suggesting that Leishmania infection promotes IL-10 production that would favour disease progression [47]. This study is in line with a previous report indicating that p38MAPK-dependant CD40 signalling is impaired in Leishmania-infected macrophages [35]. Overall, the involvement of CD40 signalling in the host-parasite interaction further exemplifies the refined nature of the host-pathogen cross-talk.
Figure 2.
Strength of CD40-CD40L interaction can influence the outcome of parasite infection. (a) Cross-linking of CD40 with lower doses of agonistic anti-CD40 antibody (< 3 μg/mL) on noninfected macrophages increases phosphorylation of extracellular stress-related kinase 1/2 (ERK 1/2) and consequently stimulates high production of IL-10, whereas cross-linking with higher doses (> 3 μg/mL) increases phosphorylation of p38-mitogen activated protein kinase (p38MAPK) and therefore stimulates high production of IL-12. (b) Cross-linking of Leishmania-infected macrophages with intermediate dose of anti-CD40 antibody (3 μg/mL) leads to production of more IL-10 and less IL-12 than uninfected macrophages. This suggests the potential involvement of parasite factors in disease progression through stimulation of IL-10 production.
4. CONCLUDING REMARKS
Increasing evidence points towards the crucial role of CD40-CD40L for the development of a cellular host protective immune response against intracellular parasites. Although the role of CD40-CD40L signalling in B cell maturation and isotype switching is well documented, little is known about the potent stimulation of CD40 signalling that can promote humoral immune response against extracellular parasites. A study on African Trypanoma infection in a model of SCID mice reconstituted with a bovine immune system indicated that administration of an agonistic antibody against CD40 enhanced mice survival to infection with Trypanosoma congolense, and was associated with increased production of specific IgG [48]. Further studies aiming to depict accurately the CD40 signalling-dependant protective Th2 immunity against protozoan parasites are yet to be investigated. The stimulation of CD40 signalling by CD40L and its derivatives can be considered as a useful adjunct in a vaccine strategy against protozoan infections. Current knowledge in host-parasite interaction includes a breakthrough in the modulation of CD40 signalling brought about by using soluble CD40L, CD40L-transgenic microorganisms or small peptide mimetics of the CD40L. However, recent findings outlined the possible regulation of CD40 signalling by the parasite and therefore stressed the need of a further understanding of the host-pathogen crosstalk that could lead to novel approaches for disease control.
ACKNOWLEDGMENT
The author would like to thank Drs B. Vray, M. Goldman, Y. Carlier, and A. Allaoui for discussions and support.
References
- 1.Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schriever F, Freedman AS, Freeman G, et al. Isolated human follicular dendritic cells display a unique antigenic phenotype. Journal of Experimental Medicine. 1989;169(6):2043–2058. doi: 10.1084/jem.169.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. Journal of Experimental Medicine. 1993;178(2):669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollenbaugh D, Mischel-Petty N, Edwards CP, et al. Expression of functional CD40 by vascular endothelial cells. Journal of Experimental Medicine. 1995;182(1):33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Current Opinion in Immunology. 1997;9(3):330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 6.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 7.Hollenbaugh D, Grosmaire LS, Kullas CD, et al. The human T cell antigen GP39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of GP39 with B cell co-stimulatory activity. The EMBO Journal. 1992;11(12):4313–4321. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of Leukocyte Biology. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Wykes M. Why do B cells produce CD40 ligand? Immunology and Cell Biology. 2003;81(4):328–331. doi: 10.1046/j.1440-1711.2003.01171.x. [DOI] [PubMed] [Google Scholar]
- 10.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annual Review of Immunology. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 11.Clark LB, Foy TM, Noelle RJ. CD40 and its ligand. Advances in Immunology. 1996;63:43–78. doi: 10.1016/s0065-2776(08)60854-8. [DOI] [PubMed] [Google Scholar]
- 12.Grewal IS, Flavell RA. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunology Today. 1996;17(9):410–414. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- 13.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. Journal of Experimental Medicine. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grewal IS, Borrow P, Pamer EG, Oldstone MB, Flavell RA. The CD40-CD154 system in anti-infective host defense. Current Opinion in Immunology. 1997;9(4):491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- 15.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annual Review of Immunology. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 16.Notarangelo LD, Duse M, Ugazio AG. Immunodeficiency with hyper-IgM (HIM) Immunodeficiency Reviews. 1992;3(2):101–121. [PubMed] [Google Scholar]
- 17.Levy J, Espanol-Boren T, Thomas C, et al. Clinical spectrum of X-linked hyper-IgM syndrome. Journal of Pediatrics. 1997;131(1):47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 18.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nature Reviews Immunology. 2002;2(11):845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 19.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4(3):283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 20.Kamanaka M, Yu P, Yasui T, et al. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4(3):275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 21.Soong L, Xu J-C, Grewal IS, et al. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4(3):263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 22.Stout RD, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunology Today. 1996;17(10):487–492. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Tarleton RL. Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. Journal of Immunology. 2001;166(7):4596–4603. doi: 10.4049/jimmunol.166.7.4596. [DOI] [PubMed] [Google Scholar]
- 24.Martin D, Tarleton R. Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection. Immunological Reviews. 2004;201(1):304–317. doi: 10.1111/j.0105-2896.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 25.Aliberti JCS, Cardoso MAG, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infection and Immunity. 1996;64(6):1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter CA, Slifer T, Araujo F. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infection and Immunity. 1996;64(7):2381–2386. doi: 10.1128/iai.64.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaussabel D, Jacobs F, de Jonge J, et al. CD40 ligation prevents Trypanosoma cruzi infection through interleukin- 12 upregulation. Infection and Immunity. 1999;67(4):1929–1934. doi: 10.1128/iai.67.4.1929-1934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamekh M, Vercruysse V, Habib M, et al. Transfection of Trypanosoma cruzi with host CD40 ligand results in improved control of parasite infection. Infection and Immunity. 2005;73(10):6552–6561. doi: 10.1128/IAI.73.10.6552-6561.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 30.Gazzinelli RT, Wysocka M, Hayashi S, et al. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. Journal of Immunology. 1994;153(6):2533–2543. [PubMed] [Google Scholar]
- 31.Subauste CS, Wessendarp M, Sorensen RU, Leiva LE. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. Journal of Immunology. 1999;162(11):6690–6700. [PubMed] [Google Scholar]
- 32.Reichmann G, Walker W, Villegas EN, et al. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infection and Immunity. 2000;68(3):1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subauste CS, Wessendarp M. CD40 restrains in vivo growth of Toxoplasma gondii independently of gamma interferon. Infection and Immunity. 2006;74(3):1573–1579. doi: 10.1128/IAI.74.3.1573-1579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferlin WG, von der Weid T, Cottrez F, Ferrick DA, Coffman RL, Howard MC. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. European Journal of Immunology. 1998;28(2):525–531. doi: 10.1002/(SICI)1521-4141(199802)28:02<525::AID-IMMU525>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Awasthi A, Mathur R, Khan A, et al. CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response. Journal of Experimental Medicine. 2003;197(8):1037–1043. doi: 10.1084/jem.20022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzei GJ, Edgerton MD, Losberger C, et al. Recombinant soluble trimeric CD40 ligand is biologically active. Journal of Biological Chemistry. 1995;270(13):7025–7028. doi: 10.1074/jbc.270.13.7025. [DOI] [PubMed] [Google Scholar]
- 37.Kornbluth RS. The emerging role of CD40 ligand in HIV infection. Journal of Leukocyte Biology. 2000;68(3):373–382. [PubMed] [Google Scholar]
- 38.Chen G, Darrah PA, Mosser DM. Vaccination against the intracellular pathogens Leishmania major and L. amazonensis by directing CD40 ligand to macrophages. Infection and Immunity. 2001;69(5):3255–3263. doi: 10.1128/IAI.69.5.3255-3263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fanslow WC, Srinivasan S, Paxton R, Gibson MG, Spriggs MK, Armitage RJ. Structural characteristics of CD40 ligand that determine biological function. Seminars in Immunology. 1994;6(5):267–278. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- 40.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 41.Morris AE, Remmele RL, Jr, Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154) Journal of Biological Chemistry. 1999;274(1):418–423. doi: 10.1074/jbc.274.1.418. [DOI] [PubMed] [Google Scholar]
- 42.Fournel S, Wieckowski S, Sun W, et al. C3-symmetric peptide scaffolds are functional mimetics of trimeric CD40L. Nature Chemical Biology. 2005;1(7):377–382. doi: 10.1038/nchembio746. [DOI] [PubMed] [Google Scholar]
- 43.Habib M, Noval Rivas M, Chamekh M, et al. Small CD40L mimetics promote control of parasitemia and enhances T cells producing interferon-γ during experimental Trypanosoma cruzi infection. doi: 10.4049/jimmunol.178.11.6700. to appear in Journal of Immunology. [DOI] [PubMed] [Google Scholar]
- 44.Zambrano-Villa S, Rosales-Borjas D, Carrero JC, Ortiz-Ortiz L. How protozoan parasites evade the immune response. Trends in Parasitology. 2002;18(6):272–278. doi: 10.1016/s1471-4922(02)02289-4. [DOI] [PubMed] [Google Scholar]
- 45.Van Overtvelt L, Vanderheyde N, Verhasselt V, et al. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infection and Immunity. 1999;67(8):4033–4040. doi: 10.1128/iai.67.8.4033-4040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Planelles L, Thomas MC, Marañón C, Morell M, López MC. Differential CD86 and CD40 co-stimulatory molecules and cytokine expression pattern induced by Trypanosoma cruzi in APCs from resistant or susceptible mice. Clinical and Experimental Immunology. 2003;131(1):41–47. doi: 10.1046/j.1365-2249.2003.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathur RK, Awasthi A, Wadhone P, Ramanamurthy B, Saha B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nature Medicine. 2004;10(5):540–544. doi: 10.1038/nm1045. [DOI] [PubMed] [Google Scholar]
- 48.Haas KM, Taylor KA, MacHugh ND, Kreeger JM, Estes DM. Enhancing effects of anti-CD40 treatment on the immune response of SCID-bovine mice to Trypanosoma congolense infection. Journal of Leukocyte Biology. 2001;70(6):931–940. [PubMed] [Google Scholar]